Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready ‘N Able to Improve Clinical Outcome?
Abstract
1. Introduction
1.1. Dendritic Cell (DC) Vaccination
1.2. Disease Applications
1.3. Optimizing Efficacy
1.4. Ribonucleic Acid (RNA) Modification
2. RNA Sources and Products
2.1. Total RNA/mRNA
2.2. Synthetic RNA
2.3. Messenger RNA (mRNA)
2.4. Interfering RNA (shRNA/siRNA)
2.5. MicroRNA (miRNA)
3. RNA Delivery Methods
4. Clinical Trials
4.1. Diseases
4.2. Vaccination
4.3. Toxicity
4.4. Immunological Responses
4.5. Clinical Responses
5. Discussion
5.1. Shortcomings and Contributions of Published Clinical Trials
5.2. Prospects
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Ashley, D.M.; Faiola, B.; Nair, S.; Hale, L.P.; Bigner, D.D.; Gilboa, E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J. Exp. Med. 1997, 186, 1177–1182. [Google Scholar] [CrossRef]
- Decker, W.K.; Xing, D.; Li, S.; Robinson, S.N.; Yang, H.; Yao, X.; Segall, H.; McMannis, J.D.; Komanduri, K.V.; Champlin, R.E.; et al. Double loading of dendritic cell MHC class I and MHC class II with an AML antigen repertoire enhances correlates of T-cell immunity in vitro via amplification of T-cell help. Vaccine 2006, 24, 3203–3216. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, L.W.; Liu, W.C.; Cheng, J.; Si, X.M.; Ren, J. Comparative analysis of DC fused with tumor cells or transfected with tumor total RNA as potential cancer vaccines against hepatocellular carcinoma. Cytotherapy 2006, 8, 580–588. [Google Scholar] [CrossRef]
- Benencia, F.; Courreges, M.C.; Coukos, G. Whole tumor antigen vaccination using dendritic cells: Comparison of RNA electroporation and pulsing with UV-irradiated tumor cells. J. Transl. Med. 2008, 6, 21. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.Z.; Li, H.Y.; Wang, D.; Shao, X.D. Comparison of cytotoxic t lymphocyte responses against pancreatic cancer induced by dendritic cells transfected with total tumor RNA and fusion hybrided with tumor cell. Exp. Biol. Med. 2015, 240, 1310–1318. [Google Scholar] [CrossRef]
- Markov, O.V.; Mironova, N.L.; Sennikov, S.V.; Vlassov, V.V.; Zenkova, M.A. Prophylactic dendritic cell-based vaccines efficiently inhibit metastases in murine metastatic melanoma. PLoS ONE 2015, 10, e0136911. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Hu, T.; Zou, T.; Shi, Q.; Chen, G. Chronic myelocytic leukemia (CML) patient-derived dendritic cells transfected with autologous total RNA induces CML-specific cytotoxicity. Indian J. Hematol. Blood Transfus. Off. J. Indian Soc. Hematol. Blood Transfus. 2016, 32, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, E.; Nair, S.K.; Lyerly, H.K. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol. Immunother. CII 1998, 46, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Van Tendeloo, V.F.; Ponsaerts, P.; Berneman, Z.N. mRNA-based gene transfer as a tool for gene and cell therapy. Curr. Opin. Mol. Ther. 2007, 9, 423–431. [Google Scholar] [PubMed]
- Rabinovich, P.M.; Weissman, S.M. Cell engineering with synthetic messenger RNA. Methods Mol. Biol. 2013, 969, 3–28. [Google Scholar]
- Kuhn, A.N.; Diken, M.; Kreiter, S.; Vallazza, B.; Tureci, O.; Sahin, U. Determinants of intracellular RNA pharmacokinetics: Implications for RNA-based immunotherapeutics. RNA Biol. 2011, 8, 35–43. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Ponsaerts, P.; Lardon, F.; Nijs, G.; Lenjou, M.; Van Broeckhoven, C.; Van Bockstaele, D.R.; Berneman, Z.N. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: Superiority to lipofection and passive pulsing of mrna and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 2001, 98, 49–56. [Google Scholar] [CrossRef]
- Nair, S.K.; Boczkowski, D.; Morse, M.; Cumming, R.I.; Lyerly, H.K.; Gilboa, E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat. Biotechnol. 1998, 16, 364–369. [Google Scholar] [CrossRef]
- Su, Z.; Vieweg, J.; Weizer, A.Z.; Dahm, P.; Yancey, D.; Turaga, V.; Higgins, J.; Boczkowski, D.; Gilboa, E.; Dannull, J. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002, 62, 5041–5048. [Google Scholar]
- Bonehill, A.; Van Nuffel, A.M.; Corthals, J.; Tuyaerts, S.; Heirman, C.; Francois, V.; Colau, D.; van der Bruggen, P.; Neyns, B.; Thielemans, K. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 3366–3375. [Google Scholar] [CrossRef]
- Thornburg, C.; Boczkowski, D.; Gilboa, E.; Nair, S.K. Induction of cytotoxic T lymphocytes with dendritic cells transfected with human papillomavirus e6 and e7 RNA: Implications for cervical cancer immunotherapy. J. Immunother. 2000, 23, 412–418. [Google Scholar] [CrossRef]
