Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results
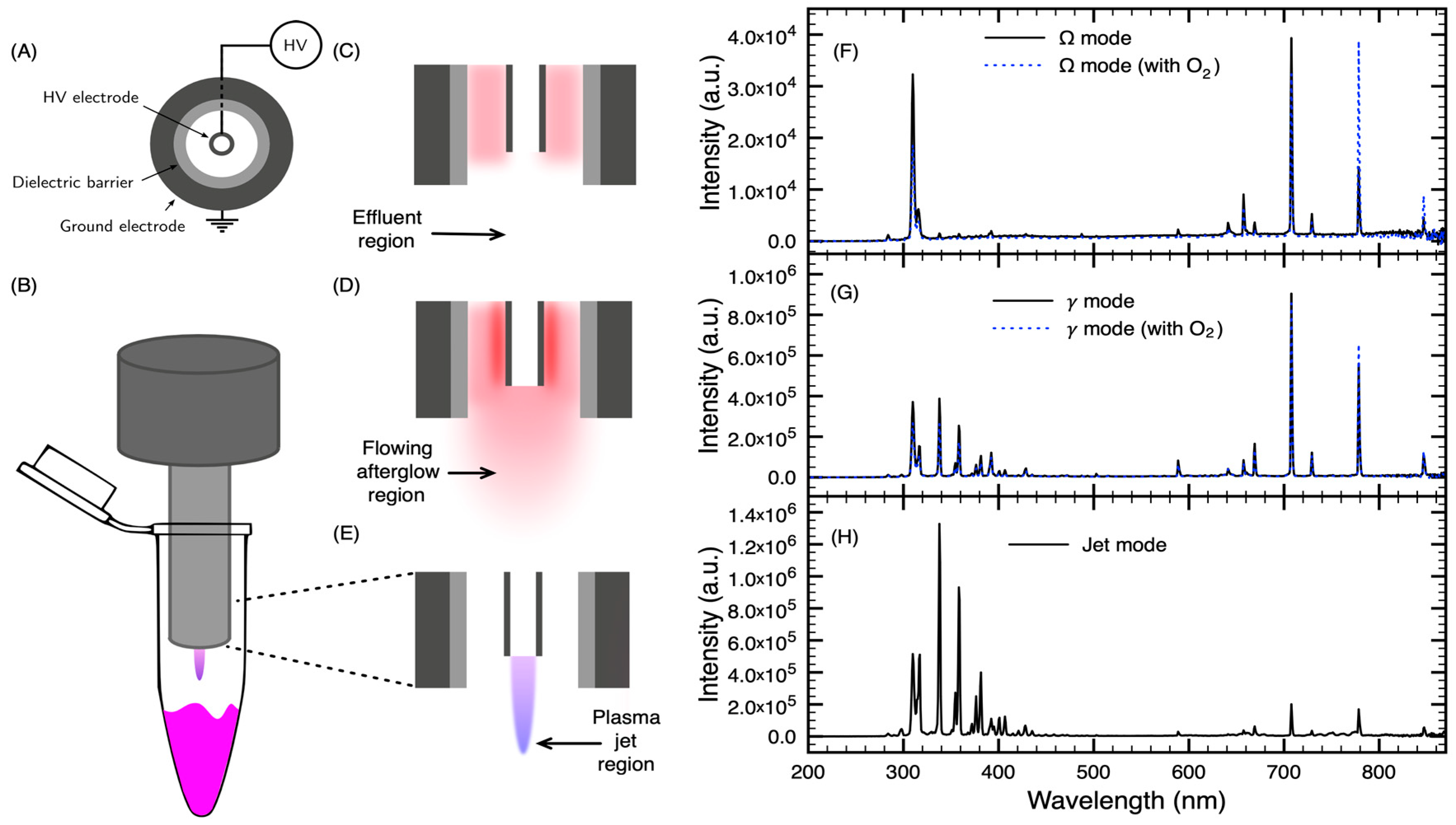
2.1. NTP Device and Experimental Setup
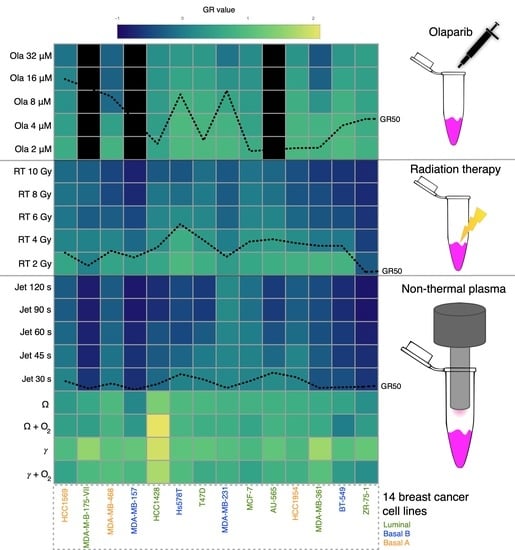
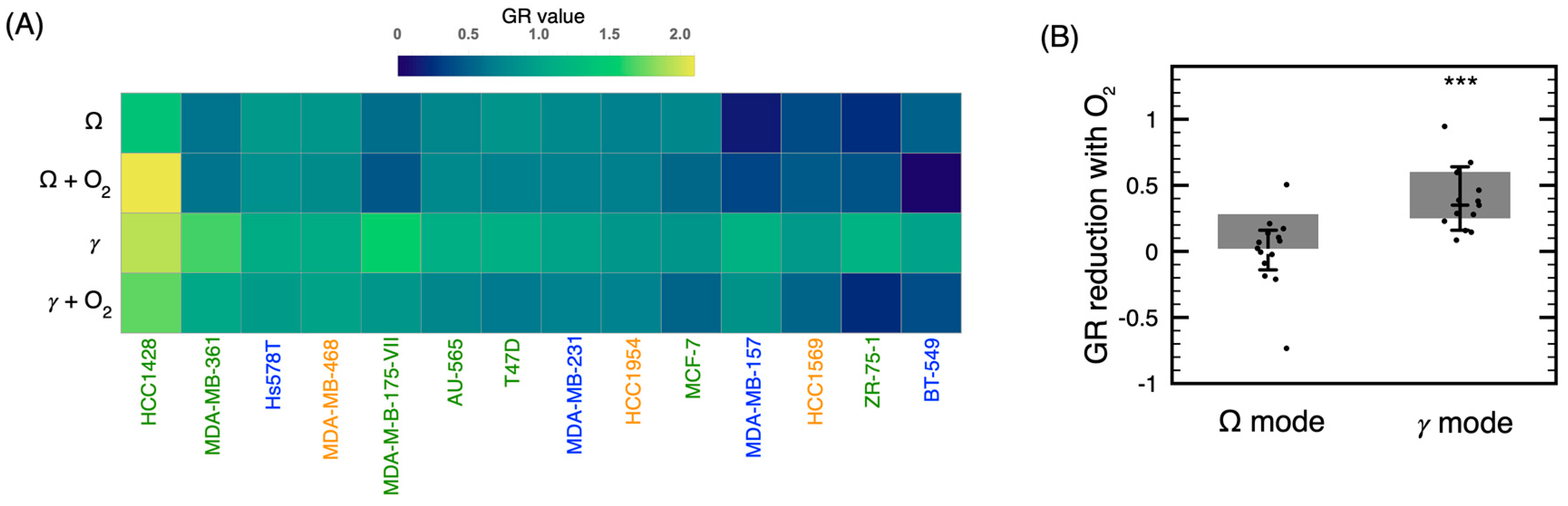
2.2. Influence of the Discharge Mode on the Cytotoxicity of the Treatment
2.3. Sensitivity of Breast Cancer Cell Lines to NTP Correlates with RT
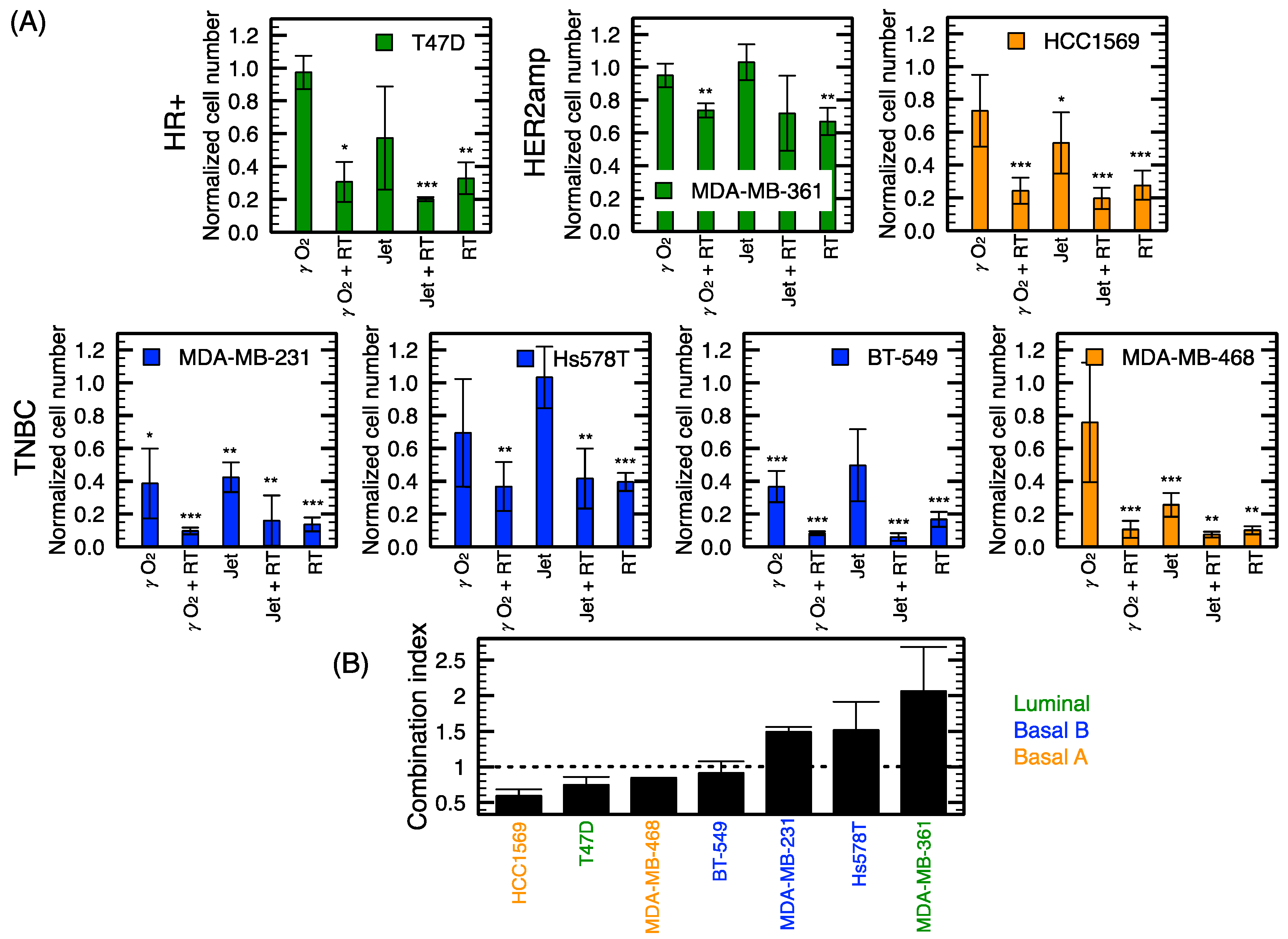
2.4. Radiosensitization of Breast Cancer Cell Lines with NTP
2.5. Olaparib Influence on NTP Growth Inhibition and DNA Damage Potential
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasma Device
4.3. Radiation Therapy and Plasma Treatment
4.4. Proliferation Assay
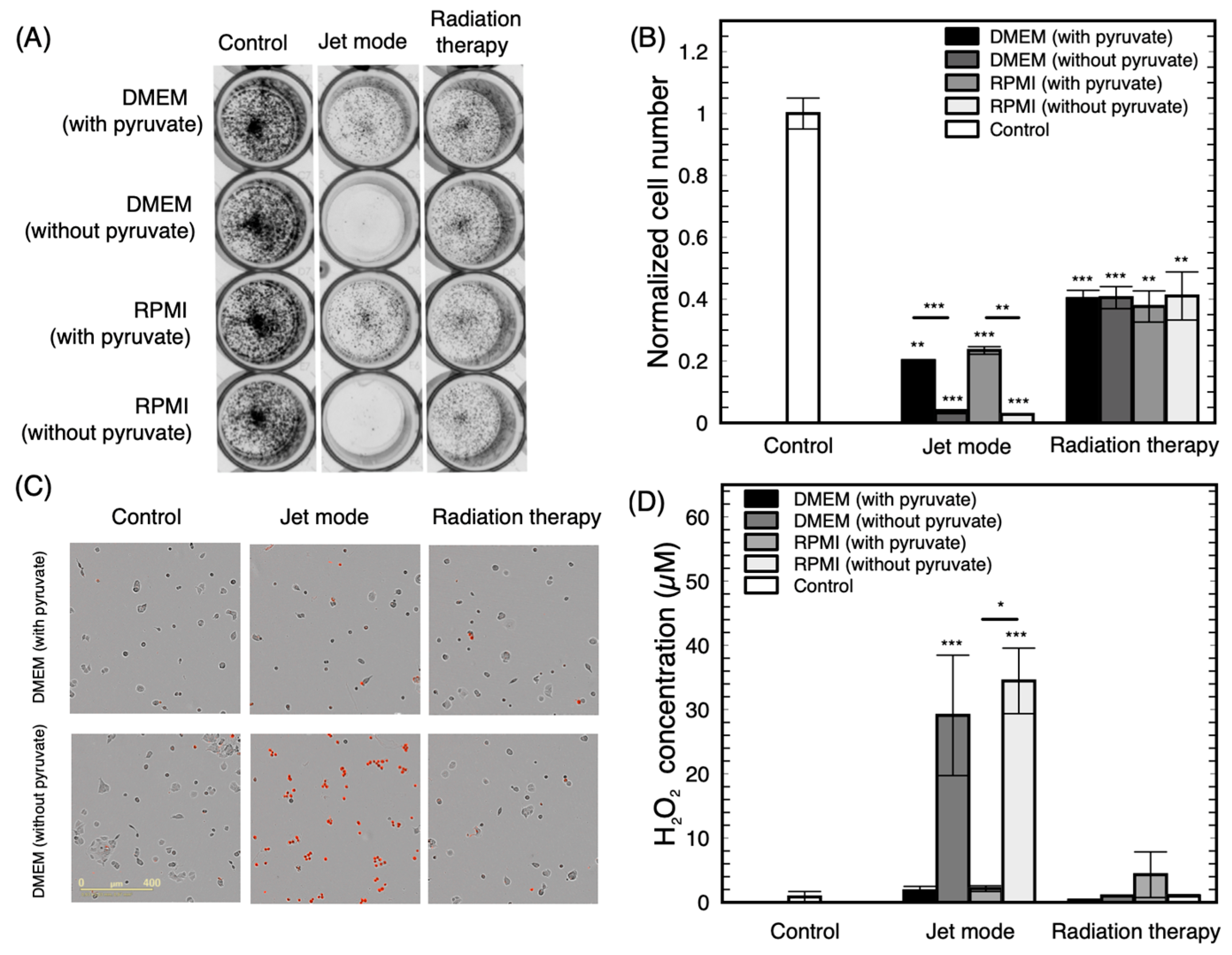
4.5. Peroxide Detection
4.6. Live Cell Imaging System
4.7. Combination Treatment
4.8. Immunofluorescence
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Cell Line | IC50 of the Jet Mode (s) | GR50 of the Jet Mode (s) | GR50 of RT (Gy) | GR50 of Olaparib (μM) | Doubling Time (d) |
