Proton Magnetic Resonance Spectroscopy Characterization of Rathke’s Cleft Cysts (RCCs): Relevance to the Differential Diagnosis of Pituitary Adenomas and RCCs
Abstract
1. Introduction
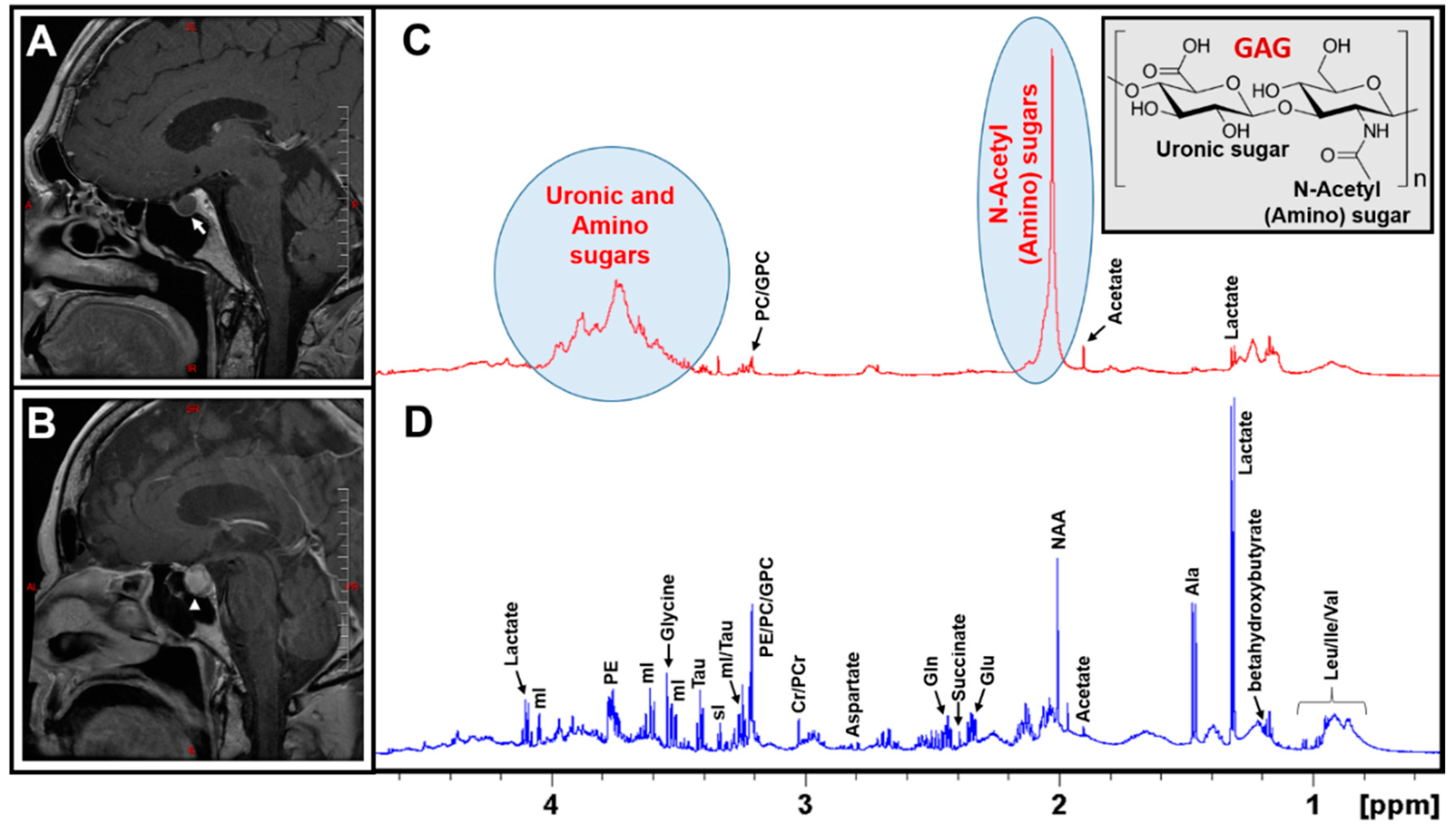
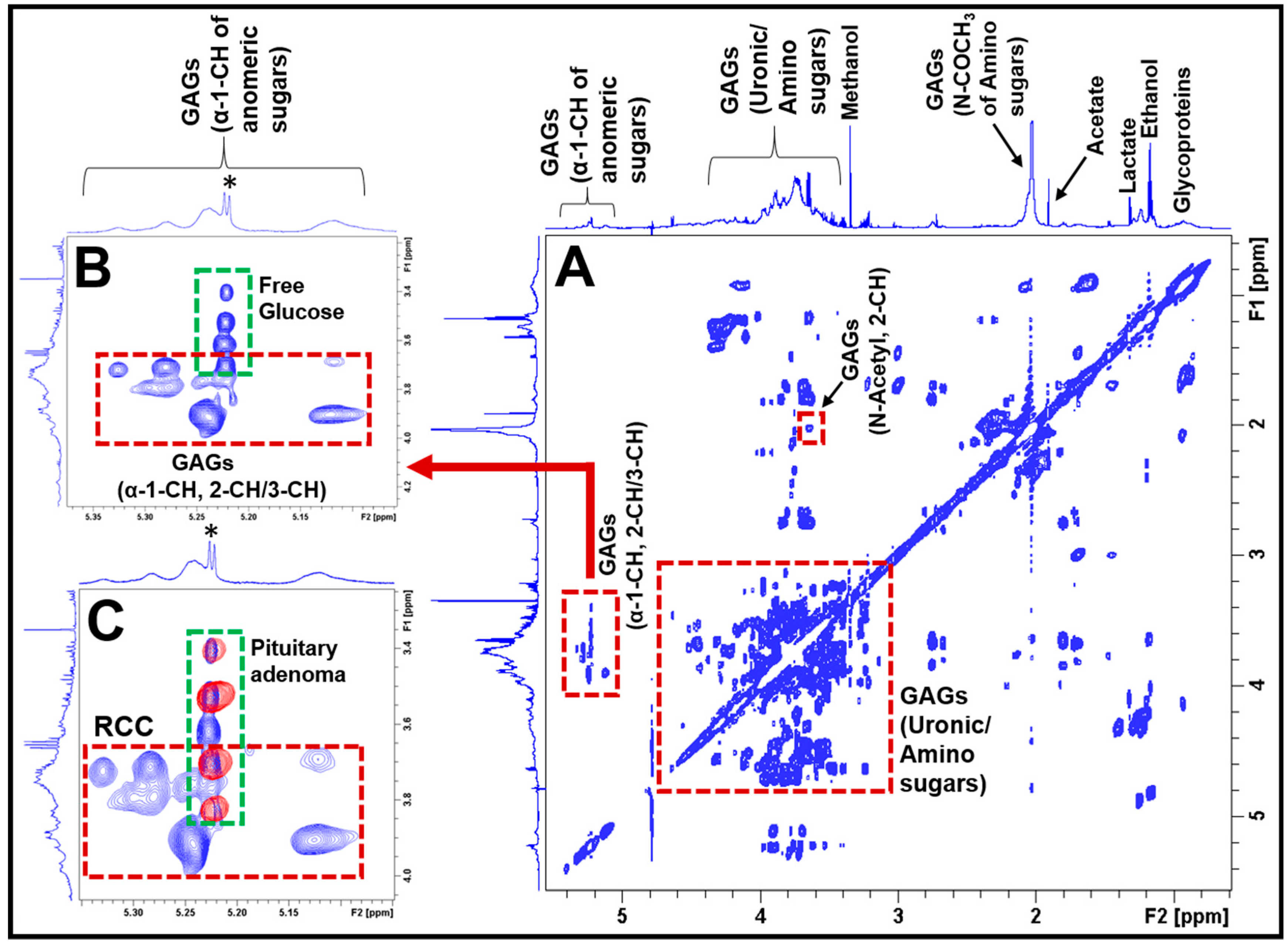
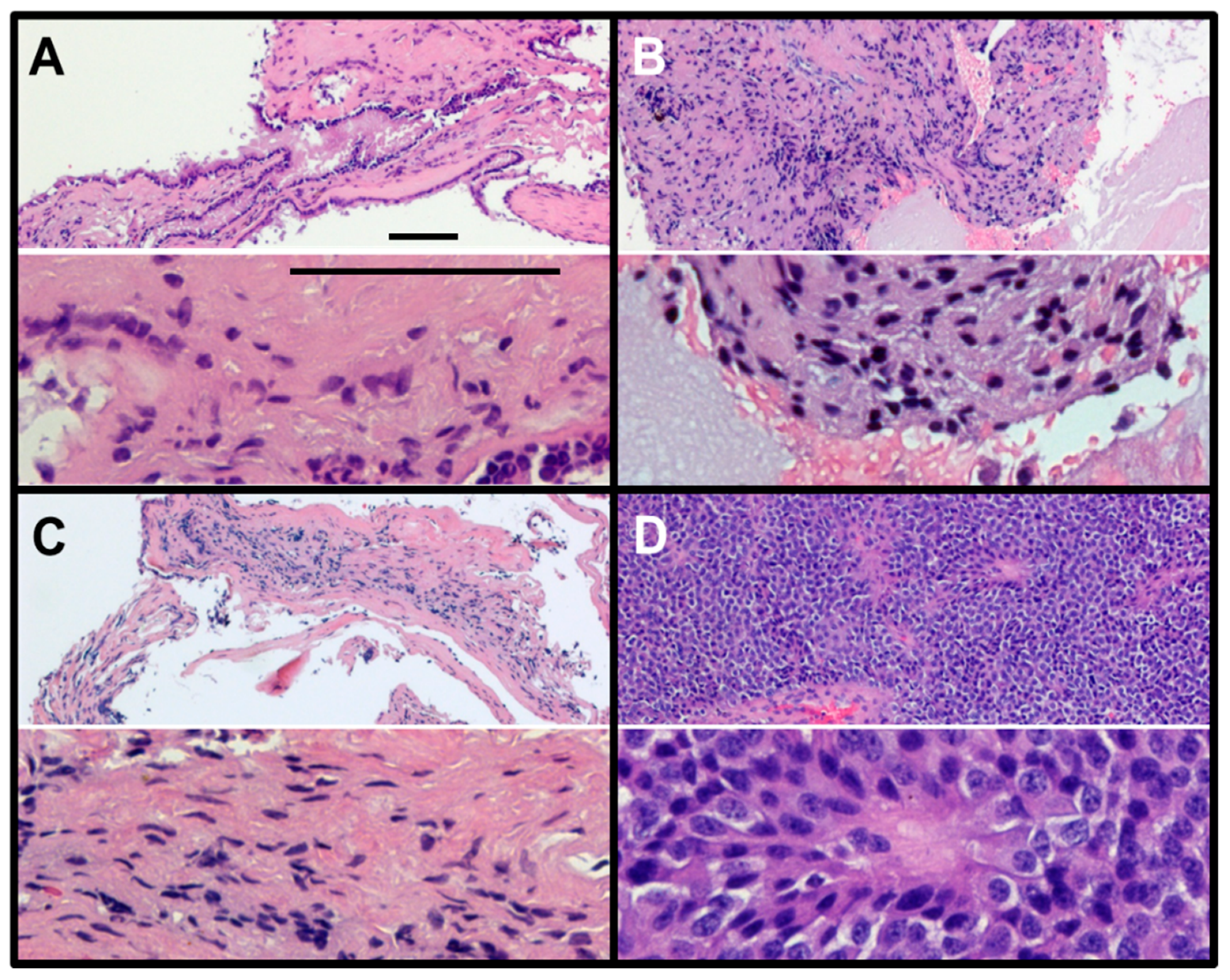
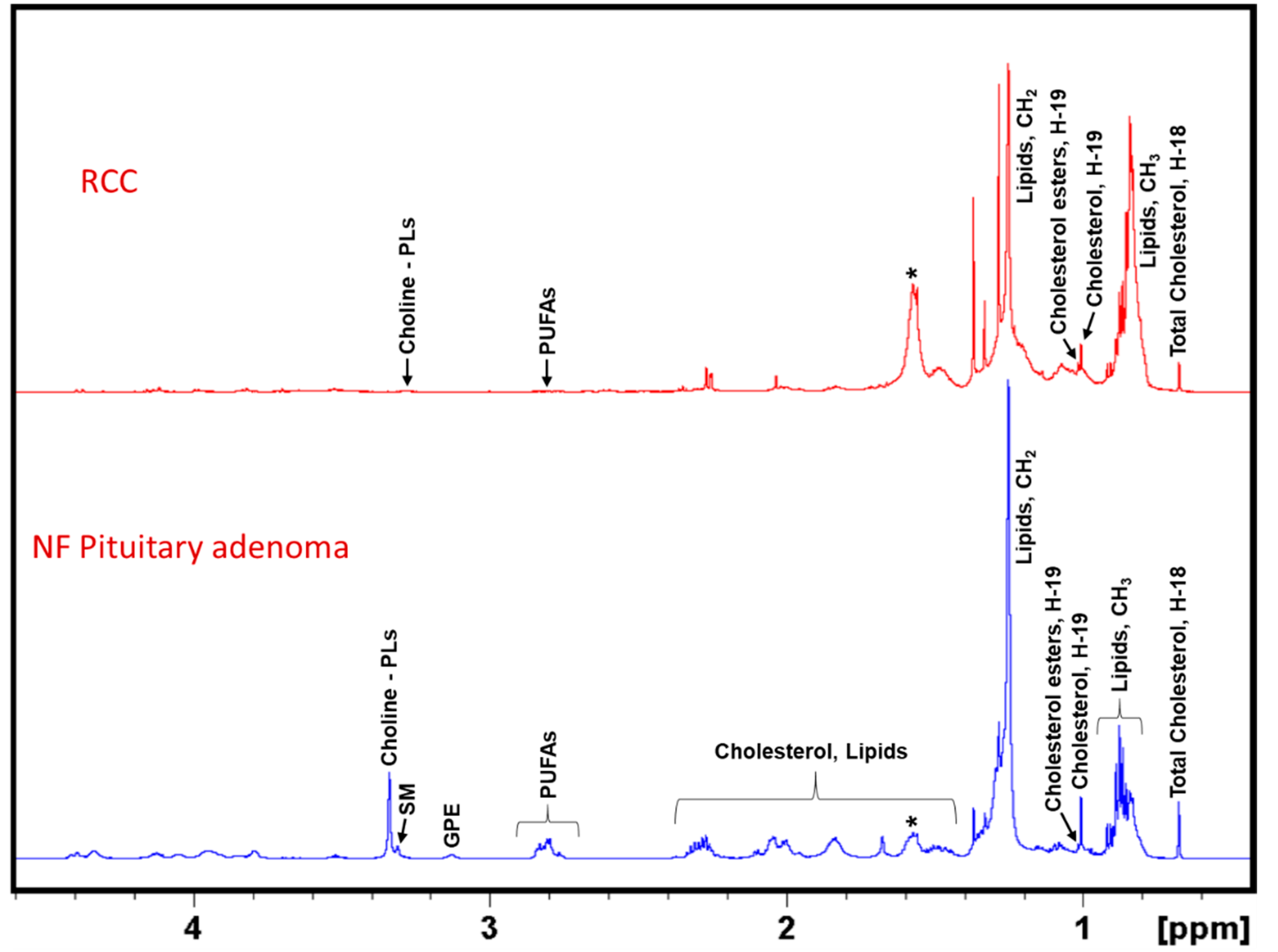
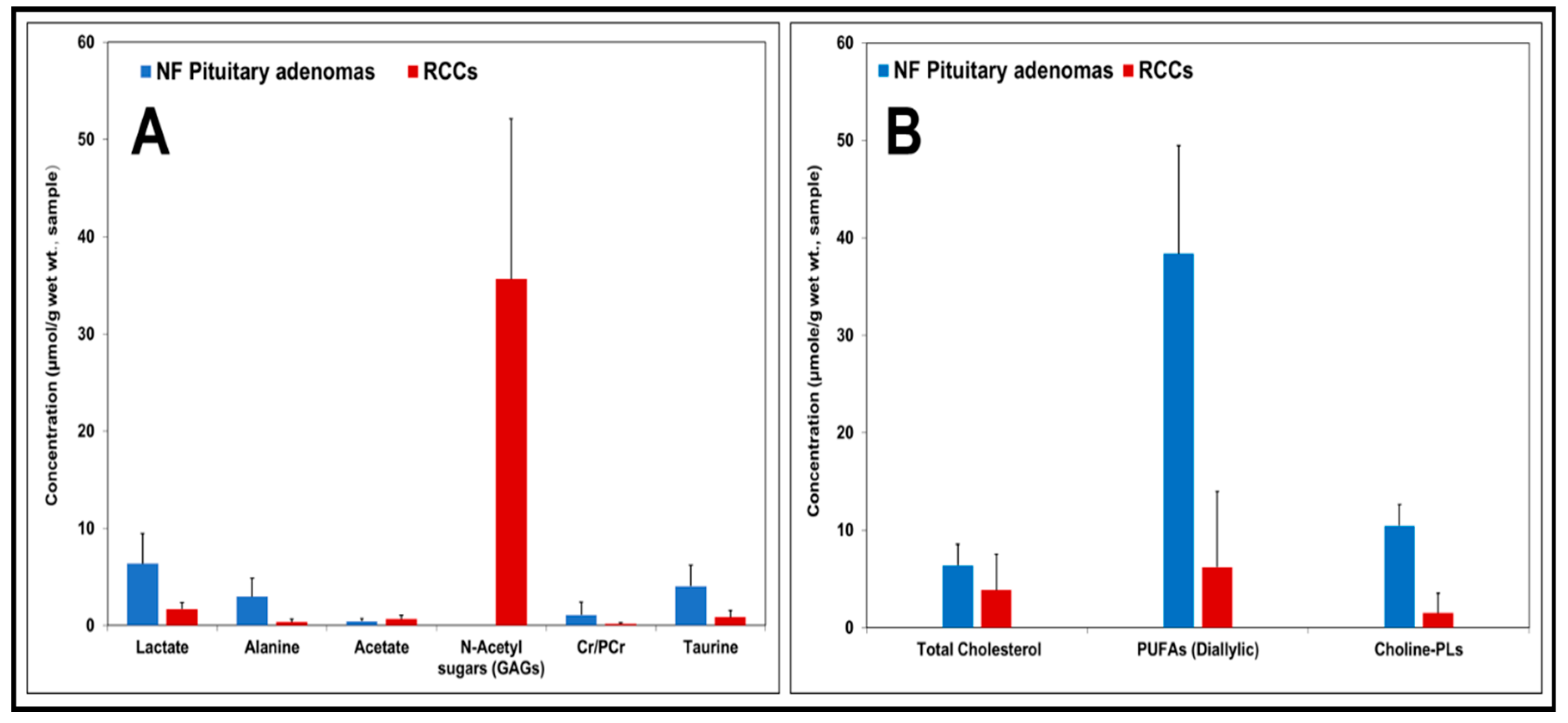
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry (IHC)
4.3. Chemicals
4.4. Methanol–Chloroform Extraction
4.5. 1D and 2D 1H NMR Experiments
4.6. Identification and Quantification of Metabolites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larkin, S.; Karavitaki, N.; Ansorge, O. Rathke’s cleft cyst. Handb. Clin. Neurol. 2014, 124, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.; Ansorge, O. Pathology and Pathogenesis of Pituitary Adenomas and Other Sellar Lesions; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ross, D.A.; Norman, D.; Wilson, C.B. Radiologic characteristics and results of surgical management of Rathke’s cysts in 43 patients. Neurosurgery 1992, 30, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Molinaro, A.M.; Tarapore, P.E.; Blevins, L., Jr.; Auguste, K.I.; Gupta, N.; Kunwar, S.; Aghi, M.K. Rathke cleft cysts in pediatric patients: presentation, surgical management, and postoperative outcomes. Neurosurg Focus. 2011, 3, E3. [Google Scholar] [CrossRef] [PubMed]
- Byun, W.M.; Kim, O.L.; Kim, D.S. MR Imaging Findings of Rathke’s Cleft Cysts: Significance of Intracystic Nodules. Am. J. Neuroradiol. 2000, 21, 485–488. [Google Scholar] [PubMed]