- Yu, H.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S. Immunity and protection by adoptive transfer of dendritic cells transfected with hepatitis C NS3/4A mRNA. Vaccine 2007, 25, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S. Strategies for loading dendritic cells with hepatitis C NS5A antigen and inducing protective immunity. J. Viral Hepat. 2008, 15, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Perruccio, K.; Bozza, S.; Montagnoli, C.; Bellocchio, S.; Aversa, F.; Martelli, M.; Bistoni, F.; Velardi, A.; Romani, L. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol. Dis. 2004, 33, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Boczkowski, D.; Fassnacht, M.; Pisetsky, D.; Gilboa, E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007, 67, 371–380. [Google Scholar] [CrossRef]
- Fassnacht, M.; Lee, J.; Milazzo, C.; Boczkowski, D.; Su, Z.; Nair, S.; Gilboa, E. Induction of CD4(+) and CD8(+) T-cell responses to the human stromal antigen, fibroblast activation protein: Implication for cancer immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 5566–5571. [Google Scholar] [CrossRef]
- Nair, S.; Boczkowski, D.; Moeller, B.; Dewhirst, M.; Vieweg, J.; Gilboa, E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood 2003, 102, 964–971. [Google Scholar] [CrossRef]
- Naka, T.; Iwahashi, M.; Nakamura, M.; Ojima, T.; Nakamori, M.; Ueda, K.; Katsuda, M.; Miyazawa, M.; Ishida, K.; Yamaue, H. Tumor vaccine therapy against recrudescent tumor using dendritic cells simultaneously transfected with tumor RNA and granulocyte macrophage colony-stimulating factor RNA. Cancer Sci. 2008, 99, 407–413. [Google Scholar] [CrossRef]
- Akiyama, Y.; Watanabe, M.; Maruyama, K.; Ruscetti, F.W.; Wiltrout, R.H.; Yamaguchi, K. Enhancement of antitumor immunity against B16 melanoma tumor using genetically modified dendritic cells to produce cytokines. Gene Ther. 2000, 7, 2113–2121. [Google Scholar] [CrossRef]
- Miller, P.W.; Sharma, S.; Stolina, M.; Butterfield, L.H.; Luo, J.; Lin, Y.; Dohadwala, M.; Batra, R.K.; Wu, L.; Economou, J.S.; et al. Intratumoral administration of adenoviral interleukin 7 gene-modified dendritic cells augments specific antitumor immunity and achieves tumor eradication. Hum. Gene Ther. 2000, 11, 53–65. [Google Scholar] [CrossRef]
- Nishioka, Y.; Hirao, M.; Robbins, P.D.; Lotze, M.T.; Tahara, H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999, 59, 4035–4041. [Google Scholar]
- Melero, I.; Duarte, M.; Ruiz, J.; Sangro, B.; Galofre, J.; Mazzolini, G.; Bustos, M.; Qian, C.; Prieto, J. Intratumoral injection of bone-marrow derived dendritic cells engineered to produce interleukin-12 induces complete regression of established murine transplantable colon adenocarcinomas. Gene Ther. 1999, 6, 1779–1784. [Google Scholar] [CrossRef]
- Kim, C.H.; Hong, M.J.; Park, S.D.; Kim, C.K.; Park, M.Y.; Sohn, H.J.; Cho, H.I.; Kim, T.G.; Hong, Y.K. Enhancement of anti-tumor immunity specific to murine glioma by vaccination with tumor cell lysate-pulsed dendritic cells engineered to produce interleukin-12. Cancer Immunol. Immunother. CII 2006, 55, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ramakrishnan, R.; Trkulja, M.; Ren, X.; Gabrilovich, D.I. Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines. Cancer Immunol. Immunother. CII 2012, 61, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yuan, X.; Belladonna, M.L.; Ong, J.M.; Wachsmann-Hogiu, S.; Farkas, D.L.; Black, K.L.; Yu, J.S. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006, 66, 8887–8896. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Taylor, J.L.; Bose, A.; Storkus, W.J. Therapeutic effectiveness of intratumorally delivered dendritic cells engineered to express the pro-inflammatory cytokine, interleukin (IL)-32. Cancer Gene Ther. 2011, 18, 663–673. [Google Scholar] [CrossRef]
- Kuwashima, N.; Nishimura, F.; Eguchi, J.; Sato, H.; Hatano, M.; Tsugawa, T.; Sakaida, T.; Dusak, J.E.; Fellows-Mayle, W.K.; Papworth, G.D.; et al. Delivery of dendritic cells engineered to secrete IFN-alpha into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: Dependence on apoptotic pathways. J. Immunol. 2005, 175, 2730–2740. [Google Scholar] [CrossRef]
- Mazzolini, G.; Alfaro, C.; Sangro, B.; Feijoo, E.; Ruiz, J.; Benito, A.; Tirapu, I.; Arina, A.; Sola, J.; Herraiz, M.; et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 999–1010. [Google Scholar] [CrossRef]
- Bontkes, H.J.; Kramer, D.; Ruizendaal, J.J.; Kueter, E.W.; van Tendeloo, V.F.; Meijer, C.J.; Hooijberg, E. Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic t cells. Gene Ther. 2007, 14, 366–375. [Google Scholar] [CrossRef]
