Integrin α7 and Extracellular Matrix Laminin 211 Interaction Promotes Proliferation of Acute Myeloid Leukemia Cells and Is Associated with Granulocytic Sarcoma
Abstract
1. Introduction
2. Results
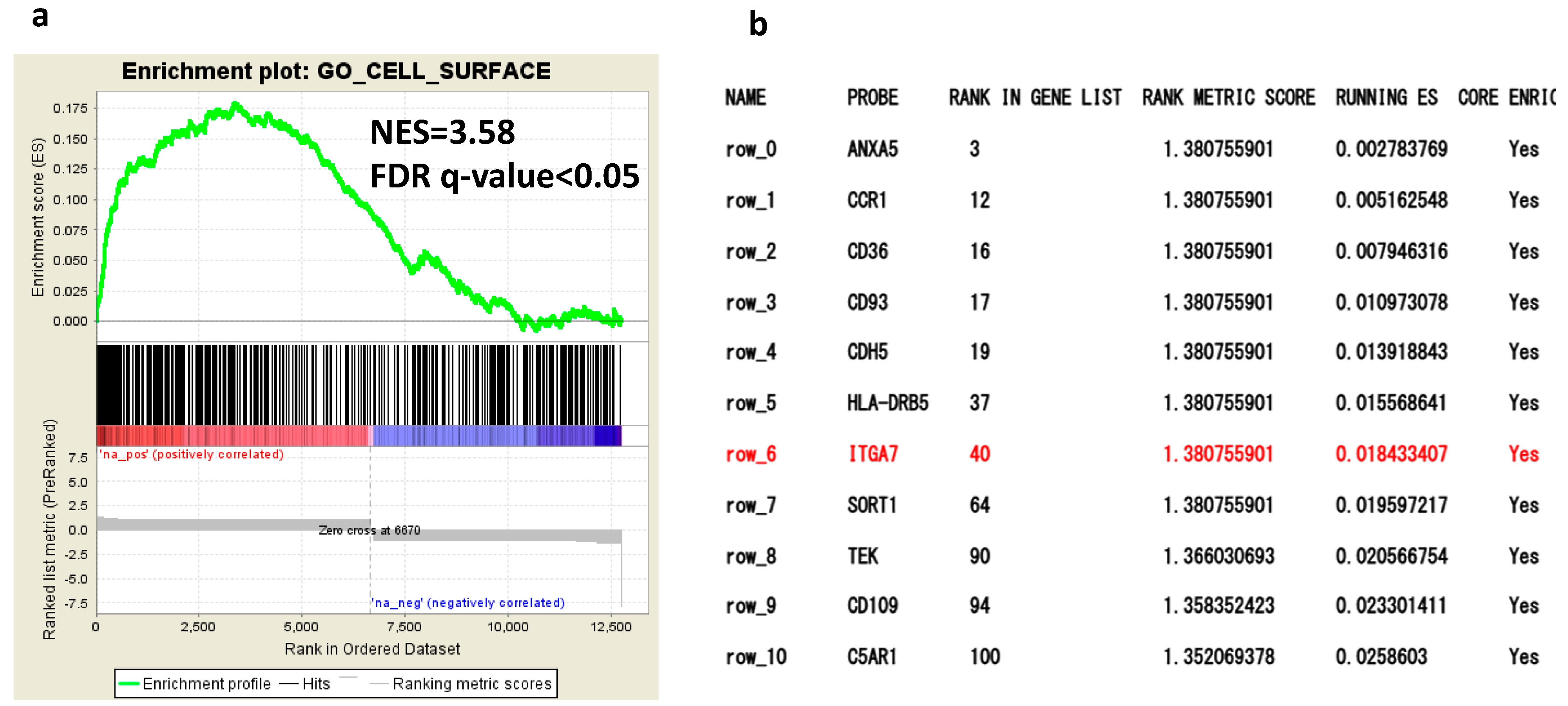
2.1. Comprehensive Gene Expression Analysis of AML Cells by RNA-Seq
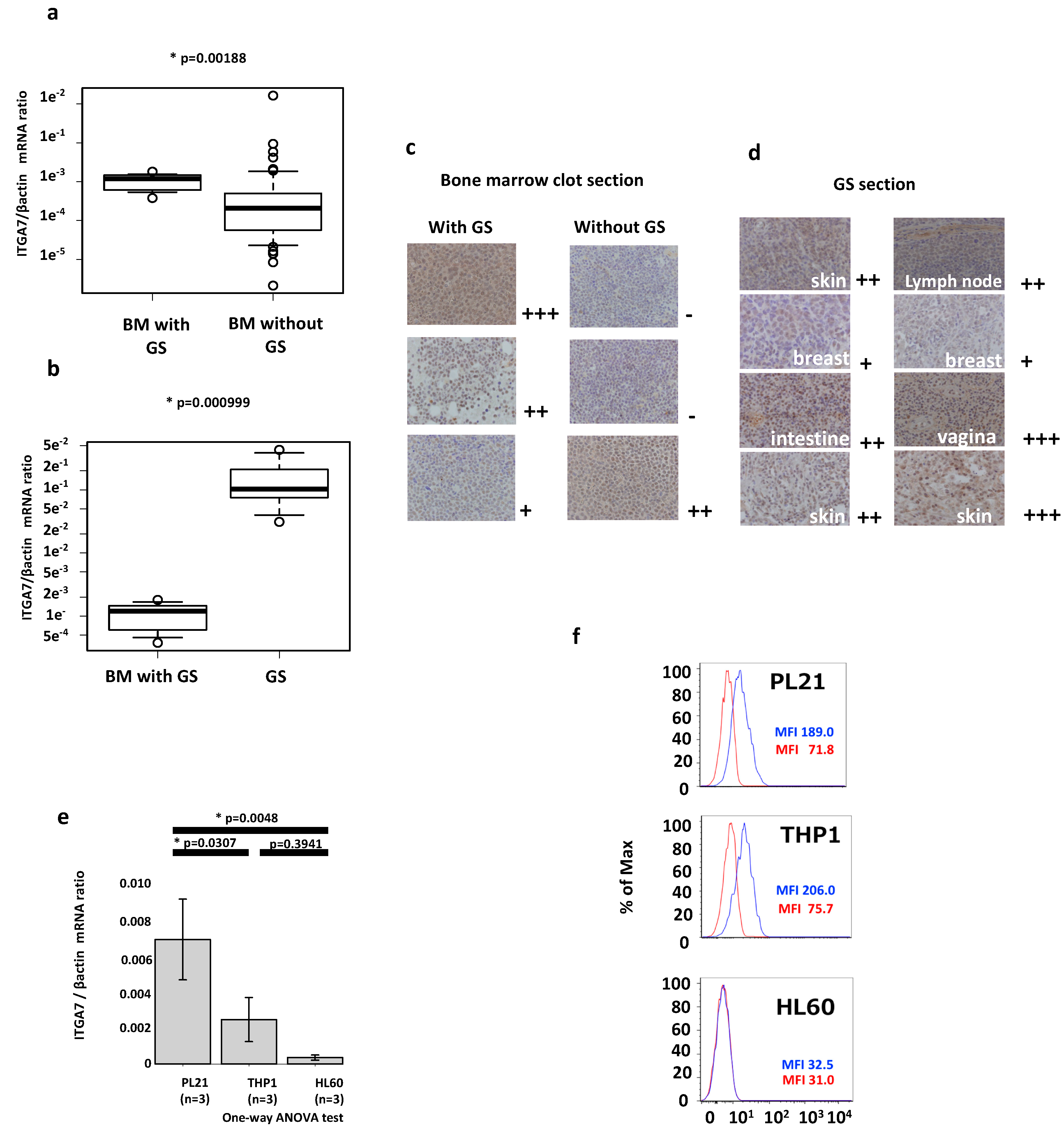
2.2. ITGA7 Is Expressed in Both AML Patients and Cell Lines
2.3. Laminin 211 Stimulates ERK Phosphorylation in AML Cell Lines Expressing Integrin α7
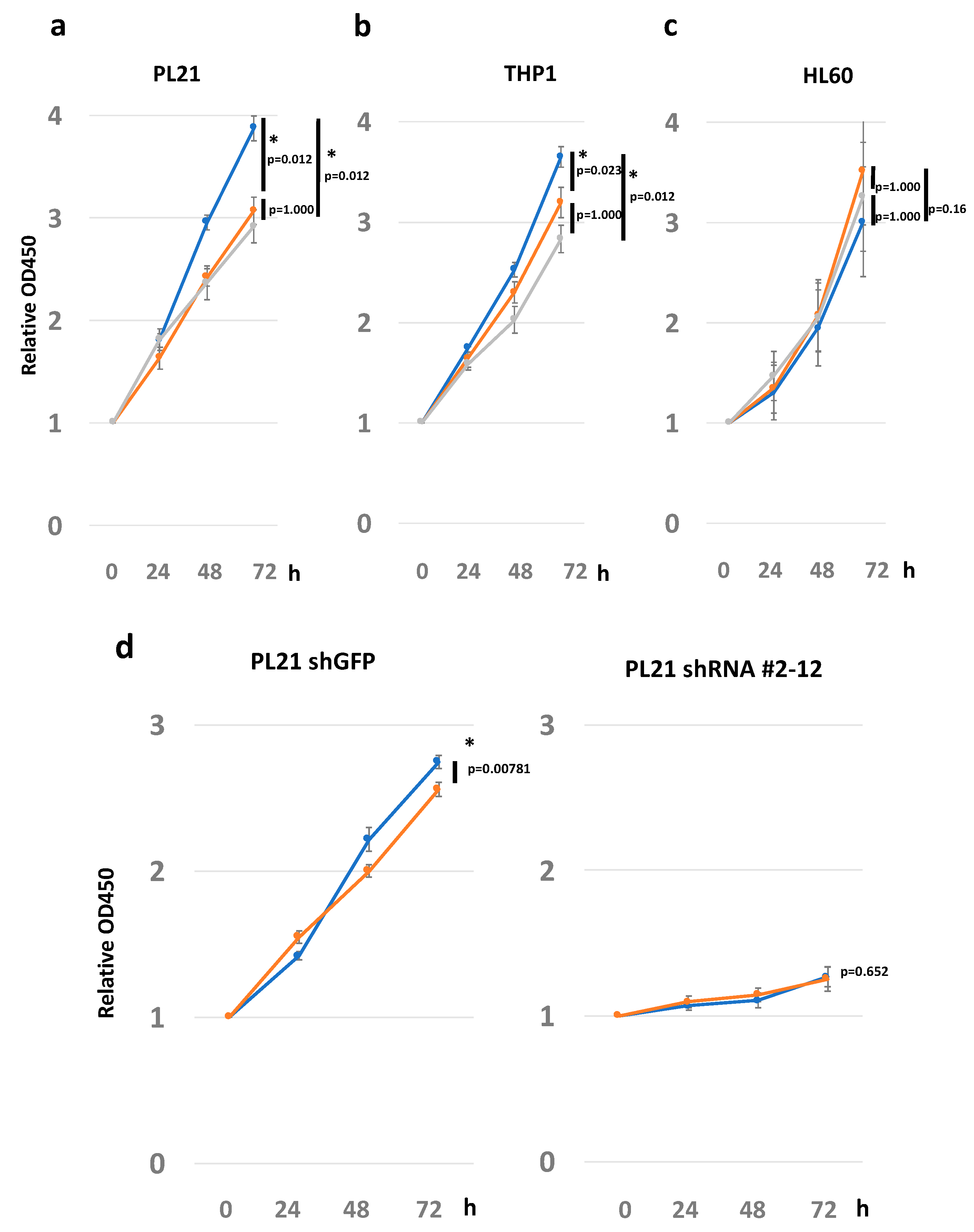
2.4. ECM Laminin 211 Promotes Proliferation of AML Cell Lines by Expressing Integrin α7
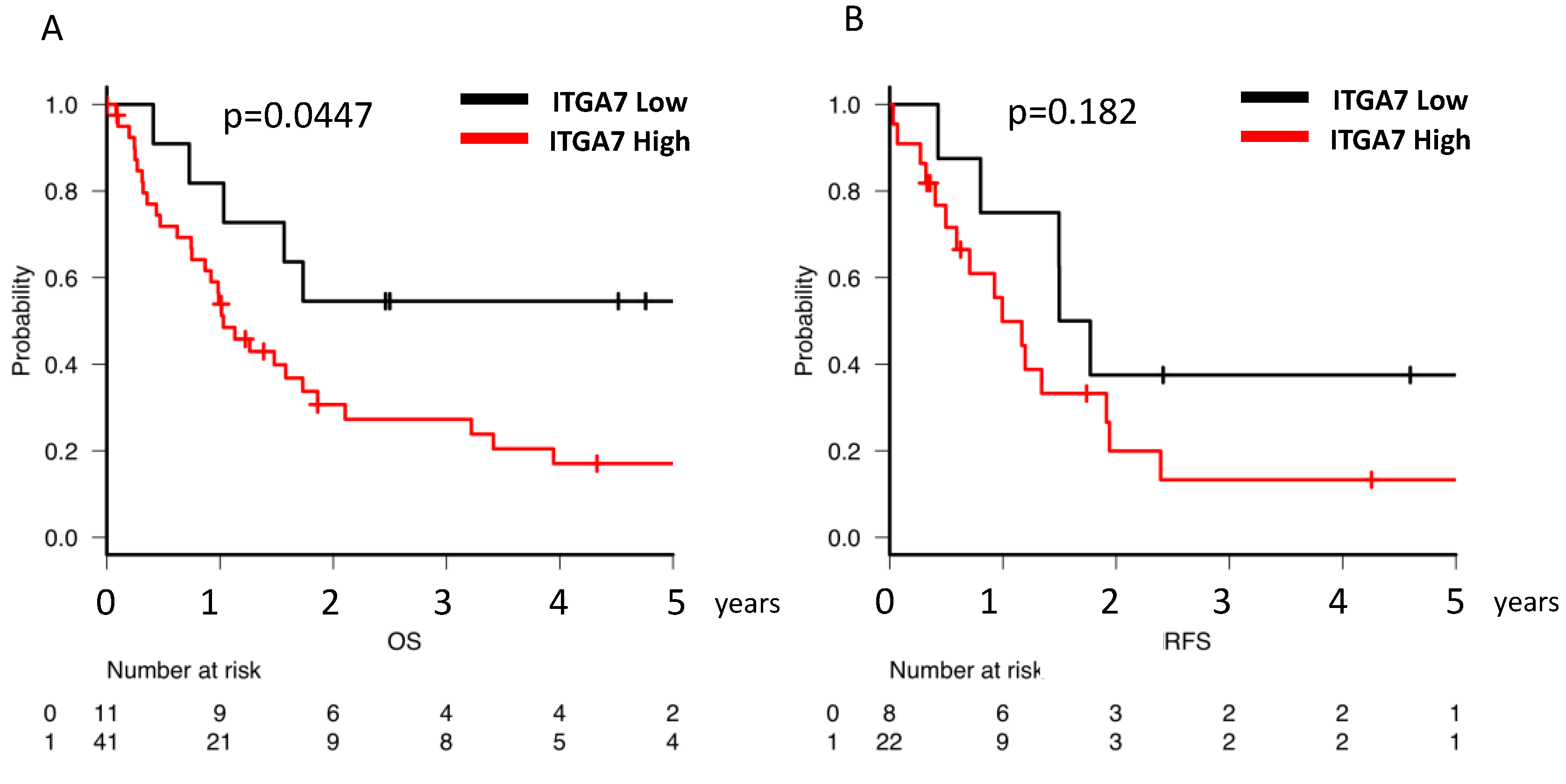
2.5. Clinical Implications of ITGA7 Expression in Bone Marrow AML Cells
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Cell Culture
4.3. Lentiviral Constructs and ITGA7 Knockdown
4.4. Cell Proliferation Assay
4.5. RNA Extraction and RNA-Seq
4.6. RT-qPCR
4.7. Flow Cytometric Analysis
4.8. Western Blot Analysis
4.9. Immunohistochemical Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; Levine, R.L.; Lo-coco, F.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel hartmut. Blood 2017, 129, 424–448. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2406. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Gardin, C. An update of current treatments for adult acute myeloid leukemia. Blood 2016, 127, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Saitoh, T.; Hatsumi, N.; Takada, S.; Yokohama, A.; Handa, H.; Jimbo, T.; Sakura, T.; Tsukamoto, N.; Murakami, H.; et al. Clinical significance of granulocytic sarcoma in adult patients with acute myeloid leukemia. Cancer Sci. 2012, 103, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Saitoh, T.; Machida, S.; Kako, S.; Doki, N.; Mori, T.; Sakura, T.; Kanda, Y.; Kanamori, H.; Miyawaki, S.; et al. Allogeneic hematopoietic stem cell transplantation for adult patients with mixed phenotype acute leukemia: Results of a matched-pair analysis. Eur. J. Haematol. 