|---|---|---|---|---|---|
| MDA-MB-231 | 1.6 | 4.9 | 1.8 | 9.6 | 1.5 |
| MDA-MB-468 | 6.0 | 10.9 | 2.3 | 3.4 | 1.6 |
| Hs578T | 18.4 | 23.3 | 4.6 | 22.3 | 1.7 |
| HCC1954 | 6.9 | 19.2 | 3.0 | 18.2 | 1.7 |
| AU-565 | 24.1 | 25.2 | 3.3 | NA | 1.8 |
| T47D | 9.6 | 16.0 | 3.2 | 17.6 | 2.1 |
| BT-549 | 7.8 | 5.8 | 2.7 | 19.2 | 2.3 |
| MCF-7 | 15.0 | 14.1 | 3.1 | 4.5 | 2.3 |
| HCC1569 | 12.1 | 13.9 | 2.1 | 3.4 | 2.4 |
| MDA-MB-157 | 4.6 | 2.6 | 1.7 | NA | 4.1 |
| ZR-75-1 | 7.9 | 6.6 | 0.5 | 11.4 | 4.5 |
| MDA-MB-361 | 31.0 | 5.1 | 2.7 | 5.5 | 5.4 |
| MDA-MB-175-VII | 13.1 | 4.3 | 1.0 | NA | 5.5 |
| HCC1428 | 48.3 | 10.2 | 2.6 | 2.5 | 7.7 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Cancer.gov Breast Cancer Treatment (Adult) (PDQ®)–Health Professional Version. Available online: www.cancer.gov/types/breast (accessed on 9 September 2019).
- Moran, M.S. Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol. 2015, 16, e113–e122. [Google Scholar] [CrossRef]