- Bontkes, H.J.; Kramer, D.; Ruizendaal, J.J.; Meijer, C.J.; Hooijberg, E. Tumor associated antigen and interleukin-12 mRNA transfected dendritic cells enhance effector function of natural killer cells and antigen specific T-cells. Clin. Immunol. 2008, 127, 375–384. [Google Scholar] [CrossRef]
- Van den Bergh, J.; Willemen, Y.; Lion, E.; Van Acker, H.; De Reu, H.; Anguille, S.; Goossens, H.; Berneman, Z.; Van Tendeloo, V.; Smits, E. Transpresentation of interleukin-15 by IL-15/IL-15ralpha mRNA-engineered human dendritic cells boosts antitumoral natural killer cell activity. Oncotarget 2015, 6, 44123–44133. [Google Scholar]
- Willemen, Y.; Van den Bergh, J.M.; Lion, E.; Anguille, S.; Roelandts, V.A.; Van Acker, H.H.; Heynderickx, S.D.; Stein, B.M.; Peeters, M.; Figdor, C.G.; et al. Engineering monocyte-derived dendritic cells to secrete interferon-alpha enhances their ability to promote adaptive and innate anti-tumor immune effector functions. Cancer Immunol. Immunother. CII 2015, 64, 831–842. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, B.; Heirman, C.; Corthals, J.; Empsen, C.; van Grunsven, L.A.; Allard, S.D.; Pen, J.; Lacor, P.; Thielemans, K.; Aerts, J.L. The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J. Leukoc. Biol. 2011, 89, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Dannull, J.; Nair, S.; Su, Z.; Boczkowski, D.; DeBeck, C.; Yang, B.; Gilboa, E.; Vieweg, J. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood 2005, 105, 3206–3213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorrie, J.; Schaft, N.; Muller, I.; Wellner, V.; Schunder, T.; Hanig, J.; Oostingh, G.J.; Schon, M.P.; Robert, C.; Kampgen, E.; et al. Introduction of functional chimeric E/L-selectin by RNA electroporation to target dendritic cells from blood to lymph nodes. Cancer Immunol. Immunother. CII 2008, 57, 467–477. [Google Scholar] [CrossRef]
- Bonehill, A.; Tuyaerts, S.; Van Nuffel, A.M.; Heirman, C.; Bos, T.J.; Fostier, K.; Neyns, B.; Thielemans, K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 1170–1180. [Google Scholar] [CrossRef]
- Peng, S.; Kim, T.W.; Lee, J.H.; Yang, M.; He, L.; Hung, C.F.; Wu, T.C. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum. Gene Ther. 2005, 16, 584–593. [Google Scholar] [CrossRef]
- Kang, T.H.; Lee, J.H.; Noh, K.H.; Han, H.D.; Shin, B.C.; Choi, E.Y.; Peng, S.; Hung, C.F.; Wu, T.C.; Kim, T.W. Enhancing dendritic cell vaccine potency by combining a BAK/BAX siRNA-mediated antiapoptotic strategy to prolong dendritic cell life with an intracellular strategy to target antigen to lysosomal compartments. Int. J. Cancer 2007, 120, 1696–1703. [Google Scholar] [CrossRef]
- Sun, H.W.; Chen, X.; Wu, H.X.; Wu, C. Dendritic cells with CED-3 and CED-4 siRNA is effective in prolonging DC lifetime and suppresses tumor growth in gastric cancer. Hepato-Gastroenterology 2013, 60, 372–376. [Google Scholar] [CrossRef]
- Hobo, W.; Novobrantseva, T.I.; Fredrix, H.; Wong, J.; Milstein, S.; Epstein-Barash, H.; Liu, J.; Schaap, N.; van der Voort, R.; Dolstra, H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol. Immunother. CII 2013, 62, 285–297. [Google Scholar] [CrossRef]
- Van den Bergh, J.M.J.; Smits, E.; Berneman, Z.N.; Hutten, T.J.A.; De Reu, H.; Van Tendeloo, V.F.I.; Dolstra, H.; Lion, E.; Hobo, W. Monocyte-derived dendritic cells with silenced PD-1 ligands and transpresenting interleukin-15 stimulate strong tumor-reactive T-cell expansion. Cancer Immunol. Res. 2017, 5, 710–715. [Google Scholar] [CrossRef]
- Gottschalk, S.; Yu, F.; Ji, M.; Kakarla, S.; Song, X.T. A vaccine that co-targets tumor cells and cancer associated fibroblasts results in enhanced antitumor activity by inducing antigen spreading. PLoS ONE 2013, 8, e82658. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.O.; Semaeva, E.; Sorensen, D.R.; Wiig, H.; Sioud, M. Dendritic cells loaded with tumor antigens and a dual immunostimulatory and anti-interleukin 10-specific small interference RNA prime T lymphocytes against leukemic cells. Transl. Oncol. 2009, 2, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Guo, C.; Yu, X.; Gao, P.; Qian, J.; Zuo, D.; Manjili, M.H.; Fisher, P.B.; Subjeck, J.R.; Wang, X.Y. Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T-cell response and antitumor immunity. Cancer Res. 2011, 71, 6611–6620. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Saeboe-Larssen, S.; Hetland, T.E.; Kaern, J.; Mobergslien, A.; Kvalheim, G. Silencing of indoleamine 2,3-dioxygenase enhances dendritic cell immunogenicity and antitumour immunity in cancer patients. Int. J. Oncol. 2013, 43, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Song, X.T.; Evel-Kabler, K.; Rollins, L.; Aldrich, M.; Gao, F.; Huang, X.F.; Chen, S.Y. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006, 3, e11. [Google Scholar] [CrossRef]
