Ocular Manifestations in Patients with Philadelphia-Negative Myeloproliferative Neoplasms
Abstract
1. Introduction
2. Method
3. Ocular Manifestations Prevalence and Pathophysiology
3.1. External Eye Involvement
Orbit, Eyelid and Lacrimal Gland Involvement
3.2. Anterior Segment Diseases
3.2.1. Cornea
3.2.2. Conjunctiva
3.2.3. Iris and Angle
3.3. Posterior Segment Diseases
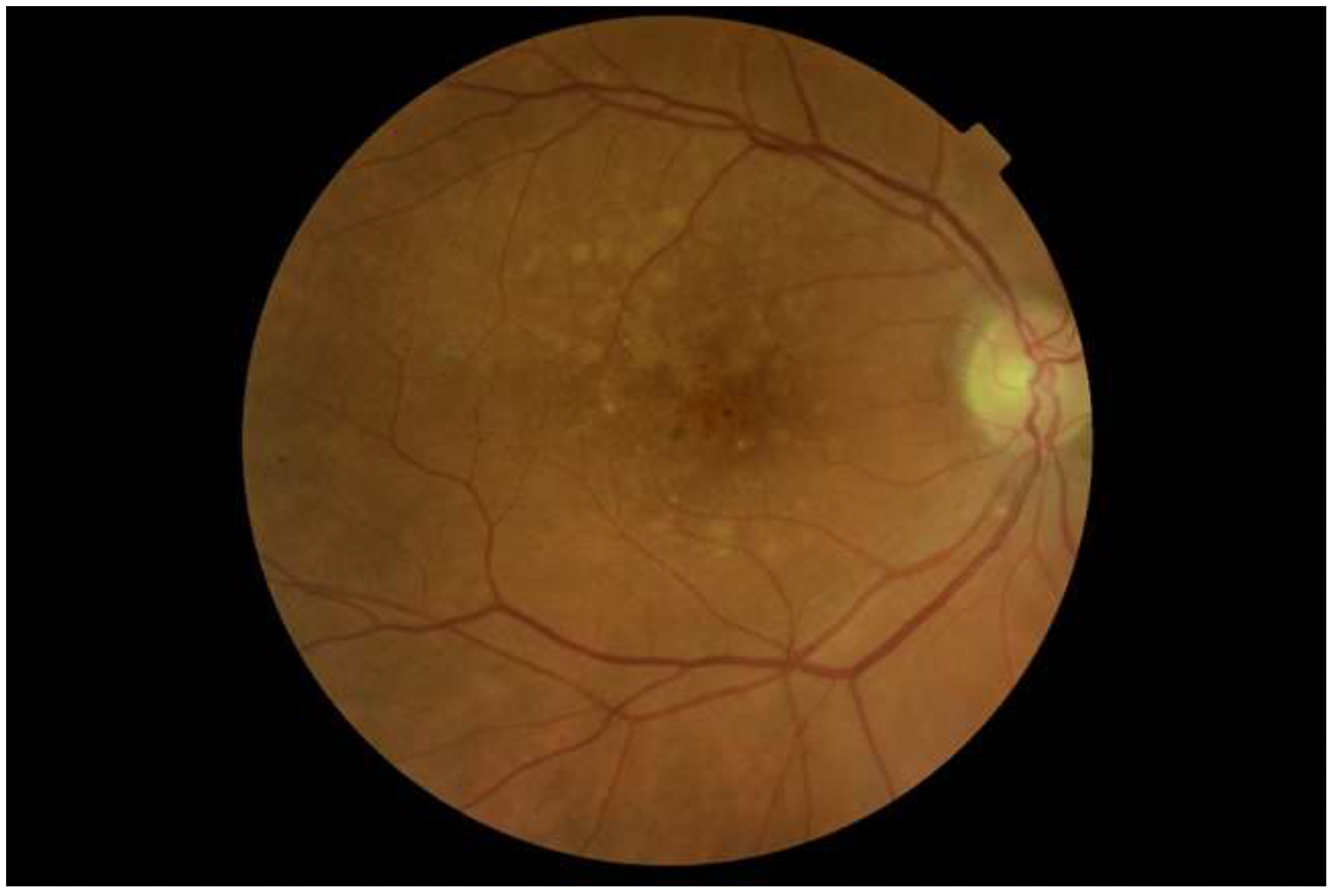
3.3.1. Retina and Choroid
3.3.2. Optic Nerve
3.4. Vaso-Occlusions, Haemorrhages and Microangiopathy
3.5. Neuro-Ophthalmologic Symptoms
3.6. Ocular Adverse Effects to Treatment
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rampal, R.; Al-Shahrour, F.; Abdel-Wahab, O.; Patel, J.P.; Brunel, J.-P.; Mermel, C.H.; Bass, A.J.; Pretz, J.; Ahn, J.; Hricik, T.; et al. Integrated Genomic Analysis Illustrates the Central Role of JAK-STAT Pathway Activation in Myeloproliferative Neoplasm Pathogenesis. Blood 2014, 123, e123–e133. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms. JAMA Oncol. 2015, 1, 97. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.; Spivak, J.L. Myeloproliferative Neoplasms. N. Engl. J. Med. 2017, 376. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.J.; Koudstaal, P.J.; Mulder, A.H.; van Vliet, H.H. Transient Neurologic and Ocular Manifestations in Primary Thrombocythemia. Neurology 1993, 43, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Jabaily, J.; Iland, H.J.; Laszlo, J.; Massey, E.W.; Faguet, G.B.; Brière, J.; Landaw, S.A.; Pisciotta, A.V. Neurologic Manifestations of Essential Thrombocythemia. Ann. Intern. Med. 1983, 99, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Joe, S.G.; Kim, J.-G.; Park, S.H.; Ko, H.S. Delayed Choroidal and Retinal Blood Flow in Polycythaemia Vera Patients with Transient Ocular Blindness: A Preliminary Study with Fluorescein Angiography. Br. J. Haematol. 2013, 161, 745–747. [Google Scholar] [CrossRef]
- Fenaux, P.; Simon, M.; Caulier, M.T.; Lai, J.L.; Goudemand, J.; Bauters, F. Clinical Course of Essential Thrombocythemia in 147 Cases. Cancer 1990, 66, 549–556. [Google Scholar] [CrossRef]
- Regev, A.; Stark, P.; Blickstein, D.; Lahav, M. Thrombotic Complications in Essential Thrombocythemia with Relatively Low Platelet Counts. Am. J. Hematol. 1997, 56, 168–172. [Google Scholar] [CrossRef]
- Billot, S.; Kouroupi, E.G.; Le Guilloux, J.; Cassinat, B.; Jardin, C.; Laperche, T.; Fenaux, P.; Carpentier, A.F.; Kiladjian, J.-J. Neurological Disorders in Essential Thrombocythemia. Haematologica 2011, 96, 1866–1869. [Google Scholar] [CrossRef]
- Michiels, J.J.; van Genderen, P.J.; Jansen, P.H.; Koudstaal, P.J. Atypical Transient Ischemic Attacks in Thrombocythemia of Various Myeloproliferative Disorders. Leuk. Lymphoma 1996, 22, 65–70. [Google Scholar] [CrossRef]
