Establishment and Characterization of Patient-Derived Xenografts (PDXs) of Different Histology from Malignant Pleural Mesothelioma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. PDX Development from MPM Patients
2.2. Biological, Histological, and Immunohistochemical Analysis
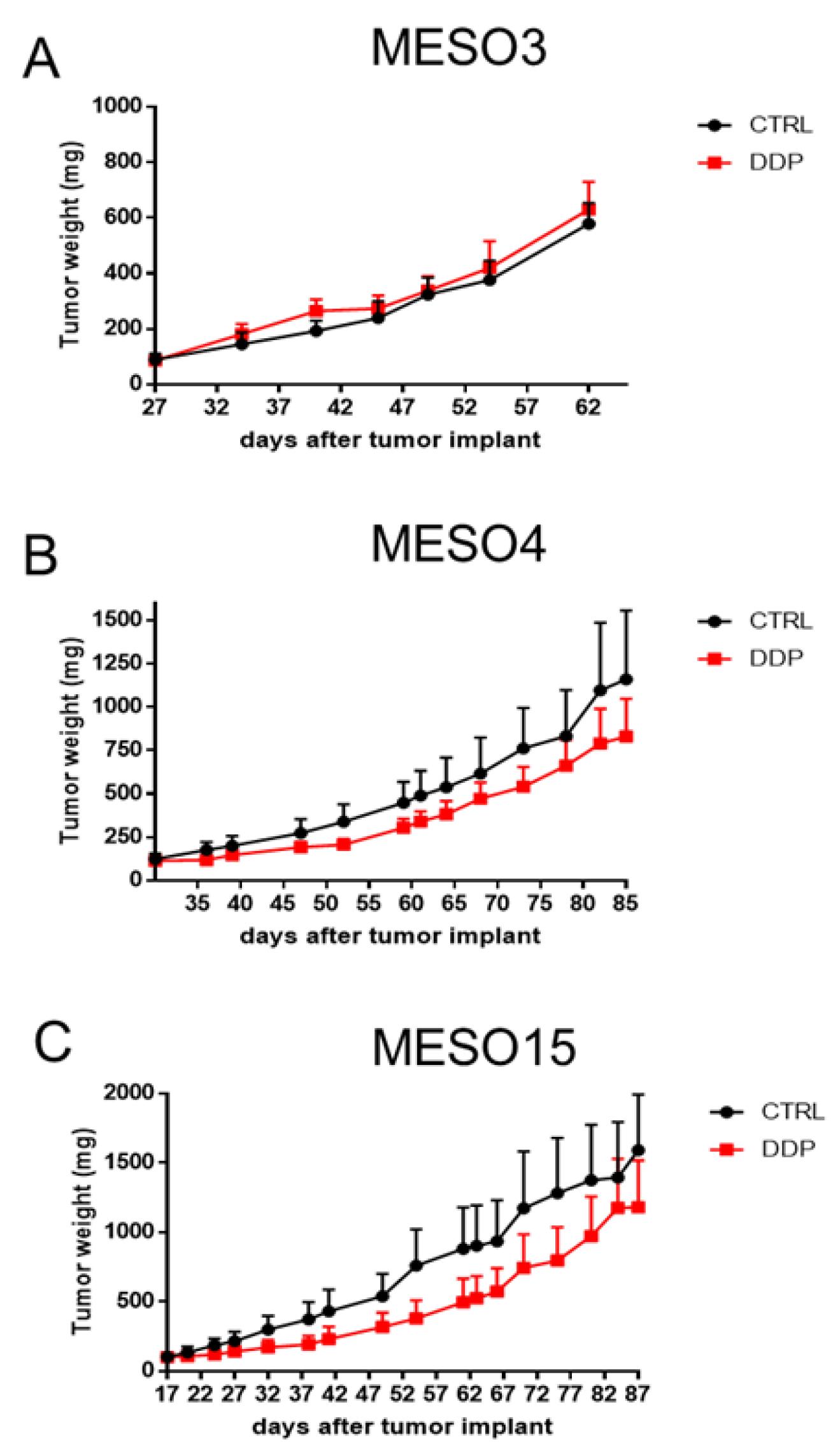
2.3. MPM PDX Response to Cisplatin Treatment
2.4. MPM In Vitro Studies
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Surgical Procedure
4.3. Histopathological Analyses
4.4. Establishment of PDX Models
4.5. In Vivo Studies
4.6. Establishment of PDX Cell Line
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [CrossRef]
- Robinson, B.W.; Musk, A.W.; Lake, R.A. Malignant mesothelioma. Lancet 2005, 366, 397–408. [Google Scholar] [CrossRef]
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell. Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalra, N.; Zhang, J.; Thomas, A.; Xi, L.; Cheung, M.; Talarchek, J.; Burkett, S.; Tsokos, M.G.; Chen, Y.; Raffeld, M.; et al. Mesothelioma patient derived tumor xenografts with defined BAP1 mutations that mimic the molecular characteristics of human malignant mesothelioma. BMC Cancer 2015, 15, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Blanquart, C.; Jaurand, M.-C.; Jean, D. The biology of malignant mesothelioma and the relevance of preclinical models. Front. Oncol. 2020, 10, 388. [Google Scholar] [CrossRef]
- Okada, M.; Kijima, T.; Aoe, K.; Kato, T.; Fujimoto, N.; Nakagawa, K.; Takeda, Y.; Hida, T.; Kanai, K.; Imamura, F.; et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open label, single arm, Japanese phase II study in Malignant Pleural Mesothelioma (MERIT). Clin. Cancer Res. 2019, 25, 5485. [Google Scholar] [CrossRef] [Green Version]
- Attanoos, R.L.; Gibbs, A.R. Pathology of malignant mesothelioma. Histopathology 1997, 30, 403–418. [Google Scholar] [CrossRef]
- Cantini, L.; Pecci, F.; Murrone, A.; Tomasetti, M.; Copparoni, C.; Fiordoliva, I.; Morgese, F.; Rinaldi, S.; Mazzanti, P.; Rubini, C.; et al. Questioning the prognostic role of BAP-1 immunohistochemistry in malignant pleural mesothelioma: A single center experience with systematic review and meta-analysis. Lung Cancer 2020, 146, 318–326. [Google Scholar] [CrossRef]
- Cigognetti, M.; Lonardi, S.; Fisogni, S.; Balzarini, P.; Pellegrini, V.; Tironi, A.; Bercich, L.; Bugatti, M.; Rossi, G.; Murer, B.; et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod. Pathol. 2015, 28, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Hylebos, M.; van Camp, G.; van Meerbeeck, J.P.; Op de Beeck, K. The genetic landscape of malignant pleural mesothelioma: Results from massively parallel sequencing. J. Thorac. Oncol. 2016, 11, 1615–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; de Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Allo, G.; John, T.; Li, M.; Tagawa, T.; Opitz, I.; Anraku, M.; Yun, Z.; Pintilie, M.; Pitcher, B.; et al. Patient-derived xenograft establishment from human malignant pleural mesothelioma. Clin. Cancer Res. 2017, 23, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinausero, M.; Rihawi, K.; Cortiula, F.; Follador, A.; Fasola, G.; Ardizzoni, A. Emerging therapies in malignant pleural mesothelioma. Crit. Rev. Oncol. Hematol. 2019, 144, 102815. [Google Scholar] [CrossRef]