- Guo, C.; Yi, H.; Yu, X.; Zuo, D.; Qian, J.; Yang, G.; Foster, B.A.; Subjeck, J.R.; Sun, X.; Mikkelsen, R.B.; et al. In situ vaccination with CD204 gene-silenced dendritic cell, not unmodified dendritic cell, enhances radiation therapy of prostate cancer. Mol. Cancer Ther. 2012, 11, 2331–2341. [Google Scholar] [CrossRef]
- Hontelez, S.; Ansems, M.; Karthaus, N.; Zuidscherwoude, M.; Looman, M.W.; Triantis, V.; Adema, G.J. Dendritic cell-specific transcript: Dendritic cell marker and regulator of TLR-induced cytokine production. J. Immunol. 2012, 189, 138–145. [Google Scholar] [CrossRef]
- de Rosa, F.; Fanini, F.; Guidoboni, M.; Vannini, I.; Amadori, D.; Ridolfi, R.; Ridolfi, L.; Fabbri, M. Micrornas and dendritic cell-based vaccination in melanoma patients. Melanoma Res. 2014, 24, 181–189. [Google Scholar] [CrossRef]
- Boczkowski, D.; Nair, S.K.; Snyder, D.; Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996, 184, 465–472. [Google Scholar] [CrossRef]
- Arthur, J.F.; Butterfield, L.H.; Roth, M.D.; Bui, L.A.; Kiertscher, S.M.; Lau, R.; Dubinett, S.; Glaspy, J.; McBride, W.H.; Economou, J.S. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997, 4, 17–25. [Google Scholar]
- Ponsaerts, P.; Van Tendeloo, V.F.; Berneman, Z.N. Cancer immunotherapy using RNA-loaded dendritic cells. Clin. Exp. Immunol. 2003, 134, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Walch, B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Delivery of functional DNA and messenger RNA to mammalian phagocytic cells by recombinant yeast. Gene Ther. 2012, 19, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Breinig, F.; Breinig, T.; Schmitt, M.J. mRNA delivery to human dendritic cells by recombinant yeast and activation of antigen-specific memory T cells. Methods Mol. Biol. 2013, 969, 163–184. [Google Scholar] [PubMed]
- Lundqvist, A.; Noffz, G.; Pavlenko, M.; Saeboe-Larssen, S.; Fong, T.; Maitland, N.; Pisa, P. Nonviral and viral gene transfer into different subsets of human dendritic cells yield comparable efficiency of transfection. J. Immunother. 2002, 25, 445–454. [Google Scholar] [CrossRef]
- Dullaers, M.; Breckpot, K.; Van Meirvenne, S.; Bonehill, A.; Tuyaerts, S.; Michiels, A.; Straetman, L.; Heirman, C.; De Greef, C.; Van Der Bruggen, P.; et al. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: Implications for cancer immunotherapy protocols. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 768–779. [Google Scholar] [CrossRef]
- Cheng, C.; Convertine, A.J.; Stayton, P.S.; Bryers, J.D. Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials 2012, 33, 6868–6876. [Google Scholar] [CrossRef]
- Schaft, N.; Wellner, V.; Wohn, C.; Schuler, G.; Dorrie, J. CD8(+) T-cell priming and boosting: More antigen-presenting DC, or more antigen per DC? Cancer Immunol. Immunother. CII 2013, 62, 1769–1780. [Google Scholar] [CrossRef]
- De Temmerman, M.L.; Dewitte, H.; Vandenbroucke, R.E.; Lucas, B.; Libert, C.; Demeester, J.; De Smedt, S.C.; Lentacker, I.; Rejman, J. mRNA-lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials 2011, 32, 9128–9135. [Google Scholar] [CrossRef]
- Dewitte, H.; Van Lint, S.; Heirman, C.; Thielemans, K.; De Smedt, S.C.; Breckpot, K.; Lentacker, I. The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J. Control. Release 2014, 194, 28–36. [Google Scholar] [CrossRef]
- Sever, A.R.; Mills, P.; Jones, S.E.; Cox, K.; Weeks, J.; Fish, D.; Jones, P.A. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. Am. J. Roentgenol. 2011, 196, 251–256. [Google Scholar] [CrossRef]
- Lim, S.; Koo, J.H.; Choi, J.M. Use of cell-penetrating peptides in dendritic cell-based vaccination. Immune Netw. 2016, 16, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Andaloussi, S.E.; Lehto, T.; Mager, I.; Rosenthal-Aizman, K.; Oprea, I.I.; Simonson, O.E.; Sork, H.; Ezzat, K.; Copolovici, D.M.; Kurrikoff, K.; et al. Design of a peptide-based vector, pepfect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic Acids Res. 2011, 39, 3972–3987. [Google Scholar] [CrossRef] [PubMed]
- De Vries, I.J.; Krooshoop, D.J.; Scharenborg, N.M.; Lesterhuis, W.J.; Diepstra, J.H.; Van Muijen, G.N.; Strijk, S.P.; Ruers, T.J.; Boerman, O.C.; Oyen, W.J.; et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003, 63, 12–17. [Google Scholar] [PubMed]