- Guo, S.Y.; Cai, X.Q.; Ma, J.; Wang, W.Y.; Lu, G. Diagnosis of concomitant pituitary adenoma and Rathke’s cleft cyst with magnetic resonance imaging. Int. J. Surg. 2015, 18, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Tachibana, O.; Muramatsu, N.; Tsuchiya, H.; Tada, M.; Arakawa, Y.; Suzuki, M.; Yamashita, J. Rathke cleft cyst: MR and biomedical analysis of cyst content. J. Comput Assist Tomogr. 1999, 23, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Back, A.G.; Komisarow, J.M.; Owens, T.R.; Cummings, T.J.; Britz, G.W. Symptomatic Rathke’s cleft cyst with a co-existing pituitary tumor; Brief review of the literature. Asian J. Neurosurg. 2013, 8, 183–187. [Google Scholar] [CrossRef]
- Kunii, N.; Abe, T.; Kawamo, M.; Tanioka, D.; Izumiyama, H.; Moritani, T. Rathke’s cleft cysts: differentiation from other cystic lesions in the pituitary fossa by use of single-shot fast spin-echo diffusion-weighted MR imaging. Acta Neurochir. (Wien) 2007, 149, 759–769. [Google Scholar] [CrossRef]
- Tamura, R.; Takahashi, S.; Emoto, K.; Nagashima, H.; Toda, M.; Yoshida, K. GH-producing pituitary adenoma and concomitant Rathke’s cleft cyst: A case report and short review. Case Rep. Neurol. Med. 2015, 948025. [Google Scholar] [CrossRef]
- Park, M.; Lee, S.K.; Choi, J.; Kim, S.H.; Kim, S.H.; Shin, N.Y.; Kim, J.; Ahn, S.S. Differentiation between Cystic Pituitary Adenomas and Rathke Cleft Cysts: A Diagnostic Model Using MRI. AJNR Am. J. Neuroradiol. 2015, 36, 1866–1873. [Google Scholar] [CrossRef]
