Mechanism- and Immune Landscape-Based Ranking of Therapeutic Responsiveness of 22 Major Human Cancers to Next Generation Anti-CTLA-4 Antibodies
Abstract
1. Background
2. Methods
2.1. Clinical Patient Samples and Database
2.2. Studies with Human Samples
2.3. Experimental Animals
2.4. Antibodies
2.5. Flow Cytometry for Mouse Samples
2.6. Gene-Set Enrichment Analysis
2.7. Ranking Cancer Types for Their Response to Anti-CTLA-4
2.8. Single Cell Sequencing Data Analysis
2.9. Biostatistical Analysis
3. Results
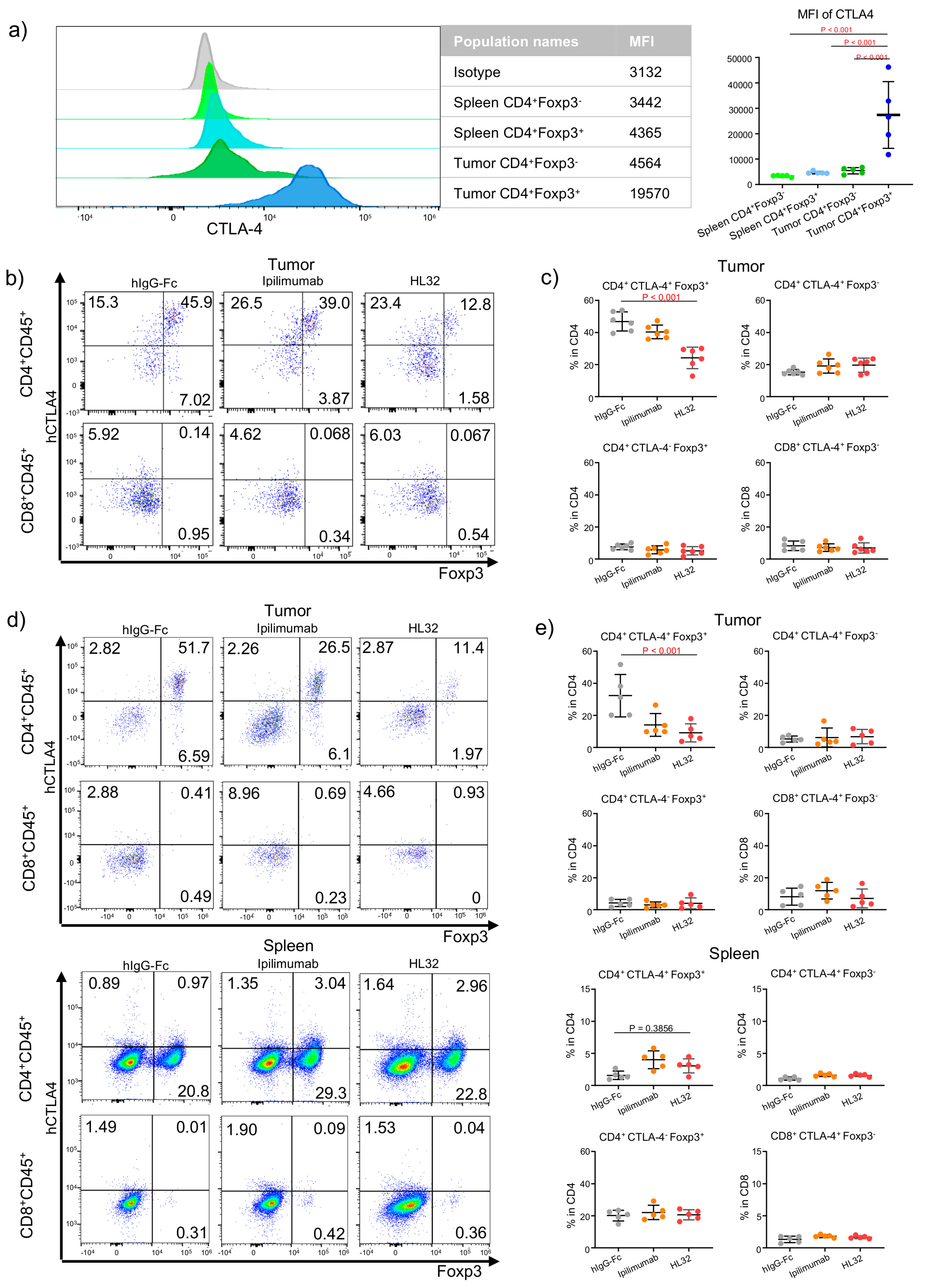
3.1. Selective Intratumoral Treg Depletion in TUMOR-BEARING MICE—SELECTIVITY in Treg Depletion by a pH-Sensitive Anti-CTLA-4 Antibody
3.2. Strategy to Rank Responsiveness of Human Cancer to anti-CTLA-4-Based Immunotherapy
3.3. Attribute 1: Ranking Human Cancer Based on CTLA4 Expression
3.4. Attribute 2: Ranking ADCC Potential of Anti-CTLA-4 Antibodies in Human Cancer Tissues
3.5. Attribute 3: Transcriptomic Features of Response to Anti-CTLA-4 Therapy
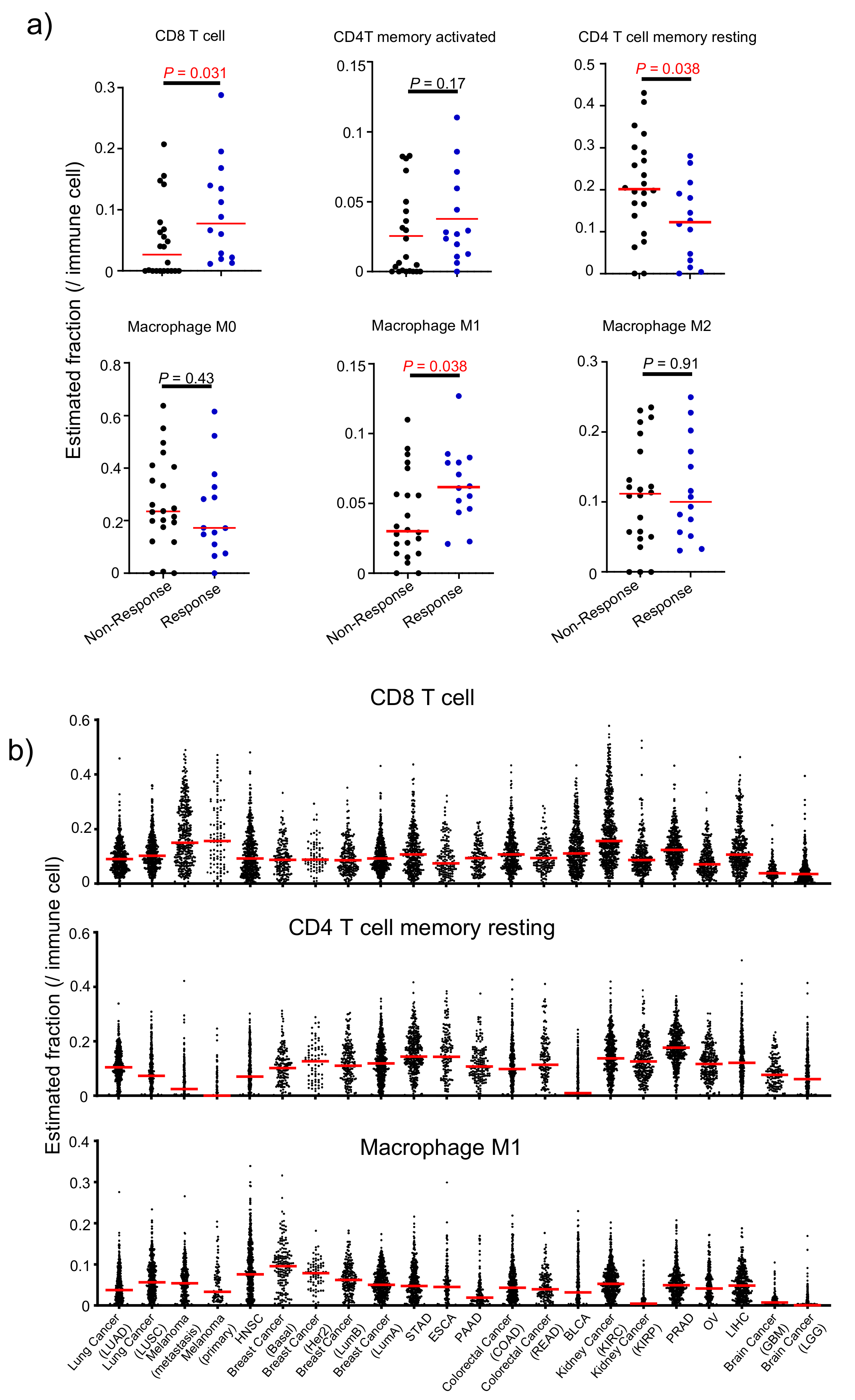
3.6. Attribute 4: Infiltrating Immune Cell Fraction-Based Ranking
3.7. Attribute 5: Tumor Mutation Burden and Neoantigen Counts
3.8. A Comprehensive Index to Rank Cancer Responsiveness
3.9. Predicting Selectivity of Treg Depletion in Human Lung Cancer Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TCGA | The Cancer Genome Atlas |
| GSEA | Gene-set Enrichment Analysis |
| ADCC | Antibody Dependent Cell-mediated Cytotoxicity/phagocytosis |
| ADCP | Antibody Dependent Cell-mediated Phagocytosis |
| NSCLC | Non Small Cell Lung Cancer |
| TMB | Tumor Mutation Burden |
References