- Di Noia, V.; Vita, E.; Ferrara, M.; Strippoli, A.; Basso, M.; Schinzari, G.; Cassano, A.; Bria, E.; Barone, C.; D’Argento, E. Malignant pleural mesothelioma: Is tailoring the second-line therapy really “raising the bar?”. Curr. Treat. Options Oncol. 2019, 20, 23. [Google Scholar] [CrossRef]
- Kindler, H.L.; Ismaila, N.; Armato, S.G.; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of malignant pleural mesothelioma: American society of clinical oncology clinical practice guideline. JCO 2018, 36, 1343–1373. [Google Scholar] [CrossRef]
- Remon, J.; Nadal, E.; Dómine, M.; Ruffinelli, J.; García, Y.; Pardo, J.C.; López, R.; Cilleruelo, A.; García-Campelo, R.; Martín, P.; et al. Malignant pleural mesothelioma: Treatment patterns and outcomes from the Spanish lung cancer group. Lung Cancer 2020, 147, 83–90. [Google Scholar] [CrossRef]
- Scherpereel, A.; Wallyn, F.; Albelda, S.M.; Munck, C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018, 19, e161–e172. [Google Scholar] [CrossRef]
- Takuwa, T.; Hasegawa, S. Current surgical strategies for malignant pleural mesothelioma. Surg. Today 2016, 46, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Cantini, L.; Belderbos, R.A.; Gooijer, C.J.; Dumoulin, D.W.; Cornelissen, R.; Baart, S.; Burgers, J.A.; Baas, P.; Aerts, J.G.J.V. Nivolumab in pre-treated malignant pleural mesothelioma: Real-world data from the Dutch expanded access program. Transl. Lung Cancer Res. 2020, 9, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- De Gooijer, C.J.; Borm, F.J.; Scherpereel, A.; Baas, P. Immunotherapy in malignant pleural mesothelioma. Front. Oncol. 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dozier, J.; Zheng, H.; Adusumilli, P.S. Immunotherapy for malignant pleural mesothelioma: Current status and future directions. Transl. Lung. Cancer Res. 2017, 6, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Hotta, K.; Fujimoto, N. Current evidence and future perspectives of immune-checkpoint inhibitors in unresectable malignant pleural mesothelioma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Metaxas, Y.; Rivalland, G.; Mauti, L.A.; Klingbiel, D.; Kao, S.; Schmid, S.; Nowak, A.K.; Gautschi, O.; Bartnick, T.; Hughes, B.G.; et al. Pembrolizumab as palliative immunotherapy in malignant pleural mesothelioma. J. Thorac. Oncol. 2018, 13, 1784–1791. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, T.; Kambayashi, Y.; Ohuchi, K.; Muto, Y.; Aiba, S. Treatment of advanced melanoma: Past, present and future. Life 2020, 10, 208. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.; Stollenwerk, H.K.; Klerings, I.; Pecherstorfer, M.; Gartlehner, G.; Singer, J. Efficacy and safety of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC): A systematic literature review. Oncoimmunology 2020, 9, 1774314. [Google Scholar] [CrossRef] [PubMed]
