Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs
Abstract
1. Introduction
2. Pathophysiology and Diagnostic Criteria for Autoimmune Cytopenias in CLL
2.1. Pathophysiology
2.2. Autoimmune Hemolytic Anemia
- Hb levels lower than or equal to 11 g/dL, in the absence of any cytotoxic treatment in the preceding month or other etiology identified;
- evidence of an underlying autoimmune mechanism, such as a positive direct antiglobulin test (DAT) for either IgG or C3 or the presence of cold agglutinin, after exclusion of alternatives (i.e., delayed hemolytic transfusion reaction);
- presence of one or more laboratory marker of hemolysis (high reticulocyte count, low serum haptoglobin levels, increased serum lactate dehydrogenase, or bilirubin levels).
2.3. Immune Thrombocytopenia
- otherwise unexplained and sudden fall in platelet count (<100 × 109/L), in the presence of normal bone marrow function (normal or increased number of megakaryocytes at bone marrow examination);
- no evidence of splenomegaly and no cytotoxic treatments within the last month;
- exclusion of other possible causes of thrombocytopenia (e.g., drug induced thrombocytopenia, infections, thrombotic thrombocytopenic purpura, disseminated intravascular coagulation).
2.4. Pure Red Cell Aplasia
- Hb levels lower than or equal to 11 g/dL, in the absence of hemolysis;
- absolute reticulocytopenia, in the absence of thrombocytopenia or neutropenia;
- exclusion of other causes of red cell aplasia, such as viral infections (e.g., parvovirus B19 or cytomegalovirus) and thymoma.
2.5. Autoimmune Granulocytopenia
- persistent neutropenia <0.5 × 109/L in the absence of cytotoxic treatments in the preceding eight weeks;
- absence of granulocyte precursors in the bone marrow.
3. Non-Hematological Autoimmune Complications in CLL
4. Prognostic Impact of Autoimmune Complications in CLL
5. Treatment of Autoimmune Complications in CLL
5.1. Standard Therapy for Autoimmune Complications in CLL
5.2. Targeted Agents for the Treatment of Autoimmune Complications in CLL
6. Drug-Induced Autoimmune Complications in CLL
6.1. Historical Data on Drug-Induced Autoimmune Complications in CLL
6.2. Drug Induced Autoimmune Complications in the Era of Targeted Agents for CLL Treatment
6.2.1. Ibrutinib
6.2.2. Idelalisib
6.2.3. Venetoclax
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2013, 88, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Burger, J.A.; O’Brien, S. Evolution of CLL treatment-from chemoimmunotherapy to targeted and individualized therapy. Nat. Rev. Clin. Oncol. 2018, 15, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Alexandrescu, D.T. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. MedGenMed 2007, 9, 35. [Google Scholar] [PubMed]
- Forconi, F.; Moss, P. Perturbation of the normal immune system in patients with CLL. Blood 2015, 126, 573–581. [Google Scholar] [CrossRef]
- Morrison, V.A. Infectious complications of chronic lymphocytic leukaemia: Pathogenesis, spectrum of infection, preventive approaches. Best Pract. Res. Clin. Haematol. 2010, 23, 145–153. [Google Scholar] [CrossRef]
- Falchi, L.; Vitale, C.; Keating, M.J.; Lerner, S.; Wang, X.; Elhor Gbito, K.Y.; Strom, S.; Wierda, W.G.; Ferrajoli, A. Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann. Oncol. 2016, 27, 1100–1106. [Google Scholar] [CrossRef]
- Hilal, T.; Gea-Banacloche, J.C.; Leis, J.F. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev. 2018, 32, 387–399. [Google Scholar] [CrossRef]
- Hamblin, T.J. Autoimmune complications of chronic lymphocytic leukemia. Semin. Oncol. 2006, 33, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Dearden, C. Disease-specific complications of chronic lymphocytic leukemia. In Hematology/the Education Program of the American Society of Hematology; American Society of Hematology: Washington, DC, USA, 2008; pp. 450–456. [Google Scholar] [CrossRef]
- Dameshek, W. Chronic lymphocytic leukemia-an accumulative disease of immunolgically incompetent lymphocytes. Blood 1967, 29, 566–584. [Google Scholar] [CrossRef]
- Barcellini, W.; Capalbo, S.; Agostinelli, R.M.; Mauro, F.R.; Ambrosetti, A.; Calori, R.; Cortelezzi, A.; Laurenti, L.; Pogliani, E.M.; Pedotti, P.; et al. Relationship between autoimmune phenomena and disease stage and therapy in B cell chronic lymphocytic leukemia. Haematologica 2006, 91, 1689–1692. [Google Scholar] [PubMed]
- Duek, A.; Shvidel, L.; Braester, A.; Berrebi, A. Clinical and immunologic aspects of B chronic lymphocytic leukemia associated with autoimmune disorders. Isr. Med. Assoc. J. 2006, 8, 828–831. [Google Scholar] [PubMed]
