Endoscopic Surveillance for Metachronous Esophageal Squamous Cell Neoplasms among Head and Neck Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
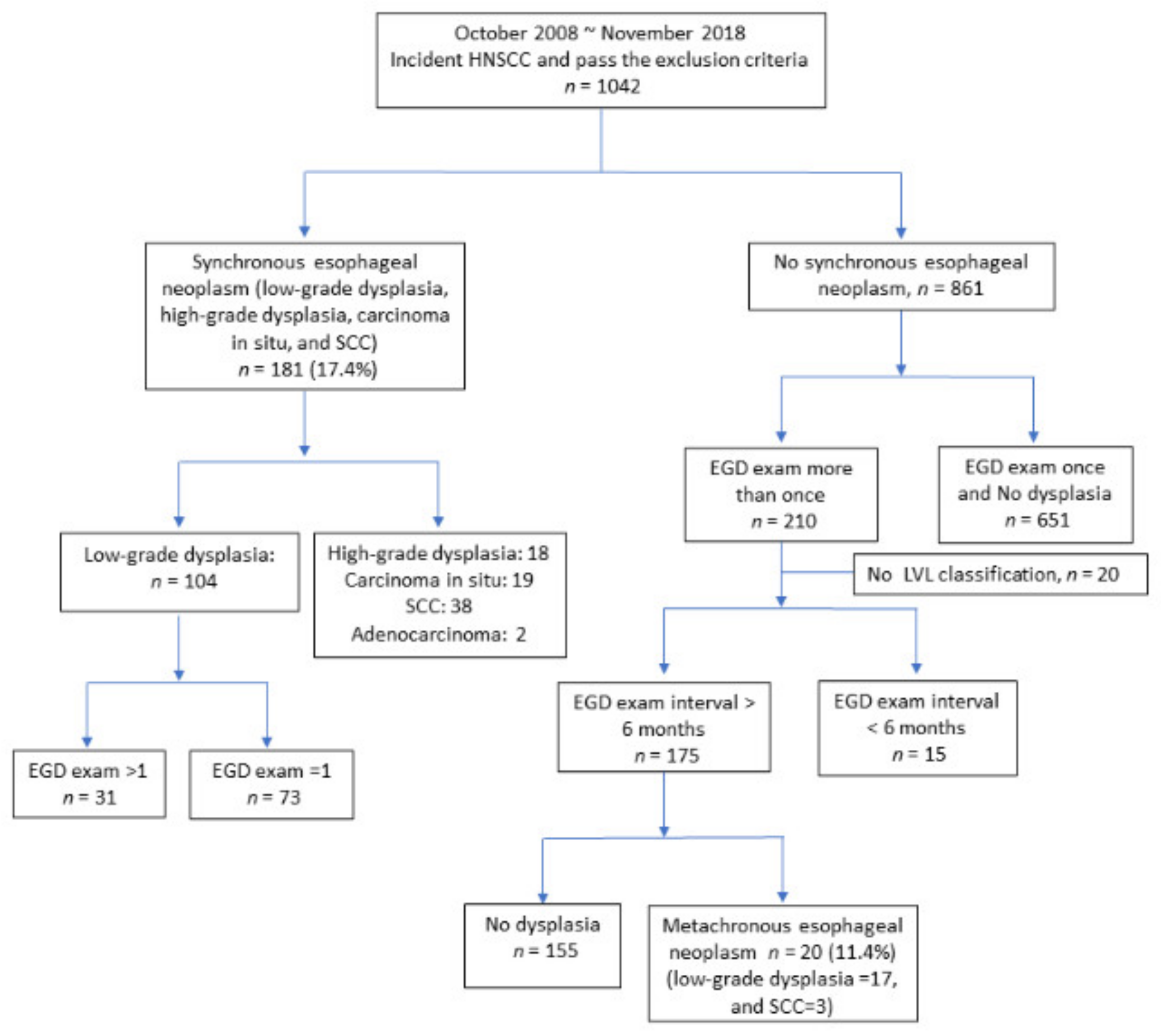
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamamoto, E.; Shibuya, H.; Yoshimura, R.; Miura, M. Site specific dependency of second primary cancer in early stage head and neck squamous cell carcinoma. Cancer 2002, 94, 2007–2014. [Google Scholar] [CrossRef]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Chung, C.S.; Lo, W.C.; Lee, Y.C.; Wu, M.S.; Wang, H.P.; Liao, L.J. Image-enhanced endoscopy for detection of second primary neoplasm in patients with esophageal and head and neck cancer: A systematic review and meta-analysis. Head Neck 2016, 38 (Suppl. S1), E2343–E2349. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Kim, D.H.; Ahn, J.Y.; Choi, K.S.; Jung, K.W.; Lee, J.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; Jung, H.Y.; et al. Routine endoscopic screening for synchronous esophageal neoplasm in patients with head and neck squamous cell carcinoma: A prospective study. Dis. Esophagus 2016, 29, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Lee, C.T.; Lee, Y.C.; Hwang, T.Z.; Wang, C.C.; Hwang, J.C.; Tai, C.M.; Chang, C.Y.; Tsai, S.S.; Wang, C.P.; et al. Risk factors for developing synchronous esophageal neoplasia in patients with head and neck cancer. Head Neck 2011, 33, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Chuang, Y.S.; Wu, T.S.; Lee, K.W.; Wu, C.W.; Wang, H.C.; Kuo, C.T.; Lee, C.H.; Kuo, W.R.; Chen, C.H.; et al. Endoscopic screening for synchronous esophageal neoplasia among patients with incident head and neck cancer: Prevalence, risk factors, and outcomes. Int. J. Cancer 2017, 141, 1987–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.; Kim, D.H.; Jung, H.Y.; Gong, E.J.; Na, H.K.; Ahn, J.Y.; Kim, M.Y.; Lee, J.H.; Choi, K.S.; Choi, K.D.; et al. Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver 2015, 9, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, M.; Hironaka, S.; Nakane, M.; Boku, N.; Ohtsu, A.; Yoshida, S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest. Endosc. 2002, 56, 517–521. [Google Scholar] [CrossRef]
- Fukuhara, T.; Hiyama, T.; Tanaka, S.; Oka, S.; Yoshihara, M.; Arihiro, K.; Chayama, K. Characteristics of esophageal squamous cell carcinomas and lugol-voiding lesions in patients with head and neck squamous cell carcinoma. J. Clin. Gastroenterol. 2010, 44, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Nishiyama, K.; Nakamura, S.; Suzuki, O.; Kawaguchi, Y.; Nakajima, A.; Imai, A.; Ishihara, R.; Uemura, H.; Fujii, T.; et al. Significance of endoscopic screening and endoscopic resection for esophageal cancer in patients with hypopharyngeal cancer. Jpn. J. Clin. Oncol. 