- Michiels, J.J.; Berneman, Z.; Gadisseur, A.; Lam, K.H.; De Raeve, H.; Schroyens, W. Aspirin-Responsive, Migraine-like Transient Cerebral and Ocular Ischemic Attacks and Erythromelalgia in JAK2-Positive Essential Thrombocythemia and Polycythemia Vera. Acta. Haematol. 2015, 133, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, P.J.; Koudstaal, A. Neurologic and Visual Symptoms in Essential Thrombocythemia: Efficacy of Low, Dose Aspirin. Semin. Thromb. Hemost. 1997, 23, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.J.; Berneman, Z.N.; Schroyens, W.; Van Vliet, H.H.D.M. Pathophysiology and Treatment of Platelet-Mediated Microvascular Disturbances, Major Thrombosis and Bleeding Complications in Essential Thrombocythaemia and Polycythaemia Vera. Platelets 2004, 15, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lee, A.G.; Rice, L.; Lambert, H.M. Bilateral Retinal Vascular Occlusive Disease in Essential Thrombocythemia. Retina 1999, 19, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; de Nully Brown, P.; Lund, B.V.; Nielsen, O.J.; Hasselbalch, H.C. Increased Platelet Activation and Abnormal Membrane Glycoprotein Content and Redistribution in Myeloproliferative Disorders. Br. J. Haematol. 2000, 110, 116–124. [Google Scholar] [CrossRef]
- de Lacerda, J.F.; Oliveira, S.N. Chronic Myeloproliferative Diseases. Handb. Clin. Neurol. 2014, 120, 1073–1081. [Google Scholar] [CrossRef]
- Carraro, M.C.; Rossetti, L.; Gerli, G.C. Prevalence of Retinopathy in Patients with Anemia or Thrombocytopenia. Eur. J. Haematol. 2001, 67, 238–244. [Google Scholar] [CrossRef]
- Haskes, C.; Gagnon, K. Retinal Manifestations of Idiopathic Myelofibrosis, a Hematologic Disorder. J. Am. Optom. Assoc. 1998, 69, 319–328. [Google Scholar]
- Charles, K.S.; Leelah, N.; Boodoo, L.; Murray, D.C. Ophthalmic Manifestations of Haematological Disorders. West Indian Med. J. 2013, 62, 99–103. [Google Scholar]
- Lin, A.L.; Burnham, J.M.; Pang, V.; Idowu, O.; Iyer, S. OCULAR MANIFESTATIONS OF PRIMARY MYELOFIBROSIS. Retin. Cases Brief Rep. 2016, 10, 364–367. [Google Scholar] [CrossRef]
- Ghazi, N.G.; Bowman, A.M.; Shields, M.D. Bilateral Lacrimal System Involvement by Sclerosing Extramedullary Hematopoietic Tumor. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Grewal, J.; Gupta, S.; Murray, P.I. Ophthalmic Manifestations of Acute Leukaemias: The Ophthalmologist’s Role. Eye 2004, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Yuen, H.K.L.; Mahesh, L.; Tse, R.K.K.; Yau, K.C.; Chan, N.; Lam, D.S.C. Orbital Sclerosing Extramedullary Hematopoietic Tumor. Arch. Ophthalmol. 2005, 123, 689–691. [Google Scholar] [CrossRef]
- Sohawon, D.; Lau, K.K.; Lau, T.; Bowden, D.K. Extra-Medullary Haematopoiesis: A Pictorial Review of Its Typical and Atypical Locations. J. Med. Imaging Radiat. Oncol. 2012, 56, 538–544. [Google Scholar] [CrossRef]
- YAMAMOTO, K.; MIWA, Y.; ABE-SUZUKI, S.; ABE, S.; KIRIMURA, S.; ONISHI, I.; KITAGAWA, M.; KURATA, M.; Kirimura, S.; Kirimura, S.; et al. Extramedullary Hematopoiesis: Elucidating the Function of the Hematopoietic Stem Cell Niche (Review). Mol. Med. Rep. 2016, 13, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.R.; Price, K.; Woodward, J.A. Massive Eyelid Thickening in Pachydermoperiostosis with Myelofibrosis. Ophthal. Plast. Reconstr. Surg. 2009, 25, 316–318. [Google Scholar] [CrossRef]