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; van Cutsem, E.; McDermott, R.; Hill, A.; et al. Faculty of 1000 evaluation for Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Nivolumab plus Ipilimumab Achieves Responses in dMMR/MSI-H Tumors. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29420181 (accessed on 1 July 2019).
- Carlino, M.S.; Long, G.V. Ipilimumab Combined with Nivolumab: A Standard of Care for the Treatment of Advanced Melanoma? Clin. Cancer Res. 2016, 22, 3992–3998. [Google Scholar] [CrossRef]
- Ledford, H. Melanoma drug wins US approval. Nature 2011, 471, 561. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; McDermott, D.F. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin. Boil. Ther. 2018, 18, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; I Scher, H.; Fizazi, K.; Bossi, A.; Eertwegh, A.J.M.V.D.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Govindan, R.; Szczesna, A.; Ahn, M.-J.; Schneider, C.-P.; Mella, P.F.G.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; A Fennell, D.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Ribas, A.; Kefford, R.; Marshall, M.A.; Punt, C.J.; Haanen, J.B.; Mármol, M.; Garbe, C.; Gogas, H.; Schachter, J.; Linette, G.; et al. Phase III Randomized Clinical Trial Comparing Tremelimumab With Standard-of-Care Chemotherapy in Patients With Advanced Melanoma. J. Clin. Oncol. 2013, 31, 616–622. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, P. How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol. 2018, 39, 953–956. [Google Scholar] [CrossRef]