- Lillehei, K.O.; Widdel, L.; Astete, C.A.; Wierman, M.E.; Kleinschmidt-DeMasters, B.K.; Kerr, J.M. Transsphenoidal resection of 82 Rathke cleft cysts: limited value of alcohol cauterization in reducing recurrence rates. J. Neurosurg. 2011, 114, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ijare, O.B.; Baskin, D.S.; Pichumani, K. Ex Vivo 1H NMR study of pituitary adenomas to differentiate various immunohistochemical subtypes. Sci. Rep. 2019, 9, 3007. [Google Scholar] [CrossRef] [PubMed]
- Bezabeh, T.; Ijare, O.B.; Marginean, E.C.; Nicholas, G. Proton magnetic resonance spectroscopy of sputum for the non-invasive diagnosis of lung cancer: Preliminary findings. J. Anal. Oncol. 2012, 1, 14–18. [Google Scholar] [CrossRef]
- Liu, Z.; Masuko, S.; Solakyildirim, K.; Pu, D.; Linhardt, R.J.; Zhang, F. Glycosaminoglycans of the porcine central nervous system. Biochemistry 2010, 49, 9839–9847. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K. Biochemistry and biology of mucopolysaccharides. Am. J. Med. 1969, 47, 664–672. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Domowicz, M.S. Chemistry and function of glycosaminoglycans in the nervous system. Adv. Neurobiol. 2014, 9, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. NMR chemical shifts in structural biology of glycosaminoglycans. Anal. Chem. 2014, 86, 65–94. [Google Scholar] [CrossRef]