- O’Rorke, M.A.; Murray, L.J.; Brand, J.S.; Bhoo-Pathy, N. The value of adjuvant radiotherapy on survival and recurrence in triple-negative breast cancer: A systematic review and meta-analysis of 5507 patients. Cancer Treat. Rev. 2016, 47, 12–21. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 5. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Sherman, J.H. Cold Plasma Cancer Therapy; Morgan & Claypool Publishers: San Rafael, CA, USA, 2019; ISBN 978-1-64327-434-8. [Google Scholar]
- Kim, S.J.; Chung, T.; Bae, S.; Leem, S. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl. Phys. Lett. 2010, 97, 023702. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.; Cerchar, E.; Azizkhan-Clifford, J. Selectivity of non-thermal atmospheric-pressure microsecond-pulsed dielectric barrier discharge plasma induced apoptosis in tumor cells over healthy cells. Plasma Med. 2011, 1, 3–4. [Google Scholar] [CrossRef]
- Park, S.-B.; Kim, B.; Bae, H.; Lee, H.; Lee, S.; Choi, E.H.; Kim, S.J. Differential epigenetic effects of atmospheric cold plasma on MCF-7 and MDA-MB-231 breast cancer cells. PLoS ONE 2015, 10, e0129931. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, S.; Zhang, H.; Kong, X.; Ding, L.; Shen, J.; Lan, Y.; Cheng, C.; Zhu, T.; Xia, W. Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways. Sci. Rep. 2017, 7, 1–2. [Google Scholar] [CrossRef]
- Mehrabifard, R.; Mehdian, H.; Bakhshzadmahmoudi, M. Effect of non-thermal atmospheric pressure plasma on MDA-MB-231 breast cancer cells. Pharm. Biomed. Res. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Pesnel, S.; Barbosa, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.-M. Antitumor Effect of Plasma Treatment on U87 Glioma Xenografts: Preliminary Results. Plasma Process. Polym. 2010, 7, 264–273. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Piroozmand, S.; Soleimani, N.; Faharani, N.J.; Ghomi, H.; Eskandari, H.F.; Sharifi, A.M.; Mirpour, S.; Eftekhari, M.; Nikkhah, M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016, 6, 29048. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Zheng, Q.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Micro-sized cold atmospheric plasma source for brain and breast cancer treatment. Plasma Med. 2018, 8. [Google Scholar] [CrossRef]
- Chagpar, A.B.; Killelea, B.K.; Tsangaris, T.N.; Butler, M.; Stavris, K.; Li, F.; Yao, X.; Bossuyt, V.; Harigopal, M.; Lannin, D.R.; et al. A randomized, controlled trial of cavity shave margins in breast cancer. N. Engl. J. Med. 2015, 373, 503–510. [Google Scholar] [CrossRef]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells--a review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, J.-S.; Lafontaine, J.; Glory, A.; Coulombe, S.; Wong, P. Comparison of Three Radio-Frequency Discharge Modes on the Treatment of Breast Cancer Cells In Vitro. 2020. Available online: http://hdl.handle.net/1866/22968 (accessed on 14 January 2020).