- Tang, F.; Du, X.; Liu, M.; Zheng, P.; Liu, Y. Anti-CTLA-4 antibodies in cancer immunotherapy: Selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Tang, F.; Liu, M.; Su, J.; Zhang, Y.; Wu, W.; Devenport, M.; A Lazarski, C.; Zhang, P.; Wang, X.; et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018, 28, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.A.; Furness, A.J.; Litchfield, K.; Joshi, K.; Rosenthal, R.; Ghorani, E.; Solomon, I.; Lesko, M.H.; Ruef, N.; Roddie, C.; et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018, 33, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef]
- Bulliard, Y.; Jolicoeur, R.; Windman, M.; Rue, S.M.; Ettenberg, S.; Knee, D.A.; Wilson, N.S.; Dranoff, G.; Brogdon, J.L. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 2013, 210, 1685–1693. [Google Scholar] [CrossRef]
- Du, X.; Liu, M.; Su, J.; Zhang, P.; Tang, F.; Ye, P.; Devenport, M.; Wang, X.; Zhang, Y.; Liu, Y.; et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018, 28, 433–447. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Liu, M.; Tang, F.; Zhang, P.; Ai, C.; Fields, J.K.; Sundberg, E.J.; Latinovic, O.S.; Devenport, M.; et al. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 2019, 29, 609–627. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; Van De Wiel, B.; Kvistborg, P.; Krijgsman, O.; Braber, M.V.D.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Price, K.D.; Simutis, F.; Fletcher, A.; Ramaiah, L.; Srour, R.; Kozlosky, J.; Sathish, J.; Engelhardt, J.; Capozzi, A.; Crona, J.; et al. Abstract LB-B33: Nonclinical safety evaluation of two distinct second generation variants of anti-CTLA4 monoclonal antibody, ipilimumab, in monkeys. In Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, Philadelphia, PA, USA, 26–30 October 2017. [Google Scholar]
- Harmonized Cancer Datasets. Genomic Data Commons Data Portal. Available online: https://portal.gdc.cancer.gov/ (accessed on 1 February 2019).