- Wilgenhof, S.; Corthals, J.; Van Nuffel, A.M.; Benteyn, D.; Heirman, C.; Bonehill, A.; Thielemans, K.; Neyns, B. Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol. Immunother. 2015, 64, 381–388. [Google Scholar] [CrossRef]
- Kyte, J.A.; Aamdal, S.; Dueland, S.; Saeboe-Larsen, S.; Inderberg, E.M.; Madsbu, U.E.; Skovlund, E.; Gaudernack, G.; Kvalheim, G. Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells. Oncoimmunology 2016, 5, e1232237. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef]
- Khoury, H.J.; Collins, R.H., Jr.; Blum, W.; Stiff, P.S.; Elias, L.; Lebkowski, J.S.; Reddy, A.; Nishimoto, K.P.; Sen, D.; Wirth, E.D., 3rd; et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer 2017, 123, 3061–3072. [Google Scholar] [CrossRef]
- Wilgenhof, S.; Corthals, J.; Heirman, C.; van Baren, N.; Lucas, S.; Kvistborg, P.; Thielemans, K.; Neyns, B. Phase ii study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 2016, 34, 1330–1338. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Sayour, E.J.; Reap, E.; Schmittling, R.; DeLeon, G.; Norberg, P.; Desjardins, A.; Friedman, A.H.; Friedman, H.S.; Archer, G.; et al. Severe adverse immunologic reaction in a patient with glioblastoma receiving autologous dendritic cell vaccines combined with GM-CSF and dose-intensified temozolomide. Cancer Immunol. Res. 2015, 3, 320–325. [Google Scholar] [CrossRef]
- Borch, T.H.; Engell-Noerregaard, L.; Zeeberg Iversen, T.; Ellebaek, E.; Met, O.; Hansen, M.; Andersen, M.H.; Thor Straten, P.; Svane, I.M. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. Oncoimmunology 2016, 5, e1207842. [Google Scholar] [CrossRef]
- Kongsted, P.; Borch, T.H.; Ellebaek, E.; Iversen, T.Z.; Andersen, R.; Met, O.; Hansen, M.; Lindberg, H.; Sengelov, L.; Svane, I.M. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy 2017, 19, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.A.; Orme, L.M.; Neale, A.M.; Radcliff, F.J.; Amor, G.M.; Maixner, W.; Downie, P.; Hassall, T.E.; Tang, M.L.; Ashley, D.M. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro-Oncology 2004, 6, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.A.; Orme, L.M.; Amor, G.M.; Neale, A.M.; Radcliff, F.J.; Downie, P.; Tang, M.L.; Ashley, D.M. Results of a phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with stage 4 neuroblastoma. Cancer 2005, 103, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.N.; Dietz, A.B.; Greiner, C.W.; Maas, M.L.; Butler, G.W.; Padley, D.J.; Bulur, P.A.; Allred, J.B.; Creagan, E.T.; Ingle, J.N.; et al. Preparing clinical-grade myeloid dendritic cells by electroporation-mediated transfection of in vitro amplified tumor-derived mRNA and safety testing in stage IV malignant melanoma. J. Transl. Med. 2006, 4, 35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lesterhuis, W.J.; De Vries, I.J.; Schreibelt, G.; Schuurhuis, D.H.; Aarntzen, E.H.; De Boer, A.; Scharenborg, N.M.; Van De Rakt, M.; Hesselink, E.J.; Figdor, C.G.; et al. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010, 30, 5091–5097. [Google Scholar]
- Gandhi, R.T.; Kwon, D.S.; Macklin, E.A.; Shopis, J.R.; McLean, A.P.; McBrine, N.; Flynn, T.; Peter, L.; Sbrolla, A.; Kaufmann, D.E.; et al. Immunization of HIV-1-infected persons with autologous dendritic cells transfected with mRNA encoding HIV-1 Gag and Nef: Results of a randomized, placebo-controlled clinical trial. J. Acquir. Immune Defic. Syndr. 2016, 71, 246–253. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Cannon, M.J.; Block, M.S.; Morehead, L.C.; Knutson, K.L. The evolving clinical landscape for dendritic cell vaccines and cancer immunotherapy. Immunotherapy 2019, 11, 75–79. [Google Scholar] [CrossRef]
- Bassani-Sternberg, M.; Digklia, A.; Huber, F.; Wagner, D.; Sempoux, C.; Stevenson, B.J.; Thierry, A.C.; Michaux, J.; Pak, H.; Racle, J.; et al. A phase IB study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma-a proof of antigen discovery feasibility in three patients. Front. Immunol. 2019, 10, 1832. [Google Scholar]
- Aarntzen, E.H.; Schreibelt, G.; Bol, K.; Lesterhuis, W.J.; Croockewit, A.J.; de Wilt, J.H.; van Rossum, M.M.; Blokx, W.A.; Jacobs, J.F.; Duiveman-de Boer, T.; et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin. Cancer Res. 2012, 18, 5460–5470. [Google Scholar] [CrossRef]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Dannull, J.; Heiser, A.; Yancey, D.; Pruitt, S.; Madden, J.; Coleman, D.; Niedzwiecki, D.; Gilboa, E.; Vieweg, J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003, 63, 2127–2133. [Google Scholar] [PubMed]