2015, 95, 455–460. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hamada, J.; Li, Y.-Q.; Shinobu, N.; Imamura, M.; Okada, F.; Takeuchi, N.; Hosokawa, M. A possible role of 92 kDa type IV collagenase in the extramedullary tumor formation in leukemia. Jpn. J. Cancer Res. 1995, 86, 298–303. [Google Scholar] [CrossRef]
- Bianconi, D.; Unseld, M.; Prager, G.W. Integrins in the spotlight of cancer. Int. J. Mol. Sci. 2016, 17, 2037. [Google Scholar] [CrossRef]
- Sangaletti, S.; Chiodoni, C.; Tripodo, C.; Colombo, M.P. Common extracellular matrix regulation of myeloid cell activity in the bone marrow and tumor microenvironments. Cancer Immunol. Immunother. 2017, 66, 1059–1067. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Balcioglu, H.E.; Danen, E.H.J. Integrin signaling in control of tumor growth and progression. Int. J. Biochem. Cell Biol. 2013, 45, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Ata, R.; Antonescu, C.N. Integrins and cell metabolism: An intimate relationship impacting cancer. Int. J. Mol. Sci. 2017, 18, 189. [Google Scholar] [CrossRef] [PubMed]
- Maziveyi, M.; Alahari, S.K.; Maziveyi, M.; Alahari, S.K. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget 2015, 8, 48471–48487. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Matsunaga, T.; Takemoto, N.; Sato, T.; Takimoto, R.; Tanaka, I.; Fujimi, A.; Akiyama, T.; Kuroda, H.; Kawano, Y.; Kobune, M.; et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat. Med. 2003, 9, 1158–1165. [Google Scholar] [CrossRef]
- Becker, P.S.; Kopecky, K.J.; Wilks, A.N.; Chien, S.; Harlan, J.M.; Willman, C.L.; Petersdorf, S.H.; Stirewalt, D.L.; Papayannopoulou, T.; Appelbaum, F.R. Very late antigen-4 function of myeloblasts correlates with improved overall survival for patients with acute myeloid leukemia. Blood 2009, 113, 866–874. [Google Scholar] [CrossRef]
