Early Changes in Circulating FGF19 and Ang-2 Levels as Possible Predictive Biomarkers of Clinical Response to Lenvatinib Therapy in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Treatment Response
2.2. Association between CAF Levels at Baseline and Treatment Effects
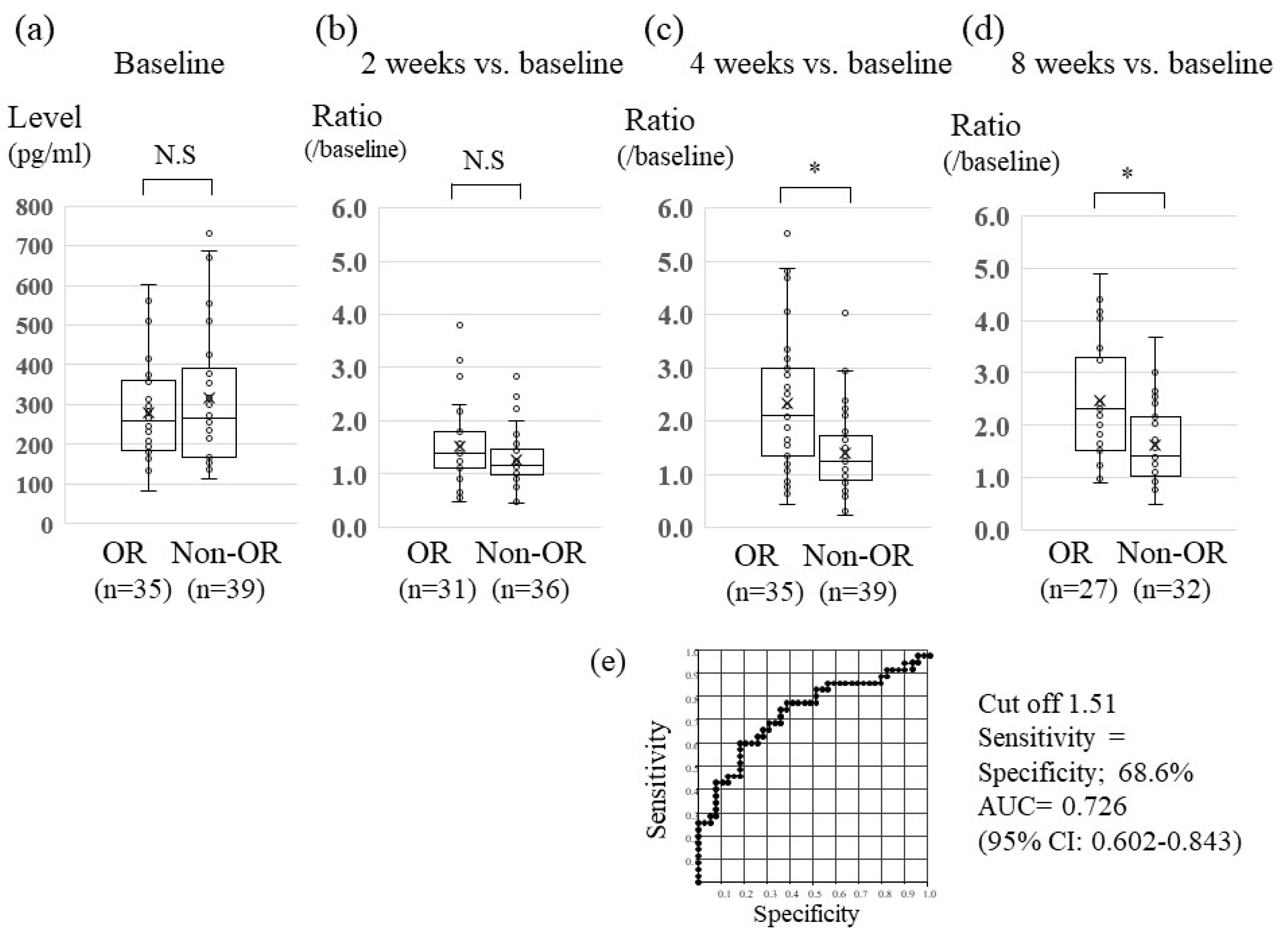
2.3. Association between Serum Changes in FGF19 Levels and Lenvatinib Treatment Effect
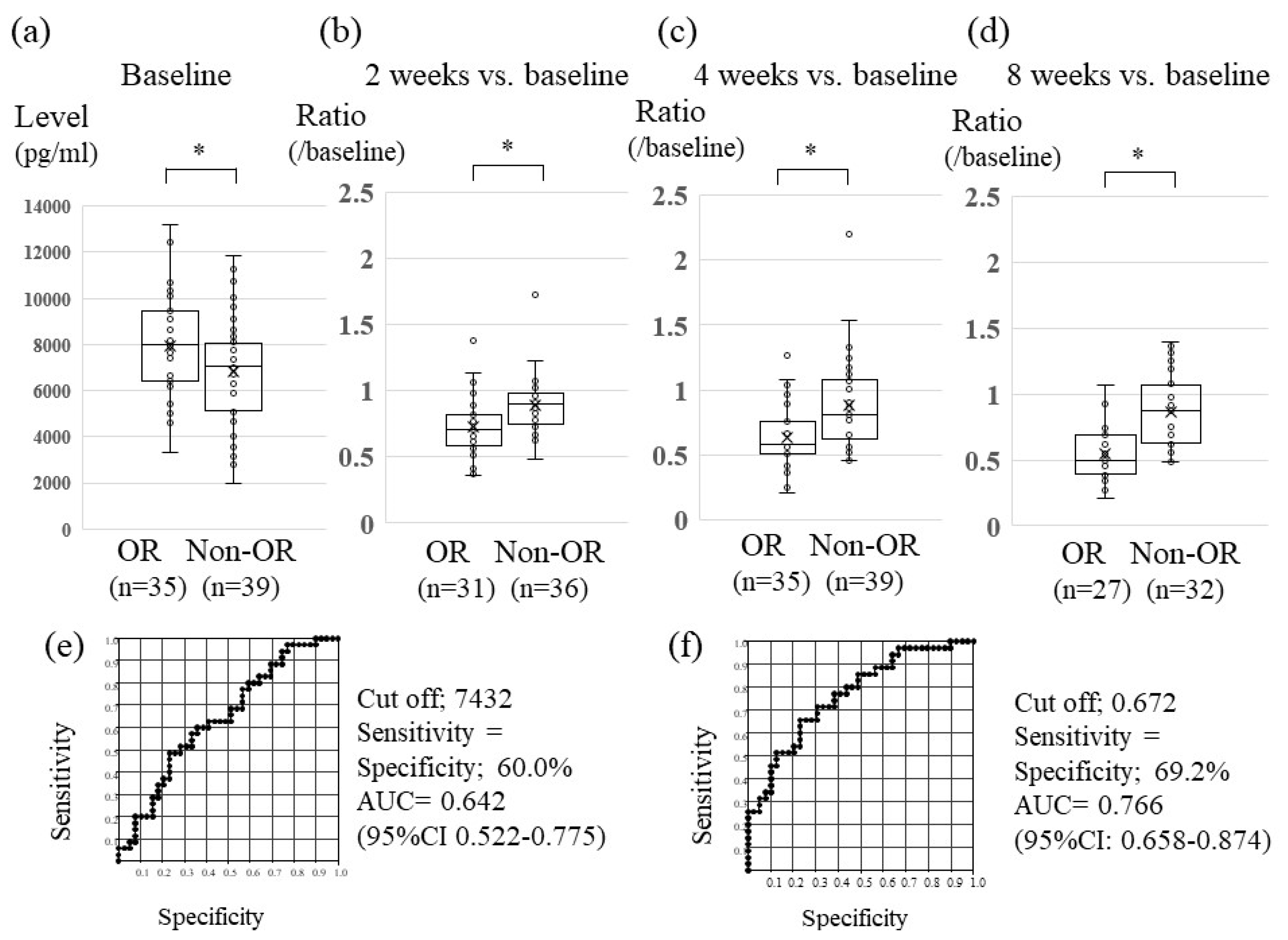
2.4. Association between Serum Changes in Ang-2 Levels and Lenvatinib Treatment Effect
2.5. Association between Serum Changes in FGF23 and VEGF Levels and Treatment Effects
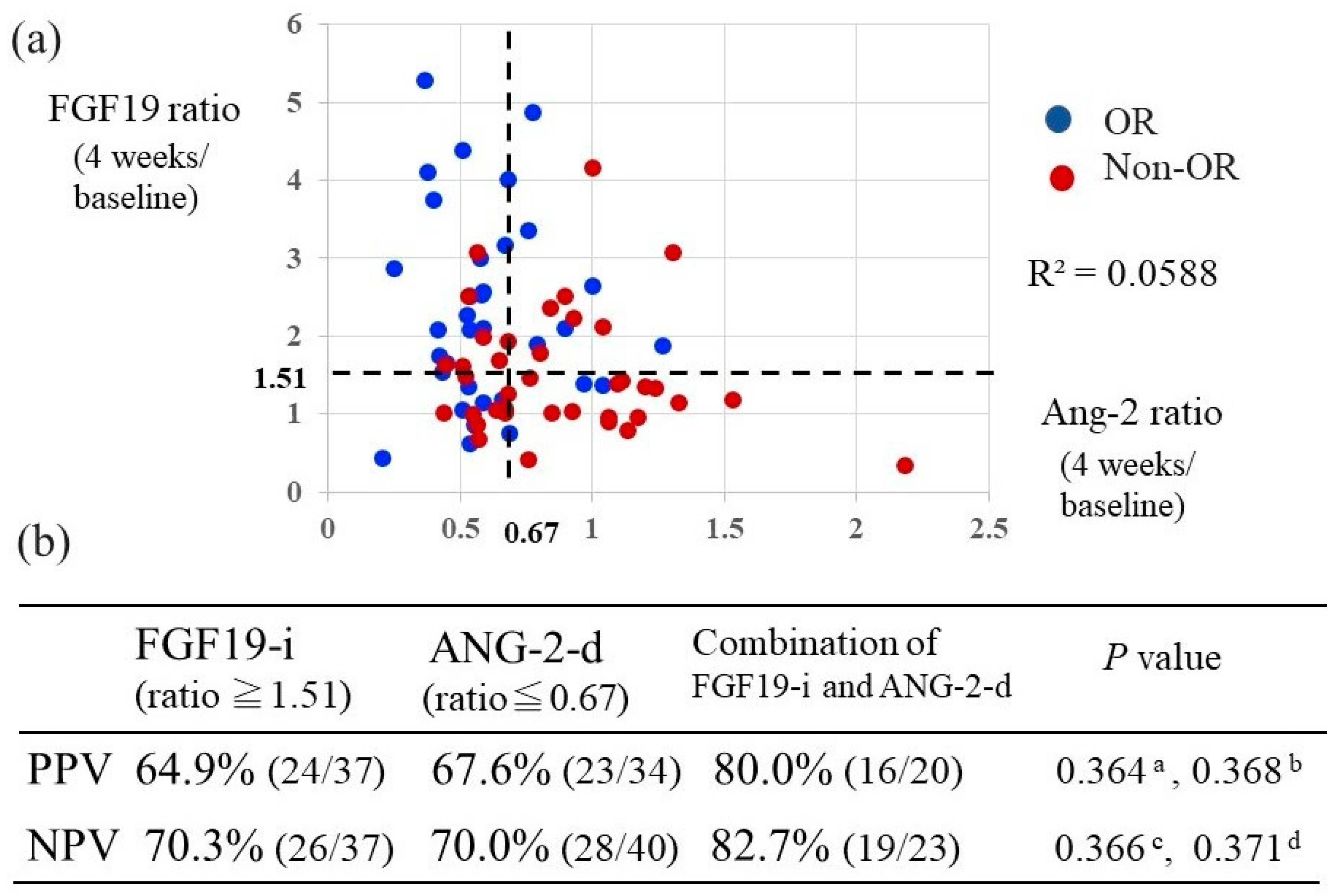
2.6. Relationship between the Combination of Changes in FGF19 and Ang-2 Levels Versus Baseline and Their Predictive Value of Response to Lenvatinib Treatment
2.7. Factors Associated with Lenvatinib Response
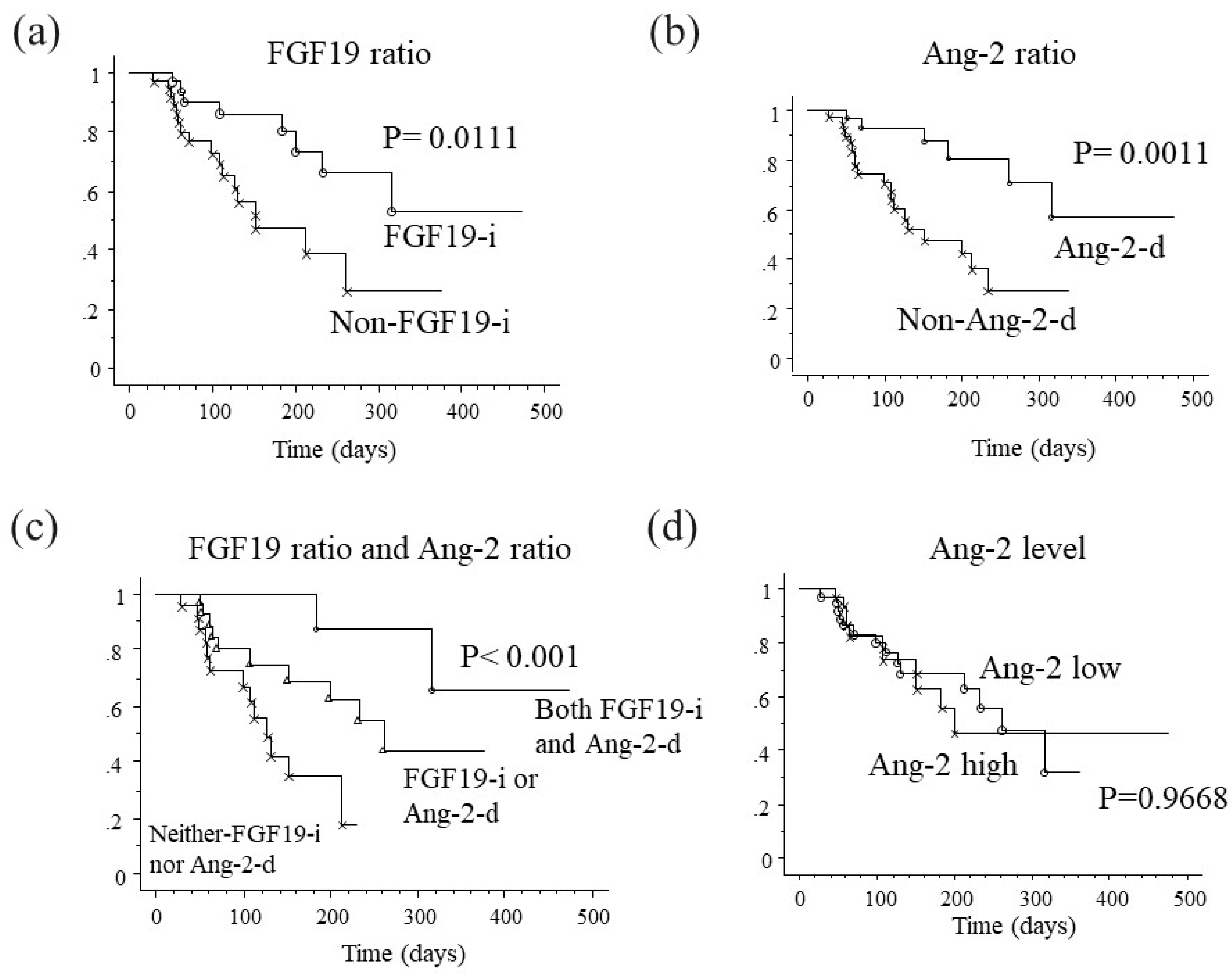
2.8. Association between Changes in FGF19 and Ang-2 Levels and Progression-Free Survival
2.9. Analysis of Progression-Free Survival Factors among Patients Receiving Lenvatinib Treatment
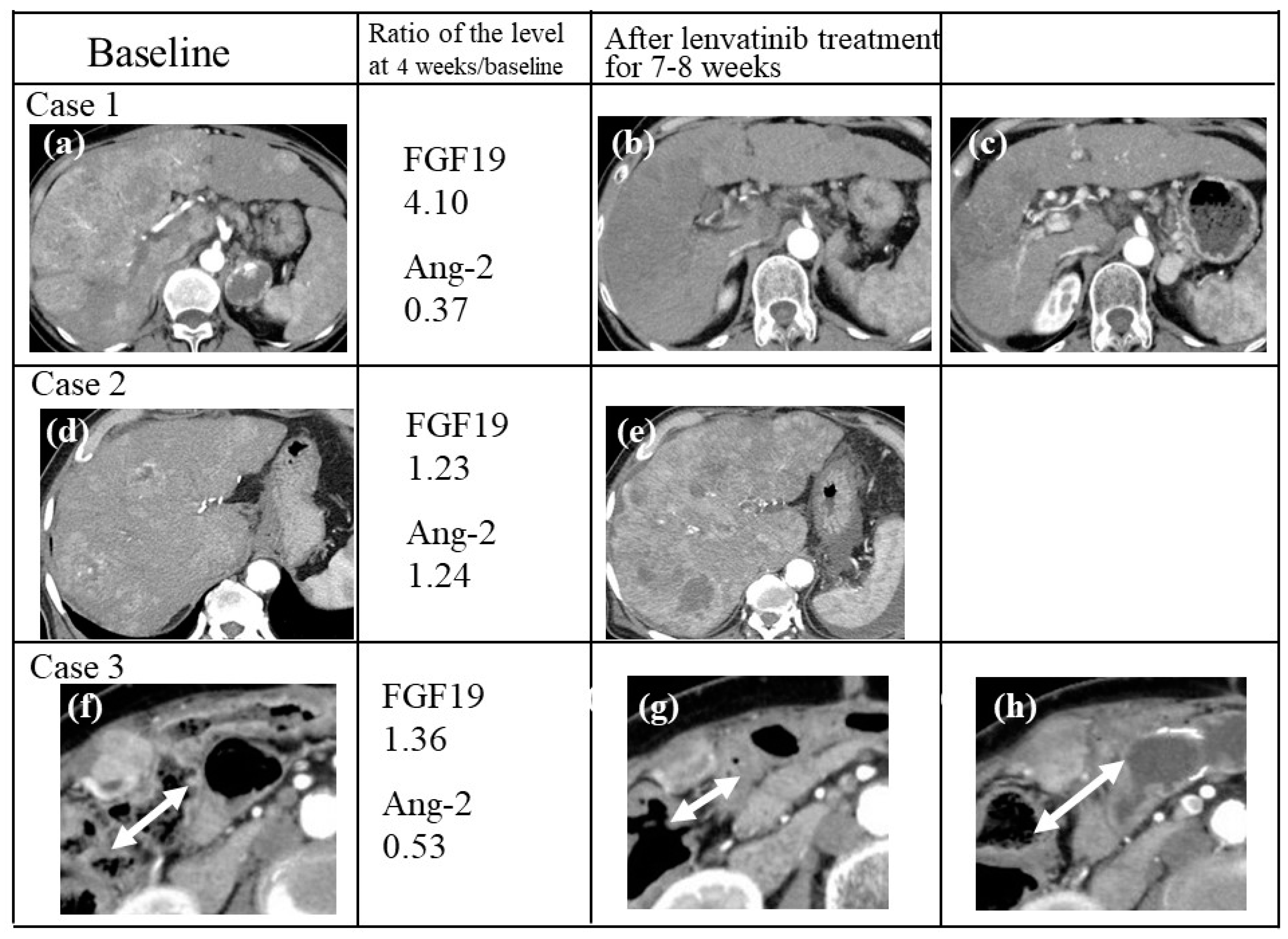
2.10. Case Presentations
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Study Design and Treatment
4.3. Diagnosis and Evaluation of Therapeutic Response
4.4. Serum Preparation and Measurement of Cytokine/Angiogenesis Factors
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef]