- Visco, C.; Ruggeri, M.; Laura Evangelista, M.; Stasi, R.; Zanotti, R.; Giaretta, I.; Ambrosetti, A.; Madeo, D.; Pizzolo, G.; Rodeghiero, F. Impact of immune thrombocytopenia on the clinical course of chronic lymphocytic leukemia. Blood 2008, 111, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Zent, C.S.; Ding, W.; Reinalda, M.S.; Schwager, S.M.; Hoyer, J.D.; Bowen, D.A.; Jelinek, D.F.; Tschumper, R.C.; Call, T.G.; Shanafelt, T.D.; et al. Autoimmune cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma: Changes in clinical presentation and prognosis. Leuk. Lymphoma 2009, 50, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Hodgson, K.; Ferrer, G.; Elena, M.; Filella, X.; Pereira, A.; Baumann, T.; Montserrat, E. Autoimmune cytopenia in chronic lymphocytic leukemia: Prevalence, clinical associations, and prognostic significance. Blood 2010, 116, 4771–4776. [Google Scholar] [CrossRef]
- Shvidel, L.; Tadmor, T.; Braester, A.; Bairey, O.; Rahimi-Levene, N.; Herishanu, Y.; Klepfish, A.; Shtalrid, M.; Berrebi, A.; Polliack, A. Pathogenesis, prevalence, and prognostic significance of cytopenias in chronic lymphocytic leukemia (CLL): A retrospective comparative study of 213 patients from a national CLL database of 1518 cases. Ann. Hematol. 2013, 92, 661–667. [Google Scholar] [CrossRef]
- Demir, C.; Ekinci, O. Clinical and serological autoimmune complications in chronic lymphocytic leukemia. Wien. Klin. Wochenschr 2017, 129, 552–557. [Google Scholar] [CrossRef]
- Visentin, A.; Imbergamo, S.; Gurrieri, C.; Frezzato, F.; Trimarco, V.; Martini, V.; Severin, F.; Raggi, F.; Scomazzon, E.; Facco, M.; et al. Major infections, secondary cancers and autoimmune diseases occur in different clinical subsets of chronic lymphocytic leukaemia patients. Eur. J. Cancer Oxf. Engl. 1990 2017, 72, 103–111. [Google Scholar] [CrossRef]
- Atef, B.; Azmy, E.; Aladle, D.; Mabed, M. The prevalence and prognostic significance of autoimmune cytopenias in a cohort of Egyptian patients with chronic lymphocytic leukemia. Hematol. Oncol. Stem Cell Ther. 2019, 12, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, T.J.; Oscier, D.G.; Young, B.J. Autoimmunity in chronic lymphocytic leukaemia. J. Clin. Pathol 1986, 39, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Carson, D.A. Autoantibodies in chronic lymphocytic leukemia and related systemic autoimmune diseases. Blood 1993, 81, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- Beaume, A.; Brizard, A.; Dreyfus, B.; Preud’homme, J.L. High incidence of serum monoclonal Igs detected by a sensitive immunoblotting technique in B cell chronic lymphocytic leukemia. Blood 1994, 84, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Caligaris-Cappio, F. B-chronic lymphocytic leukemia: A malignancy of anti-self B cells. Blood 1996, 87, 2615–2620. [Google Scholar] [CrossRef]
- Toriani-Terenzi, C.; Fagiolo, E. IL-10 and the cytokine network in the pathogenesis of human autoimmune hemolytic anemia. Ann. N. Y. Acad. Sci. 2005, 1051, 29–44. [Google Scholar] [CrossRef]
- Riches, J.C.; Ramsay, A.G.; Gribben, J.G. T cell function in chronic lymphocytic leukaemia. Semin. Cancer Biol. 2010, 20, 431–438. [Google Scholar] [CrossRef]
- Hall, A.M.; Vickers, M.A.; McLeod, E.; Barker, R.N. Rh autoantigen presentation to helper T cells in chronic lymphocytic leukemia by malignant B cells. Blood 2005, 105, 2007–2015. [Google Scholar] [CrossRef]
- Galletti, J.; Canones, C.; Morande, P.; Borge, M.; Oppezzo, P.; Geffner, J.; Bezares, R.; Gamberale, R.; Giordano, M. Chronic lymphocytic leukemia cells bind and present the erythrocyte protein band 3: Possible role as initiators of autoimmune hemolytic anemia. J. Immunol. Baltim. Md. 1950 2008, 181, 3674–3683. [Google Scholar] [CrossRef]
- D’Arena, G.; Laurenti, L.; Minervini, M.M.; Deaglio, S.; Bonello, L.; De Martino, L.; De Padua, L.; Savino, L.; Tarnani, M.; De Feo, V.; et al. Regulatory T cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leuk. Res. 2011, 35, 363–368. [Google Scholar] [CrossRef]
- Lad, D.P.; Varma, S.; Varma, N.; Sachdeva, M.U.; Bose, P.; Malhotra, P. Regulatory T cells in B cell chronic lymphocytic leukemia: Their role in disease progression and autoimmune cytopenias. Leuk. Lymphoma 2013, 54, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Go, R.S.; Li, C.Y.; Tefferi, A.; Phyliky, R.L. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood 2001, 98, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Vlachaki, E.; Diamantidis, M.D.; Klonizakis, P.; Haralambidou-Vranitsa, S.; Ioannidou-Papagiannaki, E.; Klonizakis, I. Pure Red Cell Aplasia and Lymphoproliferative Disorders: An Infrequent Association. Sci. World J. 2012, 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Rybka, J.; Butrym, A.; Wrobel, T.; Jazwiec, B.; Bogucka-Fedorczuk, A.; Poreba, R.; Kuliczkowski, K. The Expression of Toll-Like Receptors in Patients with B-Cell Chronic Lymphocytic Leukemia. Arch. Immunol. Ther. Exp. Warsz 2016, 64, 147–150. [Google Scholar] [CrossRef]