- Walter, R.B.; Alonzo, T.A.; Gerbing, R.B.; Ho, P.A.; Smith, F.O.; Raimondi, S.C.; Hirsch, B.A.; Gamis, A.S.; Franklin, J.L.; Hurwitz, C.A.; et al. High expression of the very late antigen-4 integrin independently predicts reduced risk of relapse and improved outcome in pediatric acute myeloid leukemia: A report from the children’s oncology group. J. Clin. Oncol. 2010, 28, 2831–2838. [Google Scholar] [CrossRef]
- Tavernier-Tardy, E.; Cornillon, J.; Campos, L.; Flandrin, P.; Duval, A.; Nadal, N.; Guyotat, D. Prognostic value of CXCR4 and FAK expression in acute myelogenous leukemia. Leuk. Res. 2009, 33, 764–768. [Google Scholar] [CrossRef]
- Izzi, V.; Lakkala, J.; Devarajan, R.; Ruotsalainen, H.; Savolainen, E.; Koistinen, P.; Heljasvaara, R.; Pihlajaniemi, T. An extracellular matrix signature in leukemia precursor cells and acute myeloid leukemia. Haematologica 2017, 102, e245–e248. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.A.; Bei, L.; Wang, H.; Altman, J.K.; Platanias, L.C.; Eklund, E.A. Cooperation between AlphavBeta3 integrin and the fibroblast growth factor receptor enhances proliferation of Hoxoverexpressing acute myeloid leukemia cells. Oncotarget 2016, 7, 54782–54794. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.; Brenner, A.K.; Bartaula-Brevik, S.; Reikvam, H.; Bruserud, Ø. The possible importance of β3 integrins for leukemogenesis and chemoresistance in acute myeloid leukemia. Int. J. Mol. Sci. 2018, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, A.; Majeti, R. Sticking it to the niche: CD98 mediates critical adhesive signals in AML. Cancer Cell 2016, 30, 662–664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bajaj, J.; Konuma, T.; Lytle, N.K.; Kwon, H.Y.; Ablack, J.N.; Cantor, J.M.; Rizzieri, D.; Chuah, C.; Oehler, V.G.; Broome, E.H.; et al. CD98-mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell 2016, 30, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.G.; Al-Shahrour, F.; Hartwell, K.A.; Chu, L.P.; Järås, M.; Puram, R.V.; Puissant, A.; Callahan, K.P.; Ashton, J.; McConkey, M.E.; et al. Invivo rnai screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell 2013, 24, 45–58. [Google Scholar] [CrossRef]
- Jin, L.; Hope, K.J.; Zhai, Q.; Smadja-Joffe, F.; Dick, J.E. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006, 12, 1167–1174. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Ramovs, V.; te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57, 213–243. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Wilson, C.S.; Medeiros, L.J. Extramedullary manifestations of myeloid neoplasms. Am. J. Clin. Pathol. 2015, 144, 219–239. [Google Scholar] [CrossRef]
- Pileri, S.A.; Ascani, S.; Cox, M.C.; Campidelli, C.; Bacci, F.; Piccioli, M.; Piccaluga, P.P.; Agostinelli, C.; Asioli, S.; Novero, D.; et al. Myeloid sarcoma: Clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007, 21, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhang, R.; Li, Y. Granulocytic sarcoma of abdomen in acute myeloid leukemia patient with inv(16) and t(6;17) abnormal chromosome: Case report and review of literature. Leuk. Res. 2010, 34, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Brandwein, J.; Yi, Q.L.; Chun, K.; Patterson, B.; Brien, B. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk. Res. 2004, 28, 1007–1011. [Google Scholar] [CrossRef]
- Haas, T.L.; Sciuto, M.R.; Brunetto, L.; Valvo, C.; Signore, M.; Fiori, M.E.; di Martino, S.; Giannetti, S.; Morgante, L.; Boe, A.; et al. Integrin α7 is a functional marker and potential therapeutic target in glioblastoma. Cell Stem Cell 2017, 21, 35–50. [Google Scholar] [CrossRef]