- Lu, X.; Naidis, G.; Laroussi, M.; Reuter, S.; Graves, D.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Bahn, J.H.; Lee, S.-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Joh, H.M.; Choi, J.Y.; Kim, S.J.; Chung, T.H.; Kang, T.-H. Effect of additive oxygen gas on cellular response of lung cancer cells induced by atmospheric pressure helium plasma jet. Sci. Rep. 2014, 6638. [Google Scholar] [CrossRef]
- Hafner, M.; Niepel, M.; Chung, M.; Sorger, P.K. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat. Methods 2016, 521–527. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; De Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Hirst, A.M.; Frame, F.M.; Maitland, N.J.; O’Connell, D. Low Temperature Plasma Causes Double-Strand Break DNA Damage in Primary Epithelial Cells Cultured from a Human Prostate Tumor. IEEE Trans. Plasma Sci. 2014, 42, 2740–2741. [Google Scholar] [CrossRef]
- Thompson, M.K.; Poortmans, P.; Chalmers, A.J.; Faivre-Finn, C.; Hall, E.; Huddart, R.A.; Lievens, Y.; Sebag-Montefiore, D.; Coles, C.E. Practice-changing radiation therapy trials for the treatment of cancer: Where are we 150 years after the birth of Marie Curie? Br. J. Cancer 2018, 119, 389. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy—Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Garcia, T.B.; Snedeker, J.C.; Baturin, D.; Gardner, L.; Fosmire, S.P.; Zhou, C.; Jordan, C.T.; Venkataraman, S.; Vibhakar, R.; Porter, C.C. A small-molecule inhibitor of WEE1, AZD1775, synergizes with olaparib by impairing homologous recombination and enhancing DNA damage and apoptosis in acute leukemia. Mol. Cancer Ther. 2017, 16, 2058–2068. [Google Scholar] [CrossRef]
- Carey, J.P.; Karakas, C.; Bui, T.; Chen, X.; Vijayaraghavan, S.; Zhao, Y.; Wang, J.; Mikule, K.; Litton, J.K.; Hunt, K.K.; et al. Synthetic lethality of PARP inhibitors in combination with MYC blockade is independent of BRCA status in triple-negative breast cancer. Cancer Res. 2018, 78, 742–757. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Pierce, A.; McGowan, P.M.; Cotter, M.; Mullooly, M.; O’Donovan, N.; Rani, S.; O’Driscoll, L.; Crown, J.; Duffy, M.J. Comparative antiproliferative effects of iniparib and olaparib on a panel of triple-negative and non-triple-negative breast cancer cell lines. Cancer Biol. Ther. 2013, 14, 537–545. [Google Scholar] [CrossRef]
- Fleury, H.; Malaquin, N.; Tu, V.; Gilbert, S.; Martinez, A.; Olivier, M.-A.; Sauriol, A.; Communal, L.; Leclerc-Desaulniers, K.; Carmona, E.; et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- De Feraudy, S.; Revet, I.; Bezrookove, V.; Feeney, L.; Cleaver, J.E. A minority of foci or pan-nuclear apoptotic staining of γH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc. Natl. Acad. Sci. USA 2010, 107, 6870–6875. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schütz, C.S.; Nießner, F.; Wende, K.; Weltmann, K.-D.; Gelbrich, N.; von Woedtke, T.; Schmidt, A.; Stope, M.B. Elevated H2AX Phosphorylation Observed with kINPen Plasma Treatment Is Not Caused by ROS-Mediated DNA Damage but Is the Consequence of Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Cancerbrowser.org HMS LINCS Breast Cancer Browser. Available online: www.cancerbrowser.org (accessed on 28 November 2019).