- Grandjenette, C.; Kennel, A.; Faure, G.C.; Bene, M.C.; Feugier, P. Expression of functional toll-like receptors by B-chronic lymphocytic leukemia cells. Haematologica 2007, 92, 1279–1281. [Google Scholar] [CrossRef]
- Rozkova, D.; Novotna, L.; Pytlik, R.; Hochova, I.; Kozak, T.; Bartunkova, J.; Spisek, R. Toll-like receptors on B-CLL cells: Expression and functional consequences of their stimulation. Int. J. Cancer 2010, 126, 1132–1143. [Google Scholar] [CrossRef]
- Barcellini, W.; Imperiali, F.G.; Zaninoni, A.; Reda, G.; Consonni, D.; Fattizzo, B.; Lonati, S.; Nobili, L.; Zanella, A.; Cortelezzi, A. Toll-like receptor 4 and 9 expression in B-chronic lymphocytic leukemia: Relationship with infections, autoimmunity and disease progression. Leuk. Lymphoma 2014, 55, 1768–1773. [Google Scholar] [CrossRef]
- Hill, Q.A.; Stamps, R.; Massey, E.; Grainger, J.D.; Provan, D.; Hill, A.; British Society for, H. The diagnosis and management of primary autoimmune haemolytic anaemia. Br. J. Haematol. 2017, 176, 395–411. [Google Scholar] [CrossRef]
- Hill, Q.A.; Hill, A.; Berentsen, S. Defining autoimmune hemolytic anemia: A systematic review of the terminology used for diagnosis and treatment. Blood Adv. 2019, 3, 1897–1906. [Google Scholar] [CrossRef]
- Rogers, K.A.; Woyach, J.A. Secondary autoimmune cytopenias in chronic lymphocytic leukemia. Semin. Oncol. 2016, 43, 300–310. [Google Scholar] [CrossRef]
- Zent, C.S.; Kay, N.E. Autoimmune complications in chronic lymphocytic leukaemia (CLL). Best Pract. Res. Clin. Haematol. 2010, 23, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br. J. Haematol. 2003, 120, 574–596. [CrossRef]
- Fu, R.; Zhang, T.; Liu, B.; Song, J.; Wang, G.; Li, L.; Wang, H.; Xing, L.; Wu, Y.; Guan, J.; et al. The clinical characteristics and therapy response of patients with acquired pure red cell aplasia. Hematology 2018, 23, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, F.; Sarzi-Puttini, P.; Zanella, A. Primary and secondary autoimmune neutropenia. Arthritis Res. Ther. 2005, 7, 208. [Google Scholar] [CrossRef]
- Wolach, O.; Bairey, O.; Lahav, M. Late-Onset Neutropenia After Rituximab Treatment: Case Series and Comprehensive Review of the Literature. Medicine 2010, 89, 308–318. [Google Scholar] [CrossRef]
- Viny, A.D.; Lichtin, A.; Pohlman, B.; Loughran, T.; Maciejewski, J. Chronic B cell dyscrasias are an important clinical feature of T-LGL leukemia. Leuk. Lymphoma 2008, 49, 932–938. [Google Scholar] [CrossRef]
- Hwang, K.; Park, C.-J.; Huh, H.J.; Han, S.H.; Jang, S.; Chi, H.-S. Flow Cytometric Detection of Neutrophil associated Immunoglobulin in Patients with or without Neutropenia and Establishment of the Reference Interval. Ann. Clin. Lab. Sci. 2011, 41, 144–149. [Google Scholar]
- Ito, T.; Taniuchi, S.; Tsuji, S.; Iharada, A.; Hasui, M.; Kaneko, K. Diagnosis of Autoimmune Neutropenia by Neutrophil-bound IgG and IgM Antibodies. J. Pediatric Hematol. Oncol. 2011, 33, 552–555. [Google Scholar] [CrossRef]
- Jung, M.; Rice, L. Unusual autoimmune nonhematologic complications in chronic lymphocytic leukemia. Clin. Lymphoma Myeloma Leuk. 2011, 11, S10–S13. [Google Scholar] [CrossRef]
- Alattar, M.L.; Ciccone, M.; Gaballa, M.R.; Vitale, C.; Badoux, X.C.; Manoukian, G.; Keating, M.J.; Kroll, M.H.; Ferrajoli, A. Bleeding diathesis associated with acquired von Willebrand Syndrome in three patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 3452–3454. [Google Scholar] [CrossRef]
- Landgren, O.; Engels, E.A.; Caporaso, N.E.; Gridley, G.; Mellemkjaer, L.; Hemminki, K.; Linet, M.S.; Goldin, L.R. Patterns of autoimmunity and subsequent chronic lymphocytic leukemia in Nordic countries. Blood 2006, 108, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Kane, E.; Painter, D.; Smith, A.; Crouch, S.; Oliver, S.; Patmore, R.; Roman, E. The impact of rheumatological disorders on lymphomas and myeloma: A report on risk and survival from the UK’s population-based Haematological Malignancy Research Network. Cancer Epidemiol. 2019, 59, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kyasa, M.J.; Parrish, R.S.; Schichman, S.A.; Zent, C.S. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. Am. J. Hematol. 2003, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zent, C.S.; Ding, W.; Schwager, S.M.; Reinalda, M.S.; Hoyer, J.D.; Jelinek, D.F.; Tschumper, R.C.; Bowen, D.A.; Call, T.G.; Shanafelt, T.D.; et al. The prognostic significance of cytopenia in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br. J. Haematol. 2008, 141, 615–621. [Google Scholar] [CrossRef]