- Breuhahn, K.; Gores, G.; Schirmacher, P. Strategies for hepatocellular carcinoma therapy and diagnostics: Lessons learned from high throughput and profiling approaches. Hepatology 2011, 53, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Yamamoto, Y.; Funahashi, Y.; Tsuruoka, A.; Watanabe, T.; Wakabayashi, T.; Uenaka, T.; Asada, M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 2008, 122, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yamamoto, N.; Yamada, Y.; Nokihara, H.; Fujiwara, Y.; Hirata, T.; Koizumi, F.; Nishio, K.; Koyama, N.; Tamura, T. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin. Cancer Res. 2011, 17, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Boss, D.S.; Glen, H.; Beijnen, J.H.; Keesen, M.; Morrison, R.; Tait, B.; Copalu, W.; Mazur, A.; Wanders, J.; O’Brien, J.P.; et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2012, 106, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers (Basel) 2019, 11, e952. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel) 2019, 11, e1084. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of Lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 2019, 8, 3719–3728. [Google Scholar] [CrossRef]
- Sasaki, R.; Fukushima, M.; Haraguchi, M.; Miuma, S.; Miyaaki, H.; Hidaka, M.; Eguchi, S.; Matsuo, S.; Tajima, K.; Matsuzaki, T.; et al. Response to Lenvatinib Is Associated with Optimal RelativeDose Intensity in Hepatocellular Carcinoma: Experience in Clinical Settings. Cancers (Basel) 2019, 11, e1769. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C.L. The cancer biomarker problem. Nature 2008, 452, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Sigman, C.C. Cancer biomarkers: Selecting the right drug for the right patient. Nat. Rev. Drug Discov. 2012, 11, 201–214. [Google Scholar] [CrossRef]