- Tanaka, H.; Maehama, S.; Imanaka, F.; Sakai, A.; Abe, K.; Hamada, M.; Yamashita, J.; Klmura, A.; Imamura, N.; Fujimuraand, K.; et al. Pachydermoperiostosis with Myelofibrosis and Anemia: Report of a Case of Anemiaof Multifactorial Causes and Its Improvement with Steroid Pulse and Iron Therapy. Jpn. J. Med. 1991, 30, 73–80. [Google Scholar] [CrossRef][Green Version]
- Na, J.; Choi, S.Y.; Baek, S.; Lee, H. Hemorrhage and Infarction of the Conjunctiva and Orbit in Essential Thrombocythemia. J. Craniofac. Surg. 2017, 28, 750–751. [Google Scholar] [CrossRef]
- Tefferi, A.; Fonseca, R.; Pereira, D.L.; Hoagland, H.C. A Long-Term Retrospective Study of Young Women With Essential Thrombocythemia. Mayo Clin. Proc. 2001, 76, 22–28. [Google Scholar] [CrossRef]
- Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; Antonioli, E.; et al. Risk Factors for Arterial and Venous Thrombosis in WHO-Defined Essential Thrombocythemia: An International Study of 891 Patients. Blood 2011, 117, 5857–5859. [Google Scholar] [CrossRef]
- Lindsey, J.; Insler, M.S. Polycythemia Rubra Vera and Conjunctival Vascular Congestion. Ann. Ophthalmol. 1985, 17, 62–63. [Google Scholar] [PubMed]
- Cuttler, N.; Heidemann, D.; Cendrowski, C.; Armin, A.-R.; Folberg, R. Extramedullary Hematopoiesis of the Conjunctiva Presenting as Active Systemic Disease in a Patient with Myelofibrosis. Cornea 2014, 33, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, T.L.; Mortzos, P. Iritis, Ptosis, and Sequential Severe Loss of Vision in a Patient with Essential Thrombocytosis. Eye 2010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshizumi, M.O.; Townsend-Pico, W. Essential Thrombocythemia and Central Retinal Vein Occlusion with Neovascular Glaucoma. Am. J. Ophthalmol. 1996, 121, 728–730. [Google Scholar] [CrossRef]
- Strassman, I.; Silverstone, B.Z.; Seelenfreund, M.H.; Sheer, A.; Berson, D. Essential Thrombocythemia: A Rare Case of Central Retinal Artery Occlusion. Metab. Pediatr. Syst. Ophthalmol. 1991, 14, 18–20. [Google Scholar]
- Rajagopal, R.; Apte, R.S. Seeing through Thick and through Thin: Retinal Manifestations of Thrombophilic and Hyperviscosity Syndromes. Surv. Ophthalmol. 2016, 61, 236–247. [Google Scholar] [CrossRef]
- Imasawa, M.; Iijima, H. Multiple Retinal Vein Occlusions in Essential Thrombocythemia. Am. J. Ophthalmol. 2002, 133, 152–155. [Google Scholar] [CrossRef]
- Singer, G. Migrating Emboli of Retinal Arteries in Thrombocythaemia. Br. J. Ophthalmol. 1969, 53, 279–281. [Google Scholar] [CrossRef][Green Version]
- Willerslev, A.; Hansen, M.M.; Klefter, O.N.; Bjerrum, O.W.; Hasselbalch, H.C.; Clemmensen, S.N.; Larsen, M.; Munch, I.C. Non-Invasive Imaging of Retinal Blood Flow in Myeloproliferative Neoplasms. Acta Ophthalmol. 2017, 95, 146–152. [Google Scholar] [CrossRef]