- Shi, W.D.; Meng, Z.Q.; Chen, Z.; Lin, J.H.; Zhou, Z.H.; Liu, L.M. Identification of liver metastasis-related genes in a novel human pancreatic carcinoma cell model by microarray analysis. Cancer Lett. 2009, 283, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jethanandani, P.; Ziober, B.L.; Kramer, R.H. Regulation of alpha7 integrin expression during muscle differentiation. J. Biol. Chem. 2003, 278, 49780–49788. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.N.; Montgomery, C.L.; Janssen, P.M.; Clark, K.R.; Mendell, J.R.; Rodino-Klapac, L.R. AAV-mediated overexpression of human α7 integrin leads to histological and functional improvement in dystrophic mice. Mol. Ther. 2013, 21, 520–525. [Google Scholar] [CrossRef]
- Siegel, A.L.; Atchison, K.; Fisher, K.E.; Davis, G.E.; Cornelison, D.D.W. 3D timelapse analysis of muscle satellite cell motility. Stem Cells 2009, 27, 2527–2538. [Google Scholar] [CrossRef]
- Vizirianakis, I.S.; Yao, C.C.; Chen, Y.Q.; Ziober, B.L.; Tsiftsoglou, A.S.; Kramer, R.H. Transfection of MCF-7 carcinoma cells with human integrin α7 cDNA promotes adhesion to laminin. Arch. Biochem. Biophys. 2001, 385, 108–116. [Google Scholar] [CrossRef]
- Chöber, S.; Mielenz, D.; Echtermeyer, F.; Hapke, S.; Pöschl, E.; Von der Mark, H.; Moch, H.; Von der Mark, K. The role of extracellular and cytoplasmic splice domains of α7-integrin in cell adhesion and migration on laminins. Exp. Cell Res. 2000, 255, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Boppart, M.D.; Burkin, D.J.; Kaufman, S.J. Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochim. Biophys. Acta-Mol. Basis Dis. 2011, 1812, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Yu, H.; Genersch, E.; Sanzen, N.; Sekiguchi, K.; Fässler, R.; Campbell, K.P.; Talts, J.F.; Ekblom, P. Laminin isoforms differentially regulate adhesion, spreading, proliferation, and ERK activation of β1 integrin-null cells. Exp. Cell Res. 2004, 300, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Mielenz, D.; Hapke, S.; Pöschl, E.; Von der Mark, H.; Von der Mark, K. The integrin α7 cytoplasmic domain regulates cell migration, lamellipodia formation, and p130CAS/Crk coupling. J. Biol. Chem. 2001, 276, 13417–13426. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Xia, E.; Zhou, Y.; Guan, Y.; Xiang, J.; Kong, L.; Wang, Y.; Yang, F.; Wang, O.; Zhang, X. ITGA7 functions as a tumor suppressor and regulates migration and invasion in breast cancer. Cancer Manag. Res. 2018, 10, 969–976. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Li, X.; Chen, J.; Wang, G.; Tian, Z.; Qian, M.; Chen, Z.; Guo, H.; Tang, G.; et al. Forkhead box C1 promotes colorectal cancer metastasis through transactivating ITGA7 and FGFR4 expression. Oncogene 2018, 37, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, C.; Lin, C.; Zhang, J.; Zhang, W.; Zhang, W.; Lu, Y.; Zheng, L.; Li, X. Circular RNA circITGA7 inhibits colorectal cancer growth and metastasis by modulating the Ras pathway and upregulating transcription of its host gene. ITGA7 J. Pathol. 2018, 246, 166–179. [Google Scholar] [CrossRef]
- Burkin, D.J.; Fontelonga, T.M. Mesothelioma cells breaking bad: Loss of integrin α7 promotes cell motility and poor clinical outcomes in patients. J. Pathol. 2015, 237, 282–284. [Google Scholar] [CrossRef]
- Tan, L.Z.; Song, Y.; Nelson, J.; Yu, Y.P.; Luo, J.H. Integrin α7 binds tissue inhibitor of metalloproteinase 3 to suppress growth of prostate cancer cells. Am. J. Pathol. 2013, 183, 831–840. [Google Scholar] [CrossRef]
- Ziober, B.L.; Chen, Y.Q.; Ramos, D.M.; Waleh, N.; Kramer, R.H. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell Growth Differ. 1999, 10, 479–490. [Google Scholar]