- Llovet, J.M.; Hernandez-Gea, V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin. Cancer Res. 2014, 20, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kurzrock, R.; Wheler, J.J.; Naing, A.; Falchook, G.S.; Fu, S.; Kim, K.B.; Davies, M.A.; Nguyen, L.M.; George, G.C.; et al. Phase I Dose-Escalation Study of the Multikinase Inhibitor Lenvatinib in Patients with Advanced Solid Tumors and in an Expanded Cohort of Patients with Melanoma. Clin. Cancer Res. 2015, 21, 4801–4810. [Google Scholar] [CrossRef] [PubMed]
- Niizeki, T.; Sumie, S.; Torimura, T.; Kurogi, J.; Kuromatsu, R.; Iwamoto, H.; Aino, H.; Nakano, M.; Kawaguchi, A.; Kakuma, T.; et al. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J. Gastroenterol. 2012, 47, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, H.J.; Kim, K.M.; Yu, E.S.; Kim, K.H.; Kim, S.M.; Kim, T.W.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; et al. Fibroblast growth factor receptor isotype expression and its association with overall survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 60–70. [Google Scholar] [CrossRef][Green Version]
- Qi, L.; Song, W.; Li, L.; Cao, L.; Yu, Y.; Song, C.; Wang, Y.; Zhang, F.; Li, Y.; Zhang, B.; et al. FGF4 induces epithelial-mesenchymal transition by inducing store-operated calcium entry in lung adenocarcinoma. Oncotarget 2016, 7, 74015–74030. [Google Scholar] [CrossRef]
- Miura, S.; Mitsuhashi, N.; Shimizu, H.; Kimura, F.; Yoshidome, H.; Otsuka, M.; Kato, A.; Shida, T.; Okamura, D.; Miyazaki, M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC cancer 2012, 12, e56. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Chesney, J.; Robinson, D.; Gardner, H.; Shi, M.M.; Kirkwood, J.M. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin. Cancer Res. 2011, 17, 7451–7461. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Schlumberger, M.; Elisei, R.; Habra, M.A.; Kiyota, N.; Paschke, R.; Dutcus, C.E.; Hihara, T.; McGrath, S.; Matijevic, M.; et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of Lenvatinib in differentiated cancer of the thyroid. Eur. J. Cancer 2017, 75, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.W.; Nota, N.M.; Jager, A.; Bos, M.M.; van den Bosch, J.; van der Velden, A.M.; Portielje, J.E.; Honkoop, A.H.; van Tinteren, H. ATX Trial Team. Angiogenesis- and Hypoxia-Associated Proteins as Early Indicators of the Outcome in Patients with Metastatic Breast Cancer Given First-Line Bevacizumab-Based Therapy. Clin. Cancer Res. 2016, 22, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Jarzab, B.; Cabanillas, M.E.; Robinson, B.; Pacini, F.; Ball, D.W.; McCaffrey, J.; Newbold, K.; Allison, R.; Martins, R.G.; et al. A Phase II Trial of the Multitargeted Tyrosine Kinase Inhibitor Lenvatinib (E7080) in Advanced Medullary Thyroid Cancer. Clin. Cancer Res. 2016, 22, 44–53. [Google Scholar] [CrossRef]
- Goede, V.; Coutelle, O.; Neuneier, J.; Reinacher-Schick, A.; Schnell, R.; Koslowsky, T.C.; Weihrauch, M.R.; Cremer, B.; Kashkar, H.; Odenthal, M.; et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br. J. Cancer 2010, 103, 1407–1414. [Google Scholar] [CrossRef]
- Huang, H.; Lai, J.Y.; Do, J.; Liu, D.; Li, L.; Del Rosario, J.; Doppalapudi, V.R.; Pirie-Shepherd, S.; Levin, N.; Bradshaw, C.; et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration and tumor growth. Clin. Cancer Res. 2011, 17, 1001–1011. [Google Scholar] [CrossRef]