- Su, Z.; Dannull, J.; Yang, B.K.; Dahm, P.; Coleman, D.; Yancey, D.; Sichi, S.; Niedzwiecki, D.; Boczkowski, D.; Gilboa, E.; et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J. Immunol. 2005, 174, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- Dorfel, D.; Appel, S.; Grunebach, F.; Weck, M.M.; Muller, M.R.; Heine, A.; Brossart, P. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood 2005, 105, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.A.; Mu, L.; Aamdal, S.; Kvalheim, G.; Dueland, S.; Hauser, M.; Gullestad, H.P.; Ryder, T.; Lislerud, K.; Hammerstad, H.; et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006, 13, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Wilgenhof, S.; Van Nuffel, A.M.; Benteyn, D.; Corthals, J.; Aerts, C.; Heirman, C.; Van Riet, I.; Bonehill, A.; Thielemans, K.; Neyns, B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann. Oncol. 2013, 24, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.A.; Kvalheim, G.; Lislerud, K.; thor Straten, P.; Dueland, S.; Aamdal, S.; Gaudernack, G. T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol. Immunother. 2007, 56, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P.; Boulassel, M.R.; Yassine-Diab, B.; Nicolette, C.; Healey, D.; Jain, R.; Landry, C.; Yegorov, O.; Tcherepanova, I.; Monesmith, T.; et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin. Immunol. 2010, 134, 140–147. [Google Scholar] [CrossRef]
- Van Gulck, E.; Vlieghe, E.; Vekemans, M.; Van Tendeloo, V.F.; Van De Velde, A.; Smits, E.; Anguille, S.; Cools, N.; Goossens, H.; Mertens, L.; et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS 2012, 26, F1–F12. [Google Scholar] [CrossRef]
- Allard, S.D.; De Keersmaecker, B.; de Goede, A.L.; Verschuren, E.J.; Koetsveld, J.; Reedijk, M.L.; Wylock, C.; De Bel, A.V.; Vandeloo, J.; Pistoor, F.; et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin. Immunol. 2012, 142, 252–268. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Routy, J.P.; Welles, S.; DeBenedette, M.; Tcherepanova, I.; Angel, J.B.; Asmuth, D.M.; Stein, D.K.; Baril, J.G.; McKellar, M.; et al. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: A randomized, double-blind, placebo-controlled clinical trial. J. Acquir. Immune Defic. Syndr. 2016, 72, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.L.; DeBenedette, M.A.; Tcherepanova, I.Y.; Gamble, A.; Lewis, W.E.; Cope, A.B.; Kuruc, J.D.; McGee, K.S.; Kearney, M.F.; Coffin, J.M.; et al. Immunogenicity of ags-004 dendritic cell therapy in patients treated during acute HIV infection. AIDS Res. Hum. Retrovir. 2018, 34, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Dannull, J.; Haley, N.R.; Archer, G.; Nair, S.; Boczkowski, D.; Harper, M.; De Rosa, N.; Pickett, N.; Mosca, P.J.; Burchette, J.; et al. Melanoma immunotherapy using mature DCs expressing the constitutive proteasome. J. Clin. Investig. 2013, 123, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E., 2nd; Healy, P.; et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef]
- Reap, E.A.; Suryadevara, C.M.; Batich, K.A.; Sanchez-Perez, L.; Archer, G.E.; Schmittling, R.J.; Norberg, P.K.; Herndon, J.E., 2nd; Healy, P.; Congdon, K.L.; et al. Dendritic cells enhance polyfunctionality of adoptively transferred t cells that target cytomegalovirus in glioblastoma. Cancer Res. 2018, 78, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Yoshimura, K.; Matsui, H.; Shindo, Y.; Tamesa, T.; Tokumitsu, Y.; Hashimoto, N.; Tokuhisa, Y.; Sakamoto, K.; Sakai, K.; et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis c virus-related hepatocellular carcinoma: A phase 1 dose escalation clinical trial. Cancer Immunol. Immunother. 2015, 64, 1047–1056. [Google Scholar] [CrossRef]
- Shindo, Y.; Hazama, S.; Maeda, Y.; Matsui, H.; Iida, M.; Suzuki, N.; Yoshimura, K.; Ueno, T.; Yoshino, S.; Sakai, K.; et al. Adoptive immunotherapy with MUC1-mRNA transfected dendritic cells and cytotoxic lymphocytes plus gemcitabine for unresectable pancreatic cancer. J. Transl. Med. 2014, 12, 175. [Google Scholar] [CrossRef]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef]
- Morse, M.A.; Nair, S.K.; Boczkowski, D.; Tyler, D.; Hurwitz, H.I.; Proia, A.; Clay, T.M.; Schlom, J.; Gilboa, E.; Lyerly, H.K. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int. J. Gastrointest. Cancer 2002, 32, 1–6. [Google Scholar] [CrossRef]
- Amin, A.; Dudek, A.Z.; Logan, T.F.; Lance, R.S.; Holzbeierlein, J.M.; Knox, J.J.; Master, V.A.; Pal, S.K.; Miller, W.H., Jr.; Karsh, L.I.; et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J. Immunother. Cancer 2015, 3, 14. [Google Scholar] [CrossRef]