- Ahn, B.-Y.; Choi, K.-D.; Choi, Y.-J.; Jea, S.-Y.; Lee, J.-E. Isolated Monocular Visual Loss as an Initial Manifestation of Polycythemia Vera. J. Neurol. Sci. 2007, 258, 151–153. [Google Scholar] [CrossRef]
- Raval, V.; Sudana, P.; Das, T. Idiopathic Myelofibrosis Causing Inflammatory Central Retinal Vein Occlusion Mimicking Frosted Branch Angiitis. BMJ Case Rep. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Rue, K.S.; Hirsch, L.K.; Sadun, A.A. Impending Anterior Ischemic Optic Neuropathy with Elements of Retinal Vein Occlusion in a Patient on Interferon for Polycythemia Vera. Clin. Ophthalmol. 2012, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Rodgin, S.G. Retinopathy of Blood Dyscrasias. J. Am. Optom. Assoc. 1993, 64, 769–779. [Google Scholar] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet. Glob. Heal. 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Metelitsina, T.I.; DuPont, J.C.; Ying, G.-S.; Maguire, M.G. Reduced Foveolar Choroidal Blood Flow in Eyes with Increasing AMD Severity. Investig. Opthalmology Vis. Sci. 2005, 46, 1033. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.J.; Silverman, R.H.; Rondeau, M.J.; Lloyd, H.O.; Khanifar, A.A.; Chan, R.V.P. Age-Related Macular Degeneration: Choroidal Ischaemia? Br. J. Ophthalmol. 2013, 97, 1020–1023. [Google Scholar] [CrossRef]
- Friedman, E.; Krupsky, S.; Lane, A.M.; Oak, S.S.; Friedman, E.S.; Egan, K.; Gragoudas, E.S. Ocular Blood Flow Velocity in Age-Related Macular Degeneration. Ophthalmology 1995, 102, 640–646. [Google Scholar] [CrossRef]
- Chen, J.C.; Fitzke, F.W.; Pauleikhoff, D.; Bird, A.C. Functional Loss in Age-Related Bruch’s Membrane Change with Choroidal Perfusion Defect. Invest. Ophthalmol. Vis. Sci. 1992, 33, 334–340. [Google Scholar]
- Krishnan, R. Peripheral Retinal Neovascularization Associated with Polycythemia Rubra Vera. Jpn. J. Ophthalmol. 2009, 53, 188–189. [Google Scholar] [CrossRef]
- Kim, M.J.; Yu, H.G. Case of Bilateral Retinal Neovascularization Associated with Chronic Idiopathic Myelofibrosis. Korean J. Ophthalmol. 2010, 24, 131–133. [Google Scholar] [CrossRef]
- Dapling, R.B.; Snowden, J.A.; West, J.; Talbot, J.F.; Nelson, M.E.; Greaves, M. The Microvasculature in Myeloproliferative Disease. A Study Using Retinal Fluorescein Angiography. Clin. Lab. Haematol. 1996, 18, 277–279. [Google Scholar] [PubMed]
- Ayintap, E.; Cetin, G.; Sadigov, F.; Artunay, O.; Akkan, J.C.U.; Koytak, I.A.; Tuncer, K. Peripapillary Retinal Nerve Fiber Layer Changes in Asymptomatic Essential Thrombocythemia Patients. Curr. Eye Res. 2014, 39, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Pekel, G.; Dogu, M.H.; Keskin, A.; Acer, S.; Yagci, R.; Kasikci, A.; Cetin, E.N. Subfoveal Choroidal Thickness Is Associated with Blood Hematocrit Level. Ophthalmologica. 2015, 234, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Pekel, G.; Dogu, M.H.; Sari, H.I.; Acer, S.; Kasikci, A.; Yagci, R.; Cetin, E.N. Retinal Vessel Caliber, Choroidal Thickness and Ocular Pulse Amplitude Measurements in Essential Thrombocythemia. Middle East Afr. J. Ophthalmol. 