- cBioPortal for cancer genomics. Available online: http://www.cbioportal.org/index.do (accessed on 1 February 2019).
- National Cancer Institute. Genomic Data Commons. Available online: https://gdc.cancer.gov/aboutdata/%20publications/panimmune (accessed on 1 February 2019).
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Lute, K.D.; May, K.F.; Lu, P.; Zhang, H.; Kocak, E.; Mosinger, B.; Wolford, C.; Phillips, G.; Caligiuri, M.A.; Zheng, P.; et al. Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti–CTLA-4 antibodies. Blood 2005, 106, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Gene Expression Omnibus. GSE99254. Available online: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 1 February 2019).
- The R Project for Statistical Computing. version 3.5. Available online: https://www.r-project.org/ (accessed on 1 February 2019).
- Functional Annotation Tool, DAVID Bioinformatics Resources 6.8, NIAID/NIH. Available online: https://david.ncifcrf.gov/summary.jsp (accessed on 1 February 2019).
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef]
- Zhang, W.; Gordon, M.; Schultheis, A.M.; Yang, D.Y.; Nagashima, F.; Azuma, M.; Chang, H.-M.; Borucka, E.; Lurje, G.; Sherrod, A.E.; et al. FCGR2A and FCGR3A Polymorphisms Associated With Clinical Outcome of Epidermal Growth Factor Receptor–Expressing Metastatic Colorectal Cancer Patients Treated With Single-Agent Cetuximab. J. Clin. Oncol. 2007, 25, 3712–3718. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 2018, 24, 978–985. [Google Scholar] [CrossRef]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef]
- Hiraoka, N.; Onozato, K.; Kosuge, T.; Hirohashi, S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 2006, 12, 5423–5434. [Google Scholar] [CrossRef]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those at Risk of Late Relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbé, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef]
- Pinto, J.A.; Raez, L.E.; Oliveres, H.; Rolfo, C.C. Current knowledge of Ipilimumab and its use in treating non-small cell lung cancer. Expert Opin. Boil. Ther. 2019, 19, 509–515. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Peters, S.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; Baas, P.; Dols, M.C.; Smolin, A.; Vicente, D.; et al. Abstract CT074: Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): Blood and tissue TMB analysis from MYSTIC, a Phase III study of first-line durvalumab ± tremelimumab vs. chemotherapy. Cancer Res. 2019, 79. [Google Scholar] [CrossRef]




| Order | Type | Rank Number | |||||
|---|---|---|---|---|---|---|---|