- Dearden, C.; Wade, R.; Else, M.; Richards, S.; Milligan, D.; Hamblin, T.; Catovsky, D. The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic leukemia: A beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolytic anemia. Blood 2008, 111, 1820–1826. [Google Scholar] [CrossRef]
- Visco, C.; Novella, E.; Peotta, E.; Paolini, R.; Giaretta, I.; Rodeghiero, F. Autoimmune hemolytic anemia in patients with chronic lymphocytic leukemia is associated with IgVH status. Haematologica 2010, 95, 1230–1232. [Google Scholar] [CrossRef]
- Mauro, F.R.; Foa, R.; Cerretti, R.; Giannarelli, D.; Coluzzi, S.; Mandelli, F.; Girelli, G. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: Clinical, therapeutic, and prognostic features. Blood 2000, 95, 2786–2792. [Google Scholar] [CrossRef]
- Gribben, J.G. How I treat CLL up front. Blood 2010, 115, 187–197. [Google Scholar] [CrossRef]
- Killick, S.B.; Bown, N.; Cavenagh, J.; Dokal, I.; Foukaneli, T.; Hill, A.; Hillmen, P.; Ireland, R.; Kulasekararaj, A.; Mufti, G.; et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br. J. Haematol. 2016, 172, 187–207. [Google Scholar] [CrossRef]
- Neunert, C.E.; Cooper, N. Evidence-based management of immune thrombocytopenia: ASH guideline update. In Hematology/the Education Program of the American Society of Hematology; American Society of Hematology: Washington, DC, USA, 2018; Volume 2018, pp. 568–575. [Google Scholar] [CrossRef]
- Quinquenel, A.; Willekens, C.; Dupuis, J.; Royer, B.; Ysebaert, L.; De Guibert, S.; Michallet, A.S.; Feugier, P.; Guieze, R.; Levy, V.; et al. Bendamustine and rituximab combination in the management of chronic lymphocytic leukemia associated autoimmune hemolytic anemia: A multicentric retrospective study of the French CLL intergroup (GCFLLC/MW and GOELAMS). Am. J. Hematol. 2015, 90, 204–207. [Google Scholar] [CrossRef]
- Berentsen, S.; Randen, U.; Oksman, M.; Birgens, H.; Tvedt, T.H.A.; Dalgaard, J.; Galteland, E.; Haukås, E.; Brudevold, R.; Sørbø, J.H.; et al. Bendamustine plus rituximab for chronic cold agglutinin disease: Results of a Nordic prospective multicenter trial. Blood 2017, 130, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.; Limaye, S.A.; Driscoll, N.; Johnson, C.; Caramanica, A.; Lebowicz, Y.; Patel, D.; Kohn, N.; Rai, K. A combination of rituximab, cyclophosphamide and dexamethasone effectively treats immune cytopenias of chronic lymphocytic leukemia. Leuk. Lymphoma 2009, 50, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.; Michallet, A.S.; Oberic, L.; Picard, M.; Garon, A.; Willekens, C.; Dulery, R.; Leleu, X.; Cazin, B.; Ysebaert, L. Rituximab-cyclophosphamide-dexamethasone combination in the management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia 2011, 25, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.A.; Call, T.G.; Shanafelt, T.D.; Kay, N.E.; Schwager, S.M.; Reinalda, M.S.; Rabe, K.G.; Slager, S.L.; Zent, C.S. Treatment of autoimmune cytopenia complicating progressive chronic lymphocytic leukemia/small lymphocytic lymphoma with rituximab, cyclophosphamide, vincristine, and prednisone. Leuk. Lymphoma 2010, 51, 620–627. [Google Scholar] [CrossRef]
- St Bernard, R.; Hsia, C.C. Safe utilization of ibrutinib with or without steroids in chronic lymphocytic leukemia patients with autoimmune hemolytic anemia. Ann. Hematol. 2015, 94, 2077–2079. [Google Scholar] [CrossRef]
- Manda, S.; Dunbar, N.; Marx-Wood, C.R.; Danilov, A.V. Ibrutinib is an effective treatment of autoimmune haemolytic anaemia in chronic lymphocytic leukaemia. Br. J. Haematol. 2015. [Google Scholar] [CrossRef]
- Molica, S.; Levato, L.; Mirabelli, R. Chronic lymphocytic leukemia, autoimmune hemolytic anemia and ibrutinib: A case report and review of the literature. Leuk. Lymphoma 2016, 57, 735–737. [Google Scholar] [CrossRef]
- Cavazzini, F.; Lista, E.; Quaglia, F.M.; Formigaro, L.; Cavallari, M.; Martinelli, S.; Rigolin, G.M.; Foa, R.; Cuneo, A. Response to ibrutinib of refractory life-threatening autoimmune hemolytic anemia occurring in a relapsed chronic lymphocytic leukemia patient with 17p deletion. Leuk. Lymphoma 2016, 57, 2685–2688. [Google Scholar] [CrossRef]
- Rogers, K.A.; Ruppert, A.S.; Bingman, A.; Andritsos, L.A.; Awan, F.T.; Blum, K.A.; Flynn, J.M.; Jaglowski, S.M.; Lozanski, G.; Maddocks, K.J.; et al. Incidence and description of autoimmune cytopenias during treatment with ibrutinib for chronic lymphocytic leukemia. Leukemia 2016, 30, 346–350. [Google Scholar] [CrossRef]