2016, 23, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Havelius, U.; Berglund, S.; Falke, P.; Hindfelt, B.; Krakau, T. Impaired Dark Adaptation in Polycythemia. Improvement after Treatment. Acta. Ophthalmol. Scand. 2000, 78, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Tonz, M.S.; Rigamonti, V.; Iliev, M.E. Simultaneous, Bilateral Anterior Ischemic Optic Neuropathy (AION) in Polycythemia Vera: A Case Report. Klin. Monbl. Augenheilkd. 2008, 225, 504–506. [Google Scholar] [CrossRef]
- Ganesan, S.; Raman, R.; Sharma, T. Polycythemia Causing Posterior Segment Vascular Occlusions. Oman J Ophthalmol. 2017, 10, 33–35. [Google Scholar] [CrossRef]
- Rothstein, T. Bilateral, Central Retinal Vein Closure as the Initial Manifestation of Polycythemia. Am. J. Ophthalmol. 1972, 74, 256–260. [Google Scholar] [CrossRef]
- Klein, J.P.; Cohen, A.B.; Kimberly, W.T.; Shah, A.S.; Leiderman, Y.I.; Cestari, D.M.; Dinkin, M.J. Diffusion-Weighted Magnetic Resonance Imaging of Bilateral Simultaneous Optic Nerve Infarctions. Arch. Neurol. 2009, 66, 132–133. [Google Scholar] [CrossRef]
- Gerding, H. Bilateral Non-Arteritic Anterior Ischaemic Optic Neuropathy (NAION) Associated with Essential Thrombocytosis. Klin. Monbl. Augenheilkd. 2013, 230, 430–431. [Google Scholar] [CrossRef]
- Vardizer, Y.; Linhart, Y.; Loewenstein, A.; Garzozi, H.; Mazawi, N.; Kesler, A. Interferon-Alpha-Associated Bilateral Simultaneous Ischemic Optic Neuropathy. J. Neuroophthalmol. 2003, 23, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, M.; Hayashi, K.; Fukushima, T.; Chiyoda, S.; Takahara, O. Case Report: Intracranial Extramedullary Haematopoiesis in Postpolycythemic Myelofibrosis. Br. J. Radiol. 1994, 67, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Baker, R.; Lopez, J.A.; Spencer, S. Myeloproliferative Disorders and the Hyperviscosity Syndrome. Emerg. Med. Clin. North Am. 2009, 27, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Melamed, E.; Rachmilewitz, E.A.; Reches, A.; Lavy, S. Aseptic Cavernous Sinus Thrombosis after Internal Carotid Arterial Occlusion in Polycythaemia Vera. J. Neurol. Neurosurg. Psychiatry 1976, 39, 320–324. [Google Scholar] [CrossRef]
- Kwaan, H.; Kwaan, H.C. Hyperviscosity in Polycythemia Vera and Other Red Cell Abnormalities. Semin. Thromb. Hemost. 2003, 29. [Google Scholar] [CrossRef]
- Wautier, M.-P.; El Nemer, W.; Gane, P.; Rain, J.-D.; Cartron, J.-P.; Colin, Y.; Le Van Kim, C.; Wautier, J.-L. Increased Adhesion to Endothelial Cells of Erythrocytes from Patients with Polycythemia Vera Is Mediated by Laminin Alpha5 Chain and Lu/BCAM. Blood 2007, 110, 894–901. [Google Scholar] [CrossRef]
- Thomas, D.J.; Marshall, J.; Russell, R.W.; Wetherley-Mein, G.; du Boulay, G.H.; Pearson, T.C.; Symon, L.; Zilkha, E. Effect of Haematocrit on Cerebral Blood-Flow in Man. Lancet. 1977, 2, 941–943. [Google Scholar] [CrossRef]