- Coosemans, A.; Vanderstraeten, A.; Tuyaerts, S.; Verschuere, T.; Moerman, P.; Berneman, Z.N.; Vergote, I.; Amant, F.; SW, V.A.N.G. Wilms’ tumor gene 1 (wt1)—Loaded dendritic cell immunotherapy in patients with uterine tumors: A phase I/II clinical trial. Anticancer Res. 2013, 33, 5495–5500. [Google Scholar] [PubMed]
- Hobo, W.; Strobbe, L.; Maas, F.; Fredrix, H.; Greupink-Draaisma, A.; Esendam, B.; de Witte, T.; Preijers, F.; Levenga, H.; van Rees, B.; et al. Immunogenicity of dendritic cells pulsed with MAGE3, survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer Immunol. Immunother. 2013, 62, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Suso, E.M.; Dueland, S.; Rasmussen, A.M.; Vetrhus, T.; Aamdal, S.; Kvalheim, G.; Gaudernack, G. Htert mRNA dendritic cell vaccination: Complete response in a pancreatic cancer patient associated with response against several htert epitopes. Cancer Immunol. Immunother. 2011, 60, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Rains, N.; Cannan, R.J.; Chen, W.; Stubbs, R.S. Development of a dendritic cell (dc)-based vaccine for patients with advanced colorectal cancer. Hepato-Gastroenterology 2001, 48, 347–351. [Google Scholar]
- Sioud, M.; Nyakas, M.; Saeboe-Larssen, S.; Mobergslien, A.; Aamdal, S.; Kvalheim, G. Diversification of antitumour immunity in a patient with metastatic melanoma treated with ipilimumab and an IDO-silenced dendritic cell vaccine. Case Rep. Med. 2016, 2016, 9639585. [Google Scholar] [CrossRef]
- Coosemans, A.; Vanderstraeten, A.; Tuyaerts, S.; Verschuere, T.; Moerman, P.; Berneman, Z.; Vergote, I.; Amant, F.; Van Gool, S.W. Immunological response after WT1 mRNA-loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Res. 2013, 33, 3855–3859. [Google Scholar]
- Anguille, S.; Smits, E.L.; Bryant, C.; Van Acker, H.H.; Goossens, H.; Lion, E.; Fromm, P.D.; Hart, D.N.; Van Tendeloo, V.F.; Berneman, Z.N. Dendritic cells as pharmacological tools for cancer immunotherapy. Pharmacol. Rev. 2015, 67, 731–753. [Google Scholar] [CrossRef]
- Dannull, J.; Su, Z.; Rizzieri, D.; Yang, B.K.; Coleman, D.; Yancey, D.; Zhang, A.; Dahm, P.; Chao, N.; Gilboa, E.; et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Investig. 2005, 115, 3623–3633. [Google Scholar] [CrossRef]
- Mastelic-Gavillet, B.; Balint, K.; Boudousquie, C.; Gannon, P.O.; Kandalaft, L.E. Personalized dendritic cell vaccines-recent breakthroughs and encouraging clinical results. Front. Immunol. 2019, 10, 766. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Batich, K.A.; Gunn, M.D.; Huang, M.N.; Sanchez-Perez, L.; Nair, S.K.; Congdon, K.L.; Reap, E.A.; Archer, G.E.; Desjardins, A.; et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015, 519, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Zheng, X.; Zhang, X.; Li, M.; Vladau, C.; Ichim, T.E.; Sun, H.; Min, L.R.; Garcia, B.; Min, W.P. Novel vaccination for allergy through gene silencing of CD40 using small interfering RNA. J. Immunol. 2008, 180, 8461–8469. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, S.; Goyvaerts, C.; Maenhout, S.; Goethals, L.; Disy, A.; Benteyn, D.; Pen, J.; Bonehill, A.; Heirman, C.; Breckpot, K.; et al. Preclinical evaluation of trimix and antigen mrna-based antitumor therapy. Cancer Res. 2012, 72, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, S.; Heirman, C.; Thielemans, K.; Breckpot, K. Mrna: From a chemical blueprint for protein production to an off-the-shelf therapeutic. Hum. Vaccines Immunother. 2013, 9, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Lower, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrors, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Singh, A.; Qin, H.; Fernandez, I.; Wei, J.; Lin, J.; Kwak, L.W.; Roy, K. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release 2011, 155, 184–192. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Persano, S.; Guevara, M.L.; Li, Z.; Mai, J.; Ferrari, M.; Pompa, P.P.; Shen, H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 2017, 125, 81–89. [Google Scholar] [CrossRef]
- Birkholz, K.; Schwenkert, M.; Kellner, C.; Gross, S.; Fey, G.; Schuler-Thurner, B.; Schuler, G.; Schaft, N.; Dorrie, J. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood 2010, 116, 2277–2285. [Google Scholar] [CrossRef]
- Cauwels, A.; Van Lint, S.; Paul, F.; Garcin, G.; De Koker, S.; Van Parys, A.; Wueest, T.; Gerlo, S.; Van der Heyden, J.; Bordat, Y.; et al. Delivering type I interferon to dendritic cells empowers tumor eradication and immune combination treatments. Cancer Res. 2018, 78, 463–474. [Google Scholar] [CrossRef]