- Montillo, M.; O’Brien, S.; Tedeschi, A.; Byrd, J.C.; Dearden, C.; Gill, D.; Brown, J.R.; Barrientos, J.C.; Mulligan, S.P.; Furman, R.R.; et al. Ibrutinib in previously treated chronic lymphocytic leukemia patients with autoimmune cytopenias in the RESONATE study. Blood Cancer J. 2017, 7, e524. [Google Scholar] [CrossRef]
- Hampel, P.J.; Larson, M.C.; Kabat, B.; Call, T.G.; Ding, W.; Kenderian, S.S.; Bowen, D.; Boysen, J.; Schwager, S.M.; Leis, J.F.; et al. Autoimmune cytopenias in patients with chronic lymphocytic leukaemia treated with ibrutinib in routine clinical practice at an academic medical centre. Br. J. Haematol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Quinquenel, A.; Godet, S.; Dartigeas, C.; Ysebaert, L.; Dupuis, J.; Ohanyan, H.; Collignon, A.; Gilardin, L.; Lepretre, S.; Dilhuydy, M.-S.; et al. Ibrutinib and idelalisib in the management of CLL associated autoimmune cytopenias: A study from the FILO group. Am. J. Hematol. 2019, 94, E183–E185. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Wierda, W.G.; Schuh, A.; Devereux, S.; Chaves, J.M.; Brown, J.R.; Hillmen, P.; Martin, P.; Awan, F.T.; Stephens, D.M.; et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: Updated phase 2 results. Blood 2019. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.; Parikh, S.A. A Concise Review of Autoimmune Cytopenias in Chronic Lymphocytic Leukemia. Curr. Hematol. Malig. Rep. 2017, 12, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, M.P.; Guedes, N.R.; Yamakawa, P.E.; Pereira, A.D.; Fonseca, A.; Chauffaille, M.; Goncalves, M.V.; Yamamoto, M.; Rodrigues, C.A. Treatment of refractory autoimmune hemolytic anemia with venetoclax in relapsed chronic lymphocytic leukemia with del(17p). Ann. Hematol. 2017, 96, 1577–1578. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Maldonado, E.; Danilov, A.V. Refractory Autoimmune Cytopenias Treated With Venetoclax. HemaSphere 2019, 3, e202. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.; Parsons, R.M.; Reilly, J.T.; Winfield, D.A. Autoimmune thrombocytopenia: A complication of fludarabine therapy in lymphoproliferative disorders. Clin. Lab. Haematol. 2000, 22, 175–178. [Google Scholar] [CrossRef]
- Di Raimondo, F.; Giustolisi, R.; Cacciola, E.; O’Brien, S.; Kantarjian, H.; Robertson, L.B.; Keating, M.J. Autoimmune hemolytic anemia in chronic lymphocytic leukemia patients treated with fludarabine. Leuk. Lymphoma 1993, 11, 63–68. [Google Scholar] [CrossRef]
- Myint, H.; Copplestone, J.A.; Orchard, J.; Craig, V.; Curtis, D.; Prentice, A.G.; Hamon, M.D.; Oscier, D.G.; Hamblin, T.J. Fludarabine related autoimmune haemolytic anaemia in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 1995, 91, 341–344. [Google Scholar] [CrossRef]
- Weiss, R.B.; Freiman, J.; Kweder, S.L.; Diehl, L.F.; Byrd, J.C. Hemolytic anemia after fludarabine therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 1998, 16, 1885–1889. [Google Scholar] [CrossRef]
- Robak, T.; Blasinska-Morawiec, M.; Krykowski, E.; Hellmann, A.; Konopka, L. Autoimmune haemolytic anaemia in patients with chronic lymphocytic leukaemia treated with 2-chlorodeoxyadenosine (cladribine). Eur. J. Haematol. 1997, 58, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Moya, L.; Martin, T.; Heuft, H.G.; Neubauer, A.; Herrmann, R. Allergic reaction with immune hemolytic anemia resulting from chlorambucil. Am. J. Hematol. 1989, 32, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Hertler, A.A.; Weiss, R.B.; Freiman, J.; Kweder, S.L.; Diehl, L.F. Fatal recurrence of autoimmune hemolytic anemia following pentostatin therapy in a patient with a history of fludarabine associated hemolytic anemia. Ann. Oncol. 1995, 6, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Reda, G.; Maura, F.; Gritti, G.; Gregorini, A.; Binda, F.; Guidotti, F.; Piciocchi, A.; Visco, C.; Rodeghiero, F.; Cortelezzi, A. Low-dose alemtuzumab associated immune thrombocytopenia in chronic lymphocytic leukemia. Am. J. Hematol. 2012, 87, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, N.; Gural, A.; Ben-Yehuda, D.; Gatt, M.E. Short communication: Bendamustine related hemolytic anemia in chronic lymphocytic leukemia. Cancer Chemother Pharm. 2013, 72, 709–713. [Google Scholar] [CrossRef]
- Borthakur, G.; O’Brien, S.; Wierda, W.G.; Thomas, D.A.; Cortes, J.E.; Giles, F.J.; Kantarjian, H.M.; Lerner, S.; Keating, M.J. Immune anaemias in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab--incidence and predictors. Br. J. Haematol. 2007, 136, 800–805. [Google Scholar] [CrossRef]