- Chao, J.T.; Martinez-Lemus, L.A.; Kaufman, S.J.; Meininger, G.A.; Ramos, K.S.; Wilson, E. Modulation of α7-integrin-mediated adhesion and expression by platelet-derived growth factor in vascular smooth muscle cells. Am. J. Physiol.-Cell Physiol. 2006, 290, C972–C980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M. Laminins. Cell Tissue Res. 2010, 339, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Siler, U.; Seiffert, M.; Puch, S.; Richards, A.; Torok-Storb, B.; Müller, C.A.; Sorokin, L.; Klein, G. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood 2000, 96, 4194–4203. [Google Scholar] [CrossRef] [PubMed]
- Patarroyoa, M.; Tryggvasonb, K.; Virtanenc, I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin. Cancer Biol. 2002, 14, 423–430. [Google Scholar] [CrossRef]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Franklin, R.A.; Abrams, S.L.; Chappell, W.; Kempf, C.R.; Bäsecke, J.; Stivala, F.; Donia, M.; Fagone, P.; Nicoletti, F.; et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia 2011, 25, 1080–1094. [Google Scholar] [CrossRef]
- Hatfield, K.J.; Reikvam, H.; Bruserud, O. The crosstalk between the matrix metalloprotease system and the chemokine network in acute myeloid leukemia. Curr. Med. Chem. 2010, 17, 4448–4461. [Google Scholar] [CrossRef]
- Drexler, H.G. The Leukemia-Lymphoma Cell Line Facts Book; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Oda, T.; Sekimoto, T.; Kurashima, K.; Fujimoto, M.; Nakai, A.; Yamashita, T. Acute HSF1 depletion induces cellular senescence through the MDM2-p53-p21 pathway in human diploid fibroblasts. J. Cell Sci. 2018, 131, jcs210724. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]

| With GS | Without GS | ||
|---|---|---|---|
| Number of patients | 9 | 55 | |
| Age (median) | 59 (33–82) | 65 (21–86) | |
| White blood cells (μL) | 14750 (2100–91,800) | 4800 (700–205,800) | |
| Blasts in peripheral blood (%) | 46.0 (0–99) | 18.0 (0–99) | |
| Blasts in bone marrow (%) | 56.3 (32–85.2) | 49.0 (13–99) | |
| Karyotype risk (%) | Favorable | 1 (11.1) | 8 (14.6) |
| Intermediate | 5 (56.6) | 25 (45.4) | |
| Adverse | 3 (33.3) | 21 (38.1) | |