- Rigamonti, N.; Kadioglu, E.; Keklikoglou, I.; Wyser Rmili, C.; Leow, C.C.; De Palma, M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014, 8, 696–706. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 2014, 6, e18. [Google Scholar] [CrossRef]
- Jayson, G.C.; Zhou, C.; Backen, A.; Horsley, L.; Marti-Marti, K.; Shaw, D.; Mescallado, N.; Clamp, A.; Saunders, M.P.; Valle, J.W.; et al. Plasma Tie2 isa tumor vascular response biomarker for VEGF inhibitors in metastatic colorectal cancer. Nat. Commun. 2018, 9, e4672. [Google Scholar] [CrossRef]
- Lefere, S.; Van de Velde, F.; Hoorens, A.; Raevens, S.; Van Campenhout, S.; Vandierendonck, A.; Neyt, S.; Vandeghinste, B.; Vanhove, C.; Debbaut, C.; et al. Angiopoietin-2 Promotes Pathological Angiogenesis and Is a Therapeutic Target in Murine Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Asahina, Y.; Matsuda, S.; Muraoka, M.; Nakata, T.; Suzuki, Y.; Tamaki, N.; Yasui, Y.; Suzuki, S.; Hosokawa, T.; et al. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer 2014, 120, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Kudo, M.; Cheng, A.L.; Wyrwicz, L.; Ngan, R.; Blanc, J.F.; Baron, A.D.; Vogel, A.; Ikeda, M.; Piscaglia, F.; et al. Final analysis of serum biomarkers in patients (pts) from the phase III study of Lenvatinib (LEN) vs sorafenib (SOR) in unresectable hepatocellular carcinoma (uHCC) [REFLECT]. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
| Variable | n = 74 |
|---|---|
| Median age, years | 71 (46–95) |
| Sex (Male/Female), n (%) | 49/25: 66.2/33.8% |