- Robbe, C.; Capon, C.; Maes, E.; Rousset, M.; Zweibaum, A.; Zanetta, J.P.; Michalski, J.C. Evidence of regio-specific glycosylation in human intestinal mucins: Presence of an acidic gradient along the intestinal tract. J. Biol. Chem. 2003, 278, 46337–46348. [Google Scholar] [CrossRef]
- Kleinschmidt-DeMasters, B.K.; Lillehei, K.O.; Stears, J.C. The pathologic, surgical, and MR spectrum of Rathke cleft cysts. Surg. Neurol. 1995, 44, 19–26. [Google Scholar] [CrossRef]
- Faghih, J.M.; Ghodsi, S.M.; Akhlaghpoor, S.; Mehrazin, M.; Saadat, S.; Khoshnevisan, A.; Padeganeh, T.; Aoude, A. Complementary effect of H MRS in diagnosis of suprasellar tumors. Clin. Imaging. 2012, 36, 810–815. [Google Scholar] [CrossRef]
- Einstien, A.; Virani, R.A. Clinical Relevance of Single-Voxel (1)H MRS Metabolites in Discriminating Suprasellar Tumors. J. Clin. Diagn. Res. 2016, 10, TC01–TC04. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Yue, K.; Binesh, N.; Davanzo, P.; Kumar, A.; Siegel, B.; Frye, M.; Curran, J.; Lufkin, R.; Martin, P.; et al. Localized two-dimensional shift correlated MR spectroscopy of human brain. Magn. Reson. Med. 2001, 46, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, M.; Cobianchi, B.F.; Bruno, F.; Palumbo, P.; Natella, R.; Maggialetti, N.; De Filippo, M.; Di Cesare, E.; Barile, A.; Masciocchi, C.; et al. High-resolution magnetic resonance imaging at 3T of pituitary gland: advantages and pitfalls. Gland Surg. 2019, 8, S208–S215. [Google Scholar] [CrossRef] [PubMed]