- Kanellakis, N.I.; Asciak, R.; Hamid, M.A.; Yao, X.; McCole, M.; McGowan, S.; Seraia, E.; Hatch, S.; Hallifax, R.J.; Mercer, R.M.; et al. Patient-derived malignant pleural mesothelioma cell cultures: A tool to advance biomarker-driven treatments. Thorax 2020, 75, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Wei, J.; Lin, D.; Collins, C.C.; Gout, P.W.; Wang, Y. Pre-clinical models for malignant mesothelioma research: From chemical-induced to patient-derived cancer xenografts. Front. Genet. 2018, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.R.; Rajan, S.A.P.; Votanopoulos, K.I.; Hall, A.R.; Skardal, A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci. Rep. 2018, 8, 2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkesson, E.; Niederdorfer, B.; Nakstad, V.T.; Thommesen, L.; Klinkenberg, G.; Lægreid, A.; Flobak, Å. High-throughput screening reveals higher synergistic effect of MEK inhibitor combinations in colon cancer spheroids. Sci. Rep. 2020, 10, 11574. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Commun. 2020. [Google Scholar] [CrossRef]
- Saranyutanon, S.; Deshmukh, S.K.; Dasgupta, S.; Pai, S.; Singh, S.; Singh, A.P. Cellular and molecular progression of prostate cancer: Models for basic and preclinical research. Cancers 2020, 12, 2651. [Google Scholar] [CrossRef]
- Mosaad, E.; Chambers, K.; Futrega, K.; Clements, J.; Doran, M.R. Using high throughput microtissue culture to study the difference in prostate cancer cell behavior and drug response in 2D and 3D co-cultures. BMC Cancer 2018, 18, 592. [Google Scholar] [CrossRef]
- Gonçalves, B.Ô.P.; Fialho, S.L.; Silvestrini, B.R.; Sena, I.F.G.; Dos Santos, G.S.P.; Assis Gomes, D.; Silva, L.M. Central nervous system (CNS) tumor cell heterogeneity contributes to differential platinum-based response in an in vitro 2D and 3D cell culture approach. Exp. Mol. Pathol. 2020, 116, 104520. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Sachdev, E.; Tabatabai, R.; Roy, V.; Rimel, B.J.; Mita, M.M. PARP inhibition in cancer: An update on clinical development. Target. Oncol. 2019, 14, 657–679. [Google Scholar] [CrossRef] [PubMed]
- Farago, A.F.; Yeap, B.Y.; Stanzione, M.; Hung, Y.P.; Heist, R.S.; Marcoux, J.P.; Zhong, J.; Rangachari, D.; Barbie, D.A.; Phat, S.; et al. Combination olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019, 9, 1372–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzese, E.; Diana, A.; Centonze, S.; Pignata, S.; De Vita, F.; Ciardiello, F.; Orditura, M. PARP Inhibitors in first-line therapy of ovarian cancer: Are there any doubts? Front. Oncol. 2020, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Rathkey, D.; Khanal, M.; Murai, J.; Zhang, J.; Sengupta, M.; Jiang, Q.; Morrow, B.; Evans, C.N.; Chari, R.; Fetsch, P.; et al. Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but is enhanced by temozolomide in cells with high-schlafen 11 and low-O6-methylguanine-DNA methyltransferase expression. J. Thorac. Oncol. 2020, 15, 843–859. [Google Scholar] [CrossRef]
- Ricci, F.; Bizzaro, F.; Cesca, M.; Guffanti, F.; Ganzinelli, M.; Decio, A.; Ghilardi, C.; Perego, P.; Fruscio, R.; Buda, A.; et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014, 74, 6980–6990. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.; Guffanti, F.; Affatato, R.; Brunelli, L.; Roberta, P.; Fruscio, R.; Perego, P.; Bani, M.R.; Chiorino, G.; Rinaldi, A.; et al. Establishment of patient-derived tumor xenograft models of mucinous ovarian cancer. Am. J. Cancer Res. 2020, 10, 572–580. [Google Scholar]
- Cakiroglu, E.; Senturk, S. Genomics and functional genomics of malignant pleural mesothelioma. Int. J. Mol. Sci. 2020, 21, 6342. [Google Scholar] [CrossRef]