- Smirnov, P.; Kofia, V.; Maru, A.; Freeman, M.; Ho, C.; El-Hachem, N.; Adam, G.-A.; Ba-alawi, W.; Safikhani, Z.; Haibe-Kains, B. PharmacoDB: An integrative database for mining in vitro anticancer drug screening studies. Nucleic Acids Res. 2017, 46, D994–D1002. [Google Scholar] [CrossRef]
- Léveillé, V.; Coulombe, S. Atomic Oxygen Production and Exploration of Reaction Mechanisms in a He-O2 Atmospheric Pressure Glow Discharge Torch. Plasma Process. Polym. 2006, 3, 587–596. [Google Scholar] [CrossRef]
- Neijenhuis, S.; Verwijs-Janssen, M.; van den Broek, L.J.; Begg, A.C.; Vens, C. Targeted radiosensitization of cells expressing truncated DNA polymerase β. Cancer Res. 2010, 70, 8706–8714. [Google Scholar] [CrossRef] [PubMed]
- Bridges, K.A.; Hirai, H.; Buser, C.A.; Brooks, C.; Liu, H.; Buchholz, T.A.; Molkentine, J.M.; Mason, K.A.; Meyn, R.E. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin. Cancer Res. 2011, 17, 5638–5648. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tsuno, N.H.; Sunami, E.; Kawai, K.; Shuno, Y.; Hongo, K.; Hiyoshi, M.; Kaneko, M.; Murono, K.; Tada, N.; et al. Radiosensitization of human breast cancer cells to ultraviolet light by 5-fluorouracil. Oncol. Lett. 2011, 2, 471–476. [Google Scholar] [CrossRef]
- Müller, M.; Wang, Y.; Squillante, M.R.; Held, K.D.; Anderson, R.R.; Purschke, M. UV scintillating particles as radiosensitizer enhance cell killing after X-ray excitation. Radiother. Oncol. 2018, 129, 589–594. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F. Variable susceptibility of ovarian cancer cells to non-thermal plasma-activated medium. Oncol. Rep. 2016, 35, 3169–3177. [Google Scholar] [CrossRef][Green Version]
- Bekeschus, S.; Freund, E.; Wende, K.; Gandhirajan, R.; Schmidt, A. Hmox1 upregulation is a mutual marker in human tumor cells exposed to physical plasma-derived oxidants. Antioxidants 2018, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; von Behr, M.; Bekeschus, S.; Weltmann, K.-D.; Hackbarth, C.; Heidecke, C.-D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Synergistic inhibition of tumor cell proliferation by cold plasma and gemcitabine. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Y.; Jin, C.; Fu, D. Gemcitabine-based chemotherapy as a viable option for treatment of advanced breast cancer patients: A meta-analysis and literature review. Oncotarget 2018, 9, 7148. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Seo, S.J.; Kim, Y.S.; Yang, S.S.; Lee, J.-S.; Moon, E.; Lee, K.; Kim, C.-H. Combination of NTP with cetuximab inhibited invasion/migration of cetuximab-resistant OSCC cells: Involvement of NF-κB signaling. Sci. Rep. 2015, 5, 18208. [Google Scholar] [CrossRef] [PubMed]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.-M.; Le Pape, A. Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-luc Orthotopic Pancreatic Carcinoma Model. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, l1. [Google Scholar] [CrossRef]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 503–508. [Google Scholar] [CrossRef]
- Mallet, R.T. Pyruvate: Metabolic protector of cardiac performance. Proc. Soc. Exp. Biol. Med. Minireviews 2000, 223, 136–148. [Google Scholar] [CrossRef]
- Nath, K.A.; Enright, H.; Nutter, L.; Fischereder, M.; Zou, J.; Hebbel, R.P. Effect of pyruvate on oxidant injury to isolated and cellular DNA. Kidney Int. 1994, 45, 166–176. [Google Scholar] [CrossRef]