- Meunier, P.; Castaigne, S.; Bastie, J.N.; Chosidow, O.; Aractingi, S. Cutaneous reactions after treatment with 2-chlorodeoxyadenosine. Acta Derm. Venereol. 1996, 76, 385–386. [Google Scholar] [CrossRef]
- Neste, E.V.D.; Delannoy, A.; Feremans, W.; Ferrant, A.; Michaux, L. Second Primary Tumors and Immune Phenomena After Fludarabine or 2-Chloro-2′-Deoxy adenosine Treatment. Leuk. Lymphoma 2001, 40, 541–550. [Google Scholar] [CrossRef]
- Tisler, A.; Pierratos, A.; Lipton, J.H. Crescentic glomerulonephritis associated with p-ANCA positivity in fludarabine-treated chronic lymphocytic leukaemia. In Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant; Association-European Renal Association: England and Wales, 1996; Volume 11, pp. 2306–2308. [Google Scholar] [CrossRef]
- Hodskins, J.; Houranieh, J.; Fleischman, R.A. Ibutinib-Associated Autoimmune Hemolytic Anemia in CLL. Blood 2014, 124, 5671. [Google Scholar] [CrossRef]
- Rider, T.G.; Grace, R.J.; Newman, J.A. Autoimmune haemolytic anaemia occurring during ibrutinib therapy for chronic lymphocytic leukaemia. Br. J. Haematol. 2016, 173, 326–327. [Google Scholar] [CrossRef]
- Vitale, C.; Ahn, I.E.; Sivina, M.; Ferrajoli, A.; Wierda, W.G.; Estrov, Z.; Konoplev, S.N.; Jain, N.; O’Brien, S.; Farooqui, M.; et al. Autoimmune cytopenias in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica 2016, 101, e254–e258. [Google Scholar] [CrossRef] [PubMed]
- Dimou, M.; Iliakis, T.; Pardalis, V.; Bitsani, C.; Vassilakopoulos, T.P.; Angelopoulou, M.; Tsaftaridis, P.; Papaioannou, P.; Koudouna, A.; Kalyva, S.; et al. Safety and efficacy analysis of long-term follow up real-world data with ibrutinib monotherapy in 58 patients with CLL treated in a single-center in Greece. Leuk. Lymphoma 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. New Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J. Clin. Oncol. 2018. [Google Scholar] [CrossRef]
- Davids, M.S.; Hallek, M.; Wierda, W.; Roberts, A.W.; Stilgenbauer, S.; Jones, J.A.; Gerecitano, J.F.; Kim, S.Y.; Potluri, J.; Busman, T.; et al. Comprehensive Safety Analysis of Venetoclax Monotherapy for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2018, 24, 4371–4379. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. New Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Mato, A.R.; Islam, P.; Daniel, C.; Strelec, L.; Kaye, A.H.; Brooks, S.; Ganetsky, A.; Nasta, S.; Porter, D.L.; Svoboda, J.; et al. Ibrutinib induced pneumonitis in patients with chronic lymphocytic leukemia. Blood 2016, 127, 1064–1067. [Google Scholar] [CrossRef]
- Wanner, D.; Bohn, J.P.; Rudzki, J.; Stockhammer, G.; Steurer, M. Autoimmune myelitis in a CLL patient undergoing treatment with ibrutinib. Ann. Hematol. 2019, 98, 205–207. [Google Scholar] [CrossRef]
- Shaikh, H.; Khattab, A.; Faisal, M.S.; Chilkulwar, A.; Albrethsen, M.; Sadashiv, S.; Fazal, S. Case series of unique adverse events related to the use of ibrutinib in patients with B cell malignancies—A single institution experience and a review of literature. J. Oncol. Pharm. Pract. 2019, 25, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. New Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Lamanna, N.; Kipps, T.J.; Flinn, I.; Zelenetz, A.D.; Burger, J.A.; Keating, M.; Mitra, S.; Holes, L.; Yu, A.S.; et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood 2015, 126, 2686–2694. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef]
- Lampson, B.L.; Kasar, S.N.; Matos, T.R.; Morgan, E.A.; Rassenti, L.; Davids, M.S.; Fisher, D.C.; Freedman, A.S.; Jacobson, C.A.; Armand, P.; et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 2016, 128, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Coutre, S.E.; Barrientos, J.C.; Brown, J.R.; de Vos, S.; Furman, R.R.; Keating, M.J.; Li, D.; O’Brien, S.M.; Pagel, J.M.; Poleski, M.H.; et al. Management of adverse events associated with idelalisib treatment-expert panel opinion. Leuk. Lymphoma 2015, 1–20. [Google Scholar] [CrossRef]
- Cuneo, A.; Barosi, G.; Danesi, R.; Fagiuoli, S.; Ghia, P.; Marzano, A.; Montillo, M.; Poletti, V.; Viale, P.; Zinzani, P.L. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper. Hematol. Oncol. 2019, 37, 3–14. [Google Scholar] [CrossRef]
- Robak, T.; Robak, E. Tyrosine kinase inhibitors as potential drugs for B cell lymphoid malignancies and autoimmune disorders. Expert Opin. Investig. Drugs 2012, 21, 921–947. [Google Scholar] [CrossRef]
- Hutcheson, J.; Vanarsa, K.; Bashmakov, A.; Grewal, S.; Sajitharan, D.; Chang, B.Y.; Buggy, J.J.; Zhou, X.J.; Du, Y.; Satterthwaite, A.B.; et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res. Ther. 2012, 14, R243. [Google Scholar] [CrossRef]