| Cause of HCC (HBV/HCV/NBNC), n (%) | 12/27/35: 16.2/36.5/47.3% |
| BMI | 22.7 (16.9–35.7) |
| Child-Pugh score 5/6, n: % | 52/22: 70.3/29.7% |
| mALBI grade (1,2a/2b,3) | 30/20/23/1: 40.5/27.0/31.1/1.4% |
| PS (0/1) | 67/7: 90.5/9.5% |
| Extrahepatic metastasis, n (%) | 20 (27.0%) |
| MVI, n (%) | 26 (35.1%) |
| BCLC (B/C) | 38/36: 51.4/48.6% |
| TNM (II/III/IVA/IVB) LCSGJ 6th | 4/36/14/20: 5.4/48.6/18.9/27.0% |
| TKI 1st line / 2nd line/3rd line~ | 63/8/3: 85.1/10.8/4.1% |
| Past history of TACE, n (%) | 56 (75.7%) |
| AFP (ng/mL) | 38.0 (1.0–262,413) |
| DCP (AU/mL) | 468 (10–290,000) |
| Variable | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Age (years) | <71 | 1.235 | 0.495–3.082 | 0.6505 | 0.677 | 0.227–2.017 | 0.4834 |
| Sex | Male | 0.818 | 0.311–2.155 | 0.6851 | 1.071 | 0.358–3.203 | 0.9020 |
| HCV status | Positive | 1.932 | 0.733–5.090 | 0.1828 | |||
| Child-Pugh score | 5 | 1.111 | 0.409–3.021 | 0.8364 | |||
| mALB grade | 1+2a | 1.618 | 0.594–4.404 | 0.3464 | 1.325 | 0.418–4.197 | 0.6320 |
| PS | 0 | 2.426 | 0.440–13.40 | 0.3092 | |||
| EHM | Absent | 1.135 | 0.405–3.179 | 0.8097 | |||
| MVI | Absent | 1.739 | 0.658–4.598 | 0.2645 | |||
| BCLC stage | B | 1.25 | 0.501–3.120 | 0.6325 | 0.754 | 0.325–2.455 | 0.5921 |
| TNM stgae LCSGJ 6th | II + III | 1.579 | 0.627–3.974 | 0.3320 | |||
| Past history of TKI | Naïve | 1.091 | 0.302–3.946 | 0.8945 | |||
| Past history of TACE | Naïve | 0.889 | 0.294–2.691 | 0.8349 | |||
| AFP (ng/mL ) | <38 | 0.818 | 0.327–2.046 | 0.6678 | |||
| DCP (mAU/mL) | <468 | 2.41 | 0.947–6.131 | 0.0649 | |||
| RDI | <0.8 | 0.363 | 0.141–0.934 | 0.0357 | 0.280 | 0.092–0.855 | 0.0254 |