| ADCC Feature | CTLA4 Expression | Signature Genes Score | Immune Cell Infiltration | Mutational Burden | Sum | ||
| 1 | SKCM-TM | 13.7 | 2 | 6.7 | 4.0 | 1.0 | 27.3 |
| 2 | LUAD | 12.7 | 1 | 1.3 | 13.0 | 3.5 | 31.5 |
| 3 | HNSC | 10.7 | 3 | 2.7 | 6.7 | 12.0 | 35.0 |
| 4 | LUSC | 12.7 | 8 | 5.7 | 6.7 | 4.5 | 37.5 |
| 5 | BRCA-Basal | 8.0 | 4 | 5.3 | 8.7 | 12.0 | 38.0 |
| 6 | BRCA-Her2 | 10.0 | 5 | 10.3 | 11.7 | 12.5 | 49.5 |
| 7 | KIRC | 11.0 | 15 | 2.7 | 9.3 | 13.0 | 51.0 |
| 8 | COAD | 10.3 | 10 | 11.0 | 9.0 | 12.0 | 52.3 |
| 9 | PAAD | 13.0 | 9 | 6.3 | 13.7 | 12.0 | 54.0 |
| 10 | BLCA | 5.3 | 11 | 18.3 | 8.3 | 13.0 | 56.0 |
| 11 | STAD | 21.3 | 6 | 6.7 | 12.7 | 11.0 | 57.7 |
| 12 | SKCM-TP | 15.0 | 12 | 16.0 | 6.3 | 10.5 | 59.8 |
| 13 | READ | 13.3 | 13 | 9.3 | 12.7 | 12.0 | 60.3 |
| 14 | BRCA_LumB | 8.0 | 14 | 15.3 | 10.7 | 12.5 | 60.5 |
| 15 | BRCA_LumA | 7.3 | 16 | 13.3 | 12.3 | 12.5 | 61.5 |
| 16 | ESCA | 10.7 | 7 | 15.0 | 17.0 | 13.0 | 62.7 |
| 17 | LIHC | 11.3 | 19 | 11.0 | 11.3 | 13.0 | 65.7 |
| 18 | PRAD | 13.0 | 17 | 18.7 | 11.3 | 12.5 | 72.5 |
| 19 | OV | 12.7 | 18 | 18.7 | 16.0 | 12.5 | 77.8 |
| 20 | KIRP | 11.0 | 21 | 15.7 | 18.3 | 13.0 | 79.0 |
| 21 | GBM | 10.7 | 20 | 21.0 | 16.0 | 13.0 | 80.7 |
| 22 | LGG | 11.0 | 22 | 22.0 | 16.0 | 11.5 | 82.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Xiong, X.; Rolfo, C.; Du, X.; Zhang, Y.; Yang, H.; Russo, A.; Devenport, M.; Zhou, P.; Liu, Y.; et al. Mechanism- and Immune Landscape-Based Ranking of Therapeutic Responsiveness of 22 Major Human Cancers to Next Generation Anti-CTLA-4 Antibodies. Cancers 2020, 12, 284. https://doi.org/10.3390/cancers12020284
Zhang P, Xiong X, Rolfo C, Du X, Zhang Y, Yang H, Russo A, Devenport M, Zhou P, Liu Y, et al. Mechanism- and Immune Landscape-Based Ranking of Therapeutic Responsiveness of 22 Major Human Cancers to Next Generation Anti-CTLA-4 Antibodies. Cancers. 2020; 12(2):284. https://doi.org/10.3390/cancers12020284
Chicago/Turabian StyleZhang, Peng, Xinxin Xiong, Christian Rolfo, Xuexiang Du, Yan Zhang, Han Yang, Alessandro Russo, Martin Devenport, Penghui Zhou, Yang Liu, and et al. 2020. "Mechanism- and Immune Landscape-Based Ranking of Therapeutic Responsiveness of 22 Major Human Cancers to Next Generation Anti-CTLA-4 Antibodies" Cancers 12, no. 2: 284. https://doi.org/10.3390/cancers12020284
APA StyleZhang, P., Xiong, X., Rolfo, C., Du, X., Zhang, Y., Yang, H., Russo, A., Devenport, M., Zhou, P., Liu, Y., & Zheng, P. (2020). Mechanism- and Immune Landscape-Based Ranking of Therapeutic Responsiveness of 22 Major Human Cancers to Next Generation Anti-CTLA-4 Antibodies. Cancers, 12(2), 284. https://doi.org/10.3390/cancers12020284