- Dubovsky, J.A.; Beckwith, K.A.; Natarajan, G.; Woyach, J.A.; Jaglowski, S.; Zhong, Y.; Hessler, J.D.; Liu, T.M.; Chang, B.Y.; Larkin, K.M.; et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013, 122, 2539–2549. [Google Scholar] [CrossRef]
- Fiorcari, S.; Maffei, R.; Audrito, V.; Martinelli, S.; Ten Hacken, E.; Zucchini, P.; Grisendi, G.; Potenza, L.; Luppi, M.; Burger, J.A.; et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 2016, 7, 65968–65981. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.; Okkenhaug, K.; Sasaki, T.; Penninger, J.M.; Vanhaesebroeck, B.; Cyster, J.G. Cutting Edge: Differential Roles for Phosphoinositide 3-Kinases, p110g and p110d, in Lymphocyte Chemotaxis and Homing. J. Immunol. Baltim. Md. 1950 2004, 173, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K.; Patton, D.T.; Bilancio, A.; Garcon, F.; Rowan, W.C.; Vanhaesebroeck, B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J. Immunol. Baltim. Md. 1950 2006, 177, 5122–5128. [Google Scholar] [CrossRef]
- Ali, K.; Soond, D.R.; Pineiro, R.; Hagemann, T.; Pearce, W.; Lim, E.L.; Bouabe, H.; Scudamore, C.L.; Hancox, T.; Maecker, H.; et al. Inactivation of PI(3)K p110delta breaks regulatory T cell-mediated immune tolerance to cancer. Nature 2014, 510, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Mouchemore, K.A.; Sampaio, N.G.; Murrey, M.W.; Stanley, E.R.; Lannutti, B.J.; Pixley, F.J. Specific inhibition of PI3K p110delta inhibits CSF-1 induced macrophage spreading and invasive capacity. FEBS J. 2013, 280, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Fueyo, A.; Rojas, J.M.; Cariaga, A.E.; Garcia, E.; Steiner, B.H.; Barber, D.F.; Puri, K.D.; Carrera, A.C. Inhibition of PI3Kdelta reduces kidney infiltration by macrophages and ameliorates systemic lupus in the mouse. J. Immunol. Baltimore Md. 1950 2014, 193, 544–554. [Google Scholar] [CrossRef]
- Chellappa, S.; Kushekhar, K.; Munthe, L.A.; Tjonnfjord, G.E.; Aandahl, E.M.; Okkenhaug, K.; Tasken, K. The PI3K p110delta Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function. J. Immunol. Baltimore Md. 1950 2019, 202, 1397–1405. [Google Scholar] [CrossRef]
- Bardwell, P.D.; Gu, J.; McCarthy, D.; Wallace, C.; Bryant, S.; Goess, C.; Mathieu, S.; Grinnell, C.; Erickson, J.; Rosenberg, S.H.; et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J. Immunol. Baltim. Md. 1950 2009, 182, 7482–7489. [Google Scholar] [CrossRef]
- de Weerdt, I.; Hofland, T.; de Boer, R.; Dobber, J.A.; Dubois, J.; van Nieuwenhuize, D.; Mobasher, M.; de Boer, F.; Hoogendoorn, M.; Velders, G.A.; et al. Distinct immune composition in lymph node and peripheral blood of CLL patients is reshaped during venetoclax treatment. Blood Adv. 2019, 3, 2642–2652. [Google Scholar] [CrossRef]
- Coscia, M.; Vitale, C.; Cerrano, M.; Maffini, E.; Giaccone, L.; Boccadoro, M.; Bruno, B. Adoptive immunotherapy with CAR modified T cells in cancer: Current landscape and future perspectives. Front. Biosci. Landmark Ed. 2019, 24, 1284–1315. [Google Scholar] [CrossRef]
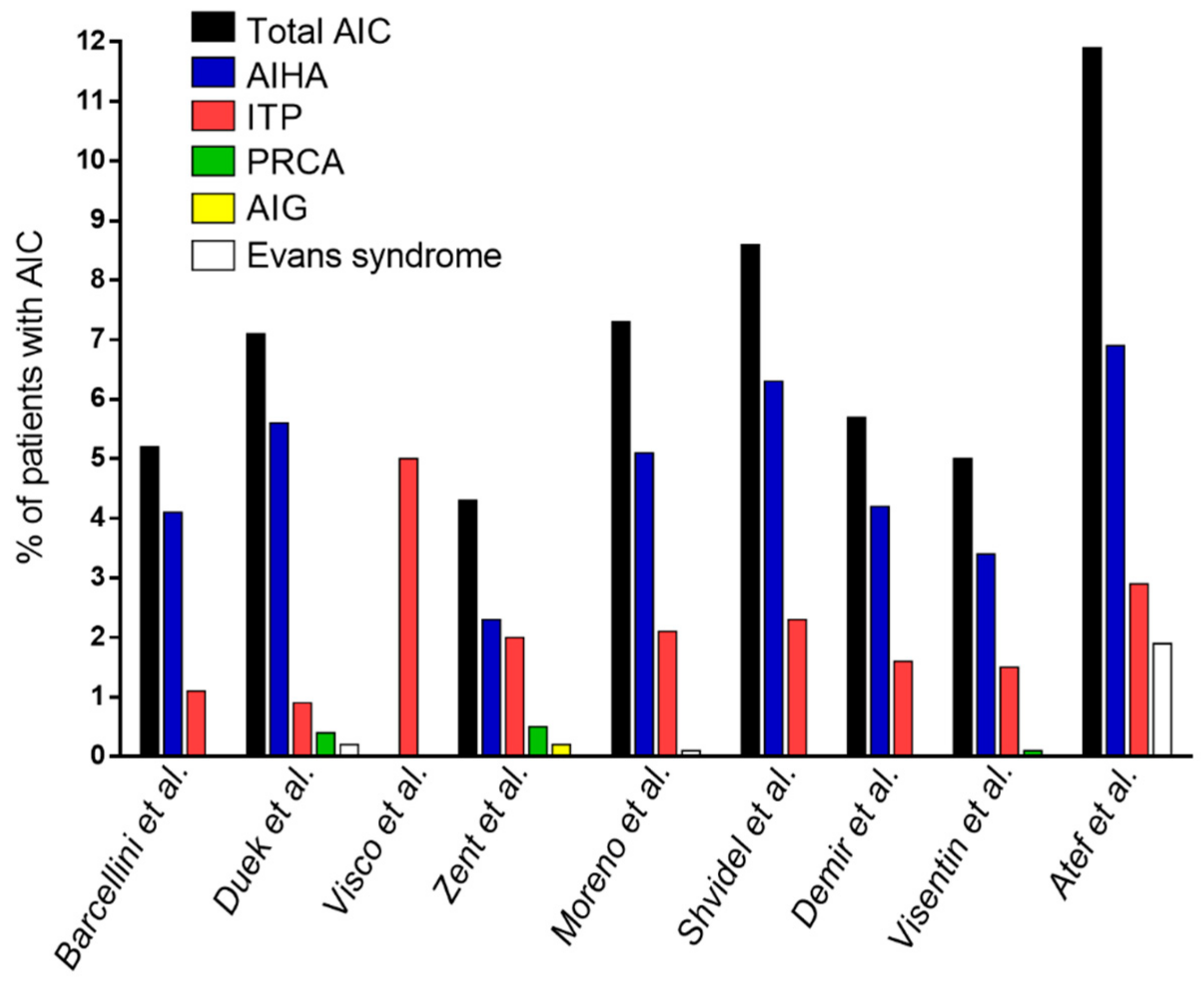
| Authors | Type of Study | Number of CLL Patients | Type of Cohort | Patients with AIC, n (%) |
|---|---|---|---|---|
| Barcellini et al. [14] | Multicentric, prospective + retrospective | 3150 | Treatment-naïve + pre-treated patients | Total 164 (5.2%) |
| AIHA 129 (4.1%) | ||||
| ITP 35 (1.1%) | ||||
| Duek et al. [15] | Single center, retrospective | 964 | Treatment-naïve + pre-treated patients | Total 69 (7.1%) |
| AIHA 54 (5.6%) | ||||
| ITP 9 (0.9%) | ||||
| PRCA 4 (0.4%) | ||||
| Evans syndrome 2 (0.2%) | ||||
| Visco et al. [16] | Multicentric, retrospective | 1278 | Treatment-naïve + pre-treated patients | ITP 64 (5%) |
| Zent et al. [17] | Single center, retrospective | 1750 | Treatment-naïve + pre-treated patients | Total 75 (4.3%) |
| AIHA 41 (2.3%) | ||||
| ITP 35 (2%) | ||||
| PRCA 9 (0.5%) | ||||
| AIG 3 (0.2%) | ||||
| (10 patients more than one type) | ||||
| Moreno et al. [18] | Single center, retrospective | 960 | Pre-treated patients | Total 70 (7.3%) |
| AIHA 49 (5.1%) | ||||
| ITP 20 (2.1%) | ||||
| Evans syndrome 1 (0.1%) | ||||
| Shvidel et al. [19] | Multicentric, retrospective | 1477 | Treatment-naïve + pre-treated patients | Total 127 (8.6%) |
| AIHA 93 (6.3%) | ||||
| ITP 34 (2.3%) | ||||
| (including 12 cases with Evans syndrome) | ||||
| Demir et al. [20] | Single center, prospective | 192 | Treatment-naïve + pre-treated patients | Total 11 (5.7%) |
| AIHA 8 (4.2%) | ||||
| ITP 3 (1.6%) | ||||
| Visentin et al. [21] | Single center, retrospective | 795 | Treatment-naïve + pre-treated patients | Total 40 (5%) |
| AIHA 27 (3.4%) | ||||
| ITP 12 (1.5%) | ||||