- Rao, K.; Shenoy, S.B.; Kamath, Y.; Kapoor, S. Central Retinal Artery Occlusion as a Presenting Manifestation of Polycythaemia Vera. BMJ Case Rep. 2016. [Google Scholar] [CrossRef]
- Barabas, A.P.; Offen, D.N.; Meinhard, E.A. The Arterial Complications of Polycythaemia Vera. Br. J. Surg. 1973, 60, 183–187. [Google Scholar] [CrossRef]
- Dhrami-Gavazi, E.; Lee, W.; Horowitz, J.D.; Odel, J.; Mukkamala, S.K.; Blumberg, D.M.; Weiss, M.; Winn, B.J. Jak2 Mutation-Positive Polycythemia Vera Presenting as Central Retinal Artery Occlusion. Retin. Cases Brief Rep. 2015, 9, 127–130. [Google Scholar] [CrossRef]
- Arikan, G.; Saatci, A.O.; Kahraman, S.; Piskin, O.; Men, S.; Undar, B. Central Retinal Artery Occlusion as the Presenting Sign of Essential Thrombocythemia. Turkish J. Hematol. 2011, 28, 146–148. [Google Scholar] [CrossRef] [PubMed]
- BIRGE, H.L. New Theories of Vascular Disease; with Special Reference to the Syndrome of Polycythemia in Ocular Pathology. Am. J. Ophthalmol. 1955, 39, 362–369. [Google Scholar] [CrossRef]
- Tache, J.E.; Saffra, N.; Marshak, H.; Aithal, S.; Novetsky, A.; Huang, Y.-W. Retinal Vein Thrombosis as the Presenting Symptom of Essential Thrombocythemia. Am. J. Med. Sci. 2005, 329, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Hermosilla, A.M.; Mendez-Muros, M.; Diaz-Granda, M.J. Retinal Venovenous Anastomoses on Swept-Source Optical Coherence Tomography Angiography in a Patient with Polycythemia. J. Fr. Ophtalmol. 2019, 42, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Elasri, F.; Souhail, H.; Reda, K.; Iferkhass, S.; Idrissi, A.; Naoumi, A.; Oubaaz, A. Isolated Cilioretinal Artery Occlusion as an Initial Manifestation of Polycythemia Vera. Middle East Afr. J. Ophthalmol. 2010, 17, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Nobacht, S.; Cruysberg, J.R.M.; Deutman, A.F. Peripheral Retinal Nonperfusion Associated with Essential Thrombocytosis. Am. J. Ophthalmol. 1999, 127, 101–102. [Google Scholar] [CrossRef]
- Rospond-Kubiak, I.; Oszkinis, G.; Kociecki, J. Retinal Intra-Arterial Thrombocytic Material Revealing Essential Thrombocythemia. JAMA Ophthalmol. 2016, 134. [Google Scholar] [CrossRef]
- Langabeer, S.E. The JAK2 V617F Mutation in Retinal Vein or Artery Occlusion. EXCLI J. 2019, 18, 127–128. [Google Scholar]
- Mateo, J.; Ascaso, F.J.; Jiménez, B.; Cristóbal, J.A. Jugular Venous Thrombosis Secondary to Idiopathic Myelofibrosis: A Rare Cause of Bilateral Optic Disc Swelling. Clin. Exp. Optom. 2015, 98, 286–289. [Google Scholar] [CrossRef]
- Parija, S.; Mohapatra, M.M.; Pattnaik, B.K. Polycythemia Vera Presenting with Bilateral Papilledema: A Rare Case Report. Indian J. Ophthalmol. 2008, 56, 327–329. [Google Scholar] [CrossRef]
- Nithyanandam, S. Polycythemia Vera Presenting with Bilateral Papilledema. Indian J. Ophthalmol. 2009, 325. [Google Scholar] [CrossRef] [PubMed]
- Blood, A.M.; Lowenthal, E.A.; Nowakowski, R.W. Retinopathy Secondary to Anemia from Myeloid Metaplasia in Polycythemia Vera. J. Am. Optom. Assoc. 1997, 68, 734–738. [Google Scholar]