| Patient | Age (y)/Sex | Clinical Characteristics | Hormone Panel | MRI Findings (Tumor Size) | Indications for Surgery | Immunohistochemistry (IHC) |
|---|---|---|---|---|---|---|
| 1 | 46/F | Blurry vision, fullness in ears | LH = 11.1 FSH = 16.9 PRL = 9.0 GH = <0.05 TSH = NA ACTH = NA | Showed a pituitary mass. (1.4 × 1.5 × 1.7 cm3) | Since serum PRL was normal, medical therapy was not an option, indicating surgery to resect tumor mass. | Exfoliation of neuroendocrine appearing cells, effacement of normal adenohypophyseal architecture and replacement by neuroendocrine appearing cells; tumor cells negative for PRL, LH, FSH, ACTH, TSH, and GH. MIB-1 = 1% |
| 2 | 37/M | Visual field deficit, stretch marks on stomach and back, weight gain suggesting Cushing’s disease. | LH = 2.1 FSH = 2.4 PRL = 7.6 GH = 0.1 TSH = NA ACTH = 110.0 Cortisol (urine/24 h) = 13.1 µg/L | Pituitary macroadenoma with suprasellar extension, (3.9 × 2.3 × 2.4 cm3) | Tumor mass was compressing optic chiasm and nerves. | Same as above. MIB-1 = 3% |
| 3 | 39/F | Pituitary tumor recurrence, previously underwent surgery 2 times. | LH = 4.8 FSH = 9.7 PRL = 17 GH = 0.31 TSH = 1.31 ACTH = 22.8 free T4 index = 1.7 | A mass in the left side of sella, protruding into suprasellar region. (1.0 × 1.0 × 0.8 cm3) | Tumor mass was protruding into the suprasellar region with mass effect on the optic nerves. | Same as above. MIB-1 = 1% |