- Oey, H.; Daniels, M.; Relan, V.; Chee, T.M.; Davidson, M.R.; Yang, I.A.; Ellis, J.J.; Fong, K.M.; Krause, L.; Bowman, R.V. Whole-genome sequencing of human malignant mesothelioma tumours and cell lines. Carcinogenesis 2019, 40, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Righi, L.; Koeppen, H.; Zou, W.; Izzo, S.; Grosso, F.; Libener, R.; Loiacono, M.; Monica, V.; Buttigliero, C.; et al. Molecular and histopathological characterization of the tumor immune microenvironment in advanced stage of malignant pleural mesothelioma. J. Thorac. Oncol. 2018, 13, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiola, E.; Salles, D.; Frapolli, R.; Lupi, M.; Rotella, G.; Ronchi, A.; Garassino, M.C.; Mattschas, N.; Colavecchio, S.; Broggini, M.; et al. Base excision repair-mediated resistance to cisplatin in KRAS(G12C) mutant NSCLC cells. Oncotarget 2015, 6, 30072–30087. [Google Scholar] [CrossRef] [Green Version]




| Patient | PDX | |||
|---|---|---|---|---|
| ID | Histology | Positive Marker | Histology | Positive Marker |
| MESO 3 | sarcomatoid | Vimentin, Cytokeratins AE1 and AE3 | sarcomatoid | Vimentin, Calretinin, Ki67 = 60% |
| MESO 4 | biphasic | Calretinin, Vimentin and WT1 (focal) | biphasic | Vimentin, Calretinin, Ki67 = 70% |
| MESO 7 | sarcomatoid | Vimentin, Cytokeratins AE1 and AE3 and WT1 (focal) | sarcomatoid | Vimentin, Calretinin, Ki67 = 60% |
| MESO 15 | epithelioid | Calretinin, WT1 (focal) and Vimentin | epithelioid | Vimentin, Calretinin, Ki67 = 40% |
| Doubling Time (Days) | |||
|---|---|---|---|
| In Vivo Passages | MESO3 | MESO4 | MESO15 |
| I | 16.0 | 43.1 | 23.6 |
| II | − | 12.6 | 10.0 |
| III | 7.7 | 14.1 | 10.4 |
| IV | 7.5 | 13.6 | 14.3 |
| V | − | − | 13.3 |
| ID | Histotype | T/C % (Day) |
|---|---|---|
| MESO3 | Sarcomatoid | 104 (49) |
| MESO4 | Biphasic | 61 (52) |
| MESO15 | Epithelioid | 50 (54) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affatato, R.; Mendogni, P.; Del Gobbo, A.; Ferrero, S.; Ricci, F.; Broggini, M.; Rosso, L. Establishment and Characterization of Patient-Derived Xenografts (PDXs) of Different Histology from Malignant Pleural Mesothelioma Patients. Cancers 2020, 12, 3846. https://doi.org/10.3390/cancers12123846
Affatato R, Mendogni P, Del Gobbo A, Ferrero S, Ricci F, Broggini M, Rosso L. Establishment and Characterization of Patient-Derived Xenografts (PDXs) of Different Histology from Malignant Pleural Mesothelioma Patients. Cancers. 2020; 12(12):3846. https://doi.org/10.3390/cancers12123846
Chicago/Turabian StyleAffatato, Roberta, Paolo Mendogni, Alessandro Del Gobbo, Stefano Ferrero, Francesca Ricci, Massimo Broggini, and Lorenzo Rosso. 2020. "Establishment and Characterization of Patient-Derived Xenografts (PDXs) of Different Histology from Malignant Pleural Mesothelioma Patients" Cancers 12, no. 12: 3846. https://doi.org/10.3390/cancers12123846
APA StyleAffatato, R., Mendogni, P., Del Gobbo, A., Ferrero, S., Ricci, F., Broggini, M., & Rosso, L. (2020). Establishment and Characterization of Patient-Derived Xenografts (PDXs) of Different Histology from Malignant Pleural Mesothelioma Patients. Cancers, 12(12), 3846. https://doi.org/10.3390/cancers12123846