| FGF19-i | Positive | 4.364 | 1.644–11.58 | 0.0031 | |||
| Ang-2-d | Positive | 4.879 | 1.819–13.09 | 0.0016 | |||
| Both FGF19–i and Ang-2-d | Positive | 7.368 | 2.154–25.21 | 0.0015 | 9.143 | 2.400–34.832 | 0.0012 |
| Baseline Ang-2 level | <7432 | 0.464 | 0.183-1.175 | 0.1053 | |||
| Variable | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Age (years) | <71 | 1.092 | 0.492–2.422 | 0.8295 | 1.150 | 0.494–2.674 | 0.7462 |
| Sex | Male | 2.142 | 0.976–4.701 | 0.0575 | 2.041 | 0.880–4.733 | 0.0964 |
| HCV status | Positive | 0.789 | 0.357–1.742 | 0.5573 | |||
| Child-Pugh score | 5 | 0.486 | 0.213–1.112 | 0.0876 | |||
| mALB grade | 1 + 2a | 0.361 | 0.159–0.823 | 0.0153 | 0.442 | 0.180–1.089 | 0.0760 |
| PS | 0 | 0.468 | 0.173–1.265 | 0.1344 | |||
| EHM | Absent | 0.524 | 0.235–1.170 | 0.1150 | |||
| MVI | Absent | 0.323 | 0.146–0.719 | 0.0056 | 0.528 | 0.167–1.673 | 0.2778 |
| BCLC stage | B | 0.293 | 0.122–0.706 | 0.0062 | 0.888 | 0.200–2.060 | 0.4563 |
| TNM stgae LCSGJ 6th | II + III | 0.357 | 0.153–0.832 | 0.0170 | |||
| Past history of TKI | Naïve | 1.52 | 0.450–5.130 | 0.5010 | |||
| Past history of TACE | Naïve | 0.776 | 0.280–2.150 | 0.6250 | |||
| AFP (ng/mL) | <38 | 0.526 | 0.237–1.163 | 0.1125 | |||
| DCP (mAU/mL) | <468 | 0.550 | 0.243–1.241 | 0.1500 | |||
| RDI | <0.8 | 1.796 | 0.805–4.008 | 0.1796 | |||
| FGF19-i | Positive | 0.346 | 0.147–0.814 | 0.015 | |||
| Ang-2-d | Positive | 0.235 | 0.092–0.602 | 0.0025 | |||
| Both FGF19-i and Ang-2-d | Positive | 0.237 | 0.100–0.561 | 0.0010 | 0.171 | 0.037–0.793 | 0.0240 |