| 4 | 42/M | Dizziness, headache, visual field disturbance | LH = 3.9 FSH = 4.5 PRL = 6.6 GH = 170.0 TSH = 0.15 ACTH = 41.2 | A suprasellar mass. (1.8 × 2.1 × 1.3 cm3) | Tumor mass was uplifting the optic chiasm and prechiasmatic optic nerve. | Same as above. MIB-1 = 1% |
| 5 | 75/F | Dizziness, forgetfulness, blurry vision | LH = 2.1 FSH = 3.9 PRL = 11.0 GH = 0.07 TSH = 0.42 ACTH = 53.5 Cortisol (urine/24h) = 27.0 µg/L | Pituitary mass in the anterior aspect of the sella, hyperintense on T1-weighted image. (1.0 × 1.4 × 1.0 cm3) | Surgery was recommended due to the size of the tumor and proximity to/possible compression of the optic apparatus. | Monomorphous expansion of neuroendocrine cells, loss of normal acinar architecture, tumor cells negative for PRL, LH, FSH, ACTH, TSH, and GH. MIB-1 = 1% |
| 6 | 58/M | Fatigue with dizziness, nausea and vomiting, hyponatremia and hypokalemia. | LH = 0.3 FSH = 2.0 PRL = 10.2 GH = 0.1 TSH = 3.41 ACTH = 5 | An intrasellar mass with suprasellar extension. Hyperintense on pre-contrast T1-weighted image. (1.0 × 1.2 × 1.0 cm3) | Tumor mass was abutting the optic chiasm. The tumor mass was hyperintense on T1-image suggesting hemorrhage, and pan hypopituitarism. | Acellular mucoid debris with intermixed eosinophilic proteinaceous globules consistent with cyst contents. Cyst wall showed the presence of ciliated epithelium consistent with RCC. No features of pituitary adenoma were identified. |