- Nagy, G.; Gat, G.; Racz, M.; Mailath, L. The Optic Fundus in Polycythaemia Vera. Acta. Med. Acad. Sci. Hung. 1969, 26, 351–355. [Google Scholar] [PubMed]
- Montalto, M.; Biolato, M.; Gallo, A.; Racco, S.; Marrone, G.; Manna, R.; Grieco, A.; Gasbarrini, G. Severe Giant Cell Arteritis Associated with Essential Thrombocythaemia. Int. J. Immunopathol. Pharmacol. 2010, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Font, J.; Muñoz-Rodríguez, F.J.; Cervera, R.; Ingelmo, M. Myelodysplastic and Myeloproliferative Syndromes Associated with Giant Cell Arteritis and Polymyalgia Rheumatica: A Coincidental Coexistence or a Causal Relationship? Clin. Rheumatol. 2002, 21, 309–313. [Google Scholar] [CrossRef]
- Mahendradas, P.; Shetty, R.; Avadhani, K.; Ross, C.; Gupta, A.; Shetty, B.K. Polycythemia Vera and Increased Hemophilic Factor VIII Causing Acute Zonal Occult Outer Retinopathy: A Case Report. Ocul. Immunol. Inflamm. 2010, 18, 319–321. [Google Scholar] [CrossRef]
- Hoyt, C.S. Acquired “Double Elevator” Palsy and Polycythemia Vera. J. Pediatr. Ophthalmol. Strabismus 1978, 15, 362–365. [Google Scholar]
- Prabhakaran, V.C.; Chohan, A.; Husain, R.; Andrew, N.C. Third Nerve Paralysis as a Presenting Sign of Essential Thrombocythaemia. Eye 2006, 1483–1484. [Google Scholar] [CrossRef]
- Michiels, J.J.; Berneman, Z.; Schroyens, W.; Koudstaal, P.J.; Lindemans, J.; Neumann, H.A.M.; van Vliet, H.H.D.M. Platelet-Mediated Erythromelalgic, Cerebral, Ocular and Coronary Microvascular Ischemic and Thrombotic Manifestations in Patients with Essential Thrombocythemia and Polycythemia Vera: A Distinct Aspirin-Responsive and Coumadin-Resistant Arterial Thromboph. Platelets 2006, 17, 528–544. [Google Scholar] [CrossRef]
- Kuriyama, M.; Ishii, N.; Umezaki, H.; Kano, M. Polycythemia Vera and Transient Monocular Blindness. Observation of the Platelet Embolic Phenomena in the Ocular Fundus. Eur. Neurol. 1977, 16. [Google Scholar] [CrossRef]
- Austin, J.H. Amaurosis Fugax and Thrombocythemia. Neurology 1987, 1887. [Google Scholar] [CrossRef] [PubMed]
- Berdel, W.E.; Theiss, W.; Fink, U.; Rastetter, J. Peripheral Arterial Occlusion and Amaurosis Fugax as the First Manifestation of Polycythemia Vera- A Case Report. Blut 1984, 48, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Lambert, C.D.; Lawton, N. Progressive Neurological Deficits in Primary Polycythaemia. Br. Med. J. 1972, 3, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; low-dose aspirin in polycythemia (ECLAP). A Prospective Analysis of Thrombotic Events in the European Collaboration Study on Low-Dose Aspirin in Polycythemia (ECLAP). Pathol. Biol. 2004, 52, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Landolfi, R.; Marchioli, R.; Kutti, J.; Gisslinger, H.; Tognoni, G.; Patrono, C.; Barbui, T. Efficacy and Safety of Low-Dose Aspirin in Polycythemia Vera. N. Engl. J. Med. 2004, 350, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Santisakultarm, T.P.; Paduano, C.Q.; Stokol, T.; Southard, T.L.; Nishimura, N.; Skoda, R.C.; Olbricht, W.L.; Schafer, A.I.; Silver, R.T.; Schaffer, C.B. Stalled Cerebral Capillary Blood Flow in Mouse Models of Essential Thrombocythemia and Polycythemia Vera Revealed by in Vivo Two-Photon Imaging. J. Thromb. Haemost. 2014, 12, 2120–2130. [Google Scholar] [CrossRef]