| PRCA 1 (0.1%) | ||||
| Atef et al. [22] | Single center, retrospective | 101 | NA | Total 12 (11.9%) |
| AIHA 7 (6.9%) | ||||
| ITP 3 (2.9%) | ||||
| Evans syndrome 2 (1.9%) | ||||
| (31 patients with combined autoimmune and infiltrative etiology, 30.7%) |
| Authors | Barcellini et al. [14] | Duek et al. [15] | Visentin et al. [21] | Demir et al. [20] | Jung et al. [50] | Alattar et al. [51] |
|---|---|---|---|---|---|---|
| CLL n = 3150 | CLL n = 964 | CLL n = 795 | CLL n = 192 | 6 cases reported | 3 cases reported | |
| Skin immune complications | n = 9 | n = 8 | n = 5 | n = 1 | - | - |
| (bullous pemphigoid) | (n = 3 bullous pemphigoid) | (bullous pemphigoid) | ||||
| Hashimoto’s thyroiditis | n = 8 | n = 15 | n = 12 | - | n = 2 | - |
| Grave’s disease | - | n = 3 | n = 4 | - | - | - |
| Rheumatoid arthritis | n = 4 | n = 4 | n = 4 | - | n = 1 | - |
| Vasculitis | n = 1 | n = 5 | n = 3 | - | n = 2 | - |
| (Horton arteritis) | ||||||
| Sjӧgren syndrome | n = 1 | n = 3 | n = 3 | - | - | - |
| Systemic lupus erythematosus | n = 1 | n = 2 | - | - | - | - |
| Angioneurotic edema | - | n = 2 | - | - | n = 1 | - |
| Multiple sclerosis | - | n = 2 | - | - | - | - |
| Acquired angioedema | - | - | - | n = 3 | - | - |
| Ulcerative colitis | n = 1 | n = 1 | - | - | - | - |
| Acquired von Willebrand disease | - | - | - | - | n = 1 | n = 3 |
| Autoimmune gastritis | n = 1 | - | - | - | - | - |
| Autoimmune hepatitis | - | - | - | n = 1 | - | - |
| Autoimmune glomerulonephritis | n = 1 | - | - | - | - | - |
| Autoimmune polyneuropathy | n = 1 | - | - | - | - | - |
| Raynaud’s disease | n = 1 | - | - | - | - | - |
| Polymyositis dermatomyositis | n = 1 | - | - | - | - | - |
| Ankylosing spondylitis | - | n = 1 | - | - | - | - |
| Pernicious anemia | - | n = 1 | - | - | - | - |
| Myasthenia gravis | - | - | - | - | n = 1 | - |
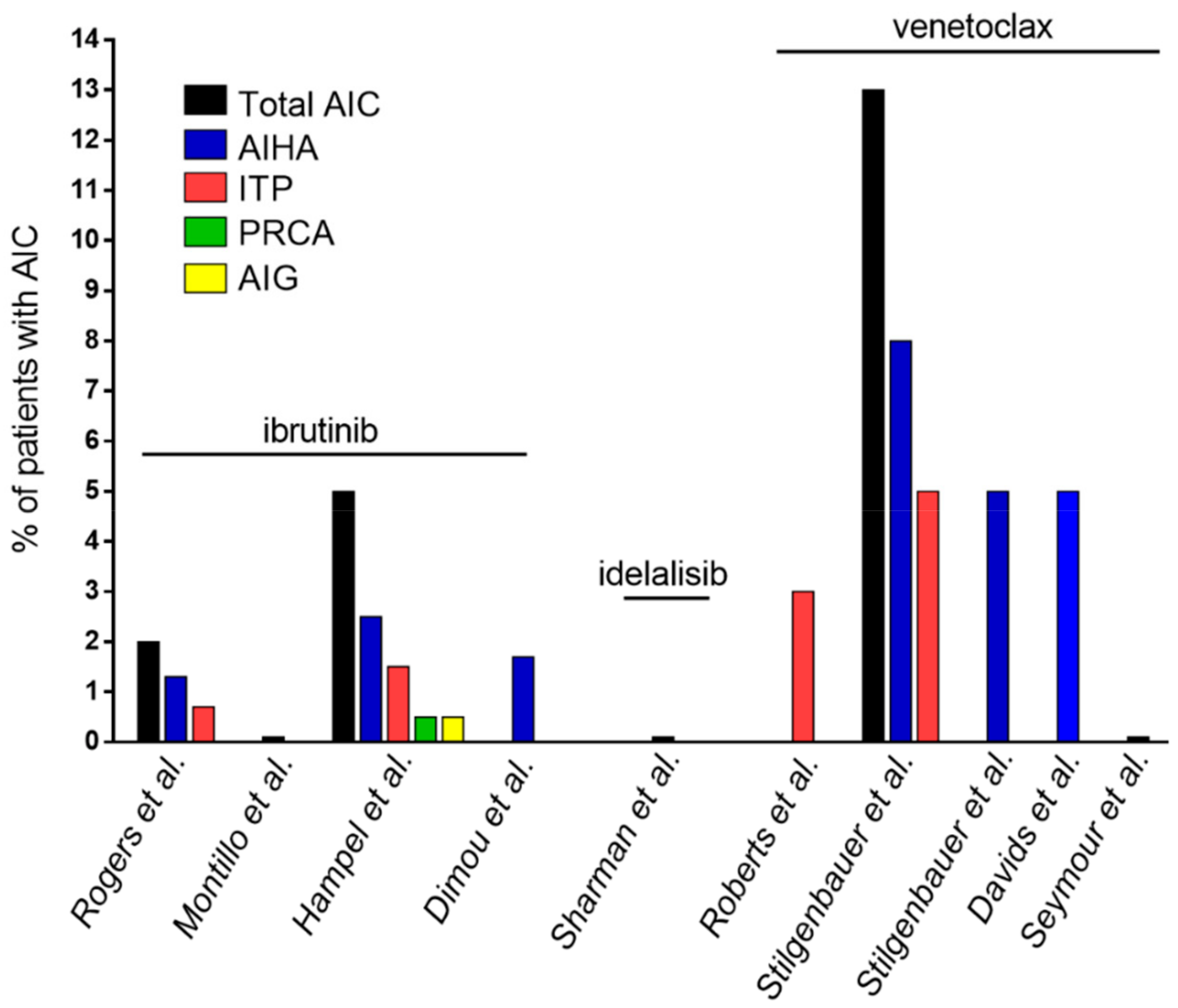
| Authors | Type of Study | Number of CLL Patients | Type of Cohort | Drug | Patients with AIC, n (%) |
|---|---|---|---|---|---|
| Rogers et al. [71] | Single center, retrospective (patients treated in 4 different clinical trials) | 301 | Treatment-naïve + pre-treated patients | Ibrutinib ± ofatumumab | Total 6 (2%) |
| AIHA 4 (1.3%) | |||||
| ITP 2 (0.7%) | |||||
| Montillo et al. [72] | Multicenter, prospective | 195 | Pre-treated patients | Ibrutinib | Total 0 |
| Hampel et al. [73] | Single center, retrospective (clinical practice) | 193 | 32 treatment-naïve (17%), 161 pre-treated patients (83%) | Ibrutinib | Total 11 (5%) |
| AIHA 5 (2.5%) | |||||
| ITP 3 (1.5%) | |||||
| PRCA 1 (0.5%) | |||||
| AIG 1 (0.5%) | |||||
| Aplastic anemia 1 (0.5%) | |||||
| Dimou et al. [95] | Single center, retrospective (clinical practice) | 58 | 11 treatment-naïve (19%), | Ibrutinib | AIHA 1 (1.7%) |
| 47 pre-treated patients (81%) | |||||
| Sharman et al. [96] | Multicenter, prospective | 110 | Pre-treated patients | Idelalisib + rituximab | None reported |
| Roberts et al. [97] | Multicenter, prospective | 116 | Pre-treated patients | Venetoclax | Among SAEs: |
| ITP 2 (3%) | |||||
| Stilgenbauer et al. [98] | Multicenter, prospective | 107 | Pre-treated patients | Venetoclax | Total 13 (13%) |
| AIHA 8 (8%) | |||||
| ITP 5 (5%) | |||||
| Stilgenbauer et al. [99] | Multicenter, prospective | 158 | 5 treatment-naïve (3%), | Venetoclax | Among SAEs: |
| 153 pre-treated patients (97%) | AIHA 8 (5%) | ||||
| Davids et al. [100] | Pooled analysis (3 prospective trials) | 350 | Pre-treated patients | Venetoclax | AIHA 17 (5%) |
| Seymour et al. [101] | Multicenter, prospective | 193 | Pre-treated patients | Venetoclax + rituximab | No grade ≥3 AIC reported |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, C.; Montalbano, M.C.; Salvetti, C.; Boccellato, E.; Griggio, V.; Boccadoro, M.; Coscia, M. Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs. Cancers 2020, 12, 282. https://doi.org/10.3390/cancers12020282
Vitale C, Montalbano MC, Salvetti C, Boccellato E, Griggio V, Boccadoro M, Coscia M. Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs. Cancers. 2020; 12(2):282. https://doi.org/10.3390/cancers12020282
Chicago/Turabian StyleVitale, Candida, Maria Chiara Montalbano, Chiara Salvetti, Elia Boccellato, Valentina Griggio, Mario Boccadoro, and Marta Coscia. 2020. "Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs" Cancers 12, no. 2: 282. https://doi.org/10.3390/cancers12020282
APA StyleVitale, C., Montalbano, M. C., Salvetti, C., Boccellato, E., Griggio, V., Boccadoro, M., & Coscia, M. (2020). Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs. Cancers, 12(2), 282. https://doi.org/10.3390/cancers12020282