| 7 | 38/F | Dizziness, right-sided facial numbness and headache. History of thyroid cancer currently on thyroid supplementation. | LH = 7.0 FSH = 6.7 PRL = 10.6 GH = 0.2 TSH = 0.02 ACTH = 11.0 | A lesion on the right side of the pituitary gland, bulging into the suprasellar cistern without mass effect Bright on T2-weighted image. (0.9 × 0.7 × 0.9 cm3) | The lesion was extended into the suprasellar cistern and abutted the optic nerve. | Same as above, findings diagnostic of RCC. |
| 8 | 25/M | Dizziness | LH = 3.2 FSH = 2.3 PRL= 5.7 GH = 0.1 TSH = 0.92 ACTH = NA | Intrasellar mass which is cystic in nature. (0.4 × 0.5 × 0.35 cm3) | Since serum PRL was normal, medical therapy was not indicated, and the surgery was recommended. | Proteinaceous debris and fibrotic tissue with rare ciliated cells compatible with RCC. No features of pituitary adenoma were identified. |
| 9 | 45/F | Prolactin secreting pituitary lesion was suspected. | LH = 10 FSH = 0.3 PRL = 55.05 # GH = NA TSH = 2.05 ACTH = NA | MRI showed a large pituitary mass bulging up into the suprasellar cistern. (1.1 × 1.2 × 2.0 cm3) | Surgery was recommended due to the presence of large pituitary mass which was extending into suprasellar cistern and compressing the optic apparatus. | Acellular debris with scattered epithelial cells consistent with RCC. No features of pituitary adenoma were identified. |
| 10 | 25/F | Irregular menstrual cycle, weight gain, prolactin secreting pituitary lesion was suspected. | LH = 5.8 FSH = 2.1 PRL = 26.9 # GH = 0.1 TSH = 1.80 ACTH = 20.8 | MRI showed a pituitary lesion, differential hypo enhancement in the posterior aspect of the pituitary gland. (0.5 × 1.0 × 0.5 cm3) | Follow up blood work confirmed that serum PRL was normal (22.4 ng/mL), and surgical excision of the lesion was recommended. | Proteinaceous debris with bland degenerated cells compatible with RCC. No features of pituitary adenoma were identified. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijare, O.B.; Sharpe, M.A.; Baskin, D.S.; Pichumani, K. Proton Magnetic Resonance Spectroscopy Characterization of Rathke’s Cleft Cysts (RCCs): Relevance to the Differential Diagnosis of Pituitary Adenomas and RCCs. Cancers 2020, 12, 360. https://doi.org/10.3390/cancers12020360
Ijare OB, Sharpe MA, Baskin DS, Pichumani K. Proton Magnetic Resonance Spectroscopy Characterization of Rathke’s Cleft Cysts (RCCs): Relevance to the Differential Diagnosis of Pituitary Adenomas and RCCs. Cancers. 2020; 12(2):360. https://doi.org/10.3390/cancers12020360
Chicago/Turabian StyleIjare, Omkar B., Martyn A. Sharpe, David S. Baskin, and Kumar Pichumani. 2020. "Proton Magnetic Resonance Spectroscopy Characterization of Rathke’s Cleft Cysts (RCCs): Relevance to the Differential Diagnosis of Pituitary Adenomas and RCCs" Cancers 12, no. 2: 360. https://doi.org/10.3390/cancers12020360
APA StyleIjare, O. B., Sharpe, M. A., Baskin, D. S., & Pichumani, K. (2020). Proton Magnetic Resonance Spectroscopy Characterization of Rathke’s Cleft Cysts (RCCs): Relevance to the Differential Diagnosis of Pituitary Adenomas and RCCs. Cancers, 12(2), 360. https://doi.org/10.3390/cancers12020360