- Fraunfelder, F.W.; Fraunfelder, F.T. Interferon Alfa-Associated Anterior Ischemic Optic Neuropathy. Ophthalmology 2011, 118, 408–411. [Google Scholar] [CrossRef]
- Lewczuk, N.; Zdebik, A.; Boguslawska, J. Interferon Alpha 2a and 2b in Ophthalmology: A Review. J. Interferon Cytokine Res. 2019, 39, 259–272. [Google Scholar] [CrossRef]
- von Hofsten, J.; Johnsson Forsberg, M.; Zetterberg, M. Cytomegalovirus Retinitis in a Patient Who Received Ruxolitinib. N. Engl. J. Med. 2016, 374, 296–297. [Google Scholar] [CrossRef]
- Goldberg, R.A.; Reichel, E.; Oshry, L.J. Bilateral Toxoplasmosis Retinitis Associated with Ruxolitinib. N. Engl. J. Med. 2013, 369, 681–683. [Google Scholar] [CrossRef]
| Site or Cause of Involvement | Ocular Manifestations |
|---|---|
| External eye involvement | |
| Orbit | EMH |
| Eyelid | Pachydermoperiostosis (PDP) |
| Lacrimal gland | EMH |
| Anterior segment diseases | |
| Conjunctiva | Haemorrhage, thrombosis, conjunctivitis/injection |
| Iris | Iritis |
| Angle | Neovascular glaucoma |
| Posterior segment diseases | |
| Retina | Haemorrhages, vascular occlusions, venous dilation and tortuosity, changes in blood flow, cotton wool spots, Roth spots, AMD, peripheral neovascularisation, thinner nerve fibre layer, neuronal hypofunction |
| Choroid | Changes in blood flow |
| Optic nerve | AION, EMH, papilledema |
| CNS related to eyes | Blurred vision, transient monocular blindness, amaurosis fugax, scintillating scotomas, hemianopsia, hallucinations |
| Secondary to treatment | |
| interferon-alpha | Possible AION, retinopathy and irritative conjunctivitis |
| Ruxolitinib | Opportunistic infections |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liisborg, C.; Hasselbalch, H.C.; Sørensen, T.L. Ocular Manifestations in Patients with Philadelphia-Negative Myeloproliferative Neoplasms. Cancers 2020, 12, 573. https://doi.org/10.3390/cancers12030573
Liisborg C, Hasselbalch HC, Sørensen TL. Ocular Manifestations in Patients with Philadelphia-Negative Myeloproliferative Neoplasms. Cancers. 2020; 12(3):573. https://doi.org/10.3390/cancers12030573
Chicago/Turabian StyleLiisborg, Charlotte, Hans Carl Hasselbalch, and Torben Lykke Sørensen. 2020. "Ocular Manifestations in Patients with Philadelphia-Negative Myeloproliferative Neoplasms" Cancers 12, no. 3: 573. https://doi.org/10.3390/cancers12030573
APA StyleLiisborg, C., Hasselbalch, H. C., & Sørensen, T. L. (2020). Ocular Manifestations in Patients with Philadelphia-Negative Myeloproliferative Neoplasms. Cancers, 12(3), 573. https://doi.org/10.3390/cancers12030573