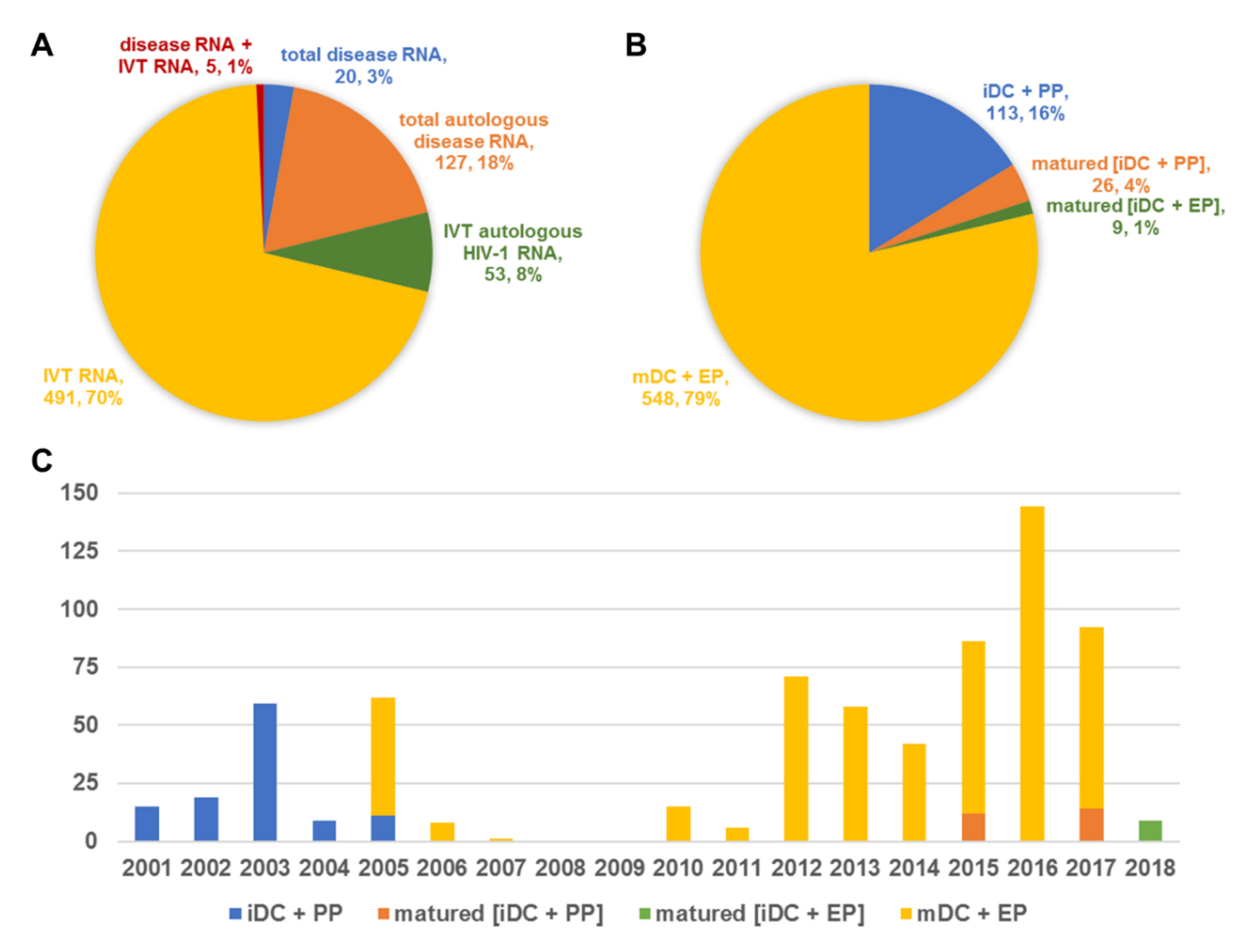
| Category | Product/Target | n Vaccinated Subjects |
|---|---|---|
| Messenger RNA | ||
| Disease-associated antigens | ||
| Melanoma-associated | tyrosinase | 144 |
| gp100 | 144 | |
| Mage-A3 | 108 | |
| Mage-C2 | 84 | |
| MelanA/Mart-1 | 42 | |
| Mage-A1 | 30 | |
| Other TAAs | hTERT | 99 |
| survivin | 69 | |
| CEA | 52 | |
| Muc1 | 42 | |
| WT1 | 38 | |
| PSA | 37 | |
| p53 | 26 | |
| PAP | 21 | |
| Hsp70 | 12 | |
| FR-α | 1 | |
| HIV-1 | Nef | 86 |
| Rev | 76 | |
| Gag | 69 | |
| Vpr | 53 | |
| Tat | 23 | |
| CMV | pp65 | 42 |
| Co-stimulatory molecules | ||
| CD70 | 68 | |
| CD40L | 68 | |
| Danger signals | ||
| cTLR4 | 68 | |
| Small interfering RNA | ||
| Three inducible iP subunits | 5 | |
| IDO | 4 | |
| Trial Author [Ref] | Disease | CR | PR | SD | MR | PD | Total | Other Treatment | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n | ||||
| Khoury [77] | AML in CR + high relapse risk | 11 (52) | - | - | - | 10 (48) | 21 | - | Sustained CRs |
| Anguille [91] | AML in CR + high relapse risk | 6 (21) | - | - | - | 22 (79) | 28 | - | Four sustained CRs |
| Wilgenhof [78] | Stage III/IV melanoma | 8 (21) | 7 (18) | 6 (15) | - | 18 (46) | 39 | Ipilimumab | - |
| Maeda [106] | HCC | 1 (8) | 1 (8) | 5 (42) | - | 5 (42) | 12 | Resection/ablation | - |
| Shindo [107] | Pancreatic cancer | 1 (2) | 3 (7) | 22 (52) | - | 16 (38) | 42 | Gemcitabine | - |
| Dannull [103] | Stage IV melanoma | 6 (50) | 1 (8) | - | - | 5 (42) | 12 | - | Five sustained CRs |
| Wilgenhof [96] | Stage III/IV melanoma | 2 (13) | 2 (13) | 4 (27) | - | 7 (47 | 15 | - | - |
| Morse [108] | CEA+ metastatic cancer MRD CEA+ metastatic cancer | 3 (25) - | - - | - 6 (25) | - - | 9 (75) 18 (75) | 12 24 | - - | Sustained CRs - |
| Morse [109] | Pancreatic cancer | 3 (100) | - | - | - | - | 3 | 5-FU + RT, resection | Sustained CRs |
| Wilgenhof [74] | Stage III/IV melanoma | 10 (33) | - | - | - | 20 (67) | 30 | Resection +/− IFN | Sustained CRs |
| Aarntzen [90] | Stage III melanoma Stage IV melanoma | 11 (42) - | - 1 (5) | - 5 (23) | - 1 (5) | 15 (58) 15 (68) | 26 22 | - - | Sustained CRs - |
| Kongsted [81] | Progressive metastatic CRPC | - | 1 (25) | 2 (50) | - | 1 (25) | 4 | Docetaxel | - |
| Kyte [75] | Stage IV melanoma | - | 1 (3) | 3 (10) | - | 25 (86) | 29 | +/− IL-2 | - |
| Amin [110] | mRCC | - | 9 (43) | 4 (19) | - | 8 (38) | 21 | Resection, sunitinib | - |
| Caruso [82] | Relapsed CNS tumors | - | 1 (14) | 4 (57) | - | 2 (29) | 7 | - | - |
| Borch [80] | Stage IV melanoma | - | - | 9 (41) | - | 13 (59) | 22 | CTX | - |
| Coosemans [111] | Uterine cancer | - | - | 1 (17) | 1 (17) | 4 (67) | 6 | - | - |
| Hobo [112] | Stage II/III myeloma | - | - | 5 (50) | - | 5 (50) | 10 | - | - |
| Suso [113] | Pancreatic cancer | - | - | 1 (100) | - | - | 1 | - | - |
| Markovic [84] | Stage IV melanoma | - | - | 1 (17) | - | 5 (83) | 6 | - | - |
| Mu [94] | Metastatic CRPC | - | - | 11 (58) | - | 8 (42) | 19 | - | SD based on PSA |
| Caruso [83] | Stage IV PNB | - | - | 1 (9) | - | 10 (91) | 11 | Resection, CRT | - |
| Rains [114] | Metastatic CRC | - | - | 6 (50) | - | 6 (50) | 12 | - | - |
| Sioud [115] | Stage IV melanoma | - | - | - | 1 (100) | - | 1 | Prior ipilimumab, RT | - |
| Coosemans [116] | Ovarian cancer | - | - | - | - | 2 (100) | 2 | - | - |
| Total | 62 (14) | 27 (6) | 96 (22) | 3 (0.7) | 249 (57) | 437 | |||
| CR + PR | 89 (20) | ||||||||
| CR + PR + SD | 185 (42) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willemen, Y.; Versteven, M.; Peeters, M.; Berneman, Z.N.; Smits, E.L.J. Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready ‘N Able to Improve Clinical Outcome? Cancers 2020, 12, 299. https://doi.org/10.3390/cancers12020299
Willemen Y, Versteven M, Peeters M, Berneman ZN, Smits ELJ. Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready ‘N Able to Improve Clinical Outcome? Cancers. 2020; 12(2):299. https://doi.org/10.3390/cancers12020299
Chicago/Turabian StyleWillemen, Yannick, Maarten Versteven, Marc Peeters, Zwi N. Berneman, and Evelien L. J. Smits. 2020. "Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready ‘N Able to Improve Clinical Outcome?" Cancers 12, no. 2: 299. https://doi.org/10.3390/cancers12020299
APA StyleWillemen, Y., Versteven, M., Peeters, M., Berneman, Z. N., & Smits, E. L. J. (2020). Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready ‘N Able to Improve Clinical Outcome? Cancers, 12(2), 299. https://doi.org/10.3390/cancers12020299