- Babich, H.; Liebling, E.J.; Burger, R.F.; Zuckerbraun, H.L.; Schuck, A.G. Choice of DMEM, formulated with or without pyruvate, plays an important role in assessing the in vitro cytotoxicity of oxidants and prooxidant nutraceuticals. Vitro Cell. Dev. Biol. Anim. 2009, 45, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Biscop, E.; Lin, A.; Boxem, W.V.; Loenhout, J.V.; Backer, J.D.; Deben, C.; Dewilde, S.; Smits, E.; Bogaerts, A. Influence of Cell Type and Culture Medium on Determining Cancer Selectivity of Cold Atmospheric Plasma Treatment. Cancers 2019, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Kelts, J.L.; Cali, J.J.; Duellman, S.J.; Shultz, J. Altered cytotoxicity of ROS-inducing compounds by sodium pyruvate in cell culture medium depends on the location of ROS generation. Springerplus 2015, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, C.; Rebl, H.; Otto, A.; Matschke, S.; Nebe, B. Pyruvate as a cell-protective agent during cold atmospheric plasma treatment in vitro: Impact on basic research for selective killing of tumor cells. Plasma Process. Polym. 2019, 16, 1900088. [Google Scholar] [CrossRef]
- Pranda, M.A.; Murugesan, B.J.; Knoll, A.J.; Oehrlein, G.S.; Stroka, K.M. Sensitivity of tumor versus normal cell migration and morphology to cold atmospheric plasma-treated media in varying culture conditions. Plasma Process. Polym. 2019, 11, e1900103. [Google Scholar] [CrossRef]




| Cell Line | Molecular Subtype | Receptor Status | Mutation Summary |
|---|---|---|---|
| AU-565 | Luminal | HER2amp | TP53, MLL3 |
| BT-549 | Basal B | TNBC | TP53, PTEN |
| HCC1428 | Luminal | HR+ | TP53 |
| HCC1569 | Basal A | HER2amp | TP53, MLL3, BRCA2, PTEN |
| HCC1954 | Basal A | HER2amp | TP53, PIK3CA |
| Hs578T | Basal B | TNBC | TP53 |
| MCF-7 | Luminal | HR+ | PIK3CA, GATA3 |
| MDA-MB-157 | Basal B | TNBC | TP53, MAP3K1 |
| MDA-MB-175-VII | Luminal | HR+ | MLL3 |
| MDA-MB-231 | Basal B | TNBC | TP53 |
| MDA-MB-361 | Luminal | HR+ | TP53, PIK3CA, BRCA2 |
| MDA-MB-468 | Basal A | TNBC | TP53, MLL3, PTEN |
| T47D | Luminal | HR+ | TP53, PIK3CA, MLL3 |
| ZR-75-1 | Luminal | HR+ | PTEN |
| Conditions | Discharge Mode | Applied Power | Treatment Time | Helium Flowrate | O2 Flowrate |
|---|---|---|---|---|---|
| Ω | Ω mode | 10 W | 4 min | 4300 mL min−1 | 0 mL min−1 |
| Ω + O2 | Ω mode | 10 W | 4 min | 4300 mL min−1 | 2 mL min−1 |
| γ | γ mode | 35 W (100 Hz@20%) | 2 min | 4300 mL min−1 | 0 mL min−1 |
| γ + O2 | γ mode | 35 W (100 Hz@20%) | 2 min | 4300 mL min−1 | 2 mL min−1 |
| Jet | Jet mode | 35 W | 10 to 120 s | 600 mL min−1 | 0 mL min−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafontaine, J.; Boisvert, J.-S.; Glory, A.; Coulombe, S.; Wong, P. Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers 2020, 12, 348. https://doi.org/10.3390/cancers12020348
Lafontaine J, Boisvert J-S, Glory A, Coulombe S, Wong P. Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers. 2020; 12(2):348. https://doi.org/10.3390/cancers12020348
Chicago/Turabian StyleLafontaine, Julie, Jean-Sébastien Boisvert, Audrey Glory, Sylvain Coulombe, and Philip Wong. 2020. "Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines" Cancers 12, no. 2: 348. https://doi.org/10.3390/cancers12020348
APA StyleLafontaine, J., Boisvert, J.-S., Glory, A., Coulombe, S., & Wong, P. (2020). Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers, 12(2), 348. https://doi.org/10.3390/cancers12020348