| N.A. | − | 1 (1.8) | |
| Chemotherapy | + | 9 (100.0) | 43 (78.1) |
| − | 0 (0.0) | 8 (14.6) | |
| Unknown | − | 4 (7.3) | |
| Response | Complete Remission (CR) | 7 (77.8) | 20 (46.5) |
| Non-CR | 2 (22.2) | 19 (44.1) | |
| Unknown | − | 4 (9.3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, N.; Oda, T.; Takizawa, M.; Ishizaki, T.; Tsukamoto, N.; Yokohama, A.; Takei, H.; Saitoh, T.; Shimizu, H.; Honma, K.; et al. Integrin α7 and Extracellular Matrix Laminin 211 Interaction Promotes Proliferation of Acute Myeloid Leukemia Cells and Is Associated with Granulocytic Sarcoma. Cancers 2020, 12, 363. https://doi.org/10.3390/cancers12020363
Kobayashi N, Oda T, Takizawa M, Ishizaki T, Tsukamoto N, Yokohama A, Takei H, Saitoh T, Shimizu H, Honma K, et al. Integrin α7 and Extracellular Matrix Laminin 211 Interaction Promotes Proliferation of Acute Myeloid Leukemia Cells and Is Associated with Granulocytic Sarcoma. Cancers. 2020; 12(2):363. https://doi.org/10.3390/cancers12020363
Chicago/Turabian StyleKobayashi, Nobuhiko, Tsukasa Oda, Makiko Takizawa, Takuma Ishizaki, Norifumi Tsukamoto, Akihiko Yokohama, Hisashi Takei, Takayuki Saitoh, Hiroaki Shimizu, Kazuki Honma, and et al. 2020. "Integrin α7 and Extracellular Matrix Laminin 211 Interaction Promotes Proliferation of Acute Myeloid Leukemia Cells and Is Associated with Granulocytic Sarcoma" Cancers 12, no. 2: 363. https://doi.org/10.3390/cancers12020363
APA StyleKobayashi, N., Oda, T., Takizawa, M., Ishizaki, T., Tsukamoto, N., Yokohama, A., Takei, H., Saitoh, T., Shimizu, H., Honma, K., Kimura-Masuda, K., Kuroda, Y., Ishihara, R., Murakami, Y., Murakami, H., & Handa, H. (2020). Integrin α7 and Extracellular Matrix Laminin 211 Interaction Promotes Proliferation of Acute Myeloid Leukemia Cells and Is Associated with Granulocytic Sarcoma. Cancers, 12(2), 363. https://doi.org/10.3390/cancers12020363