| Baseline Ang-2 level | <7432 | 0.983 | 0.444–2.179 | 0.9668 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuma, M.; Uojima, H.; Numata, K.; Hidaka, H.; Toyoda, H.; Hiraoka, A.; Tada, T.; Hirose, S.; Atsukawa, M.; Itokawa, N.; et al. Early Changes in Circulating FGF19 and Ang-2 Levels as Possible Predictive Biomarkers of Clinical Response to Lenvatinib Therapy in Hepatocellular Carcinoma. Cancers 2020, 12, 293. https://doi.org/10.3390/cancers12020293
Chuma M, Uojima H, Numata K, Hidaka H, Toyoda H, Hiraoka A, Tada T, Hirose S, Atsukawa M, Itokawa N, et al. Early Changes in Circulating FGF19 and Ang-2 Levels as Possible Predictive Biomarkers of Clinical Response to Lenvatinib Therapy in Hepatocellular Carcinoma. Cancers. 2020; 12(2):293. https://doi.org/10.3390/cancers12020293
Chicago/Turabian StyleChuma, Makoto, Haruki Uojima, Kazushi Numata, Hisashi Hidaka, Hidenori Toyoda, Atsushi Hiraoka, Toshifumi Tada, Shunji Hirose, Masanori Atsukawa, Norio Itokawa, and et al. 2020. "Early Changes in Circulating FGF19 and Ang-2 Levels as Possible Predictive Biomarkers of Clinical Response to Lenvatinib Therapy in Hepatocellular Carcinoma" Cancers 12, no. 2: 293. https://doi.org/10.3390/cancers12020293
APA StyleChuma, M., Uojima, H., Numata, K., Hidaka, H., Toyoda, H., Hiraoka, A., Tada, T., Hirose, S., Atsukawa, M., Itokawa, N., Arai, T., Kako, M., Nakazawa, T., Wada, N., Iwasaki, S., Miura, Y., Hishiki, S., Nishigori, S., Morimoto, M., ... Maeda, S. (2020). Early Changes in Circulating FGF19 and Ang-2 Levels as Possible Predictive Biomarkers of Clinical Response to Lenvatinib Therapy in Hepatocellular Carcinoma. Cancers, 12(2), 293. https://doi.org/10.3390/cancers12020293