2010, 40, 938–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.D.; Wang, T.Y.; Lu, C.H.; Huang, C.E.; Chen, M.C. The bidirectional association between oral cancer and esophageal cancer: A population-based study in Taiwan over a 28-year period. Oncotarget 2017, 8, 44567–44578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, M.; Kumashiro, R.; Kubo, N.; Nakashima, Y.; Yoshida, R.; Yoshinaga, K.; Saeki, H.; Emi, Y.; Kakeji, Y.; Sakaguchi, Y.; et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: Epidemiology, clinical findings, and prevention. Int J. Clin. Oncol. 2010, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Shinohara, S.; Takebayashi, S.; Kikuchi, M.; Fujiwara, K.; Michida, T.; Yamamoto, R.; Hayashi, K.; Saida, K.; Naito, Y. Facial flushing after alcohol intake as a predictor for a high risk of synchronous or metachronous cancer of the upper gastrointestinal tract. Jpn. J. Clin. Oncol. 2017, 47, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Ishihara, R.; Morishima, T.; Maekawa, A.; Nakagawa, K.; Arao, M.; Ohmori, M.; Iwagami, H.; Matsuno, K.; Inoue, S.; et al. Impact of age at diagnosis of head and neck cancer on incidence of metachronous cancer. BMC Cancer 2019, 19, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.M.; Wang, H.H.; Lee, C.T.; Tai, C.M.; Tseng, C.H.; Chen, C.C.; Tsai, Y.N.; Chen, T.H.; Hsu, M.H.; Wang, C.C.; et al. A nationwide population based study to access the risk of metachronous esophageal cancers in head and neck cancer survivors. Sci. Rep. 2020, 10, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, F.H.A.; Bernardo, W.M.; Ide, E.; Rocha, R.S.P.; Aquino, J.C.M.; Minata, M.K.; Yamazaki, K.; Marques, S.B.; Sakai, P.; de Moura, E.G.H. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: A systematic review and meta-analysis. BMC Cancer 2017, 17, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruner, M.; Denis, A.; Masliah, C.; Amil, M.; Metivier, E.; Kaassis, M.; Coron, E.; Le Rhun, M.; Lecleire, S.; Antonietti, M.; et al. Narrow-band imaging versus Lugol chromoendoscopy for esophageal squamous cell cancer screening in normal endoscopic practice: Randomized controlled trial. Endoscopy 2020. [Google Scholar] [CrossRef] [PubMed]
- Rennemo, E.; Zätterström, U.; Boysen, M. Impact of second primary tumors on survival in head and neck Cancer: An analysis of 2063 cases. Laryngoscope 2008, 118, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, W.; Hubert, C.; Chen, P.; Lee, K. Impact of second primary esophageal or lung cancer on survival of patients with head and neck cancer. Oral Oncol. 2010, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]


| Items | Total | No Dysplasia | Metachronous ESCN | p Values |
|---|---|---|---|---|
| (n = 175) | (n = 155) | (n = 20) | ||
| No. (%) | No. (%) | No. (%) | ||
| Age (years) | 55.21 ± 9.43 | 55.19 ± 9.45 | 55.40 ± 9.49 | 0.92 |
| Cigarette smoking | 0.66 | |||
| No | 25 (14.29%) | 21 (13.55%) | 4 (20.00%) | |
| Yes (Former/Current) | 150 (85.71%) | 134 (86.45%) | 16 (80.00%) | |
| Betel nut chewing | 0.87 | |||
| No | 33 (18.86%) | 30 (19.35%) | 3 (15.00%) | |
| Yes (Former/Current) | 142 (81.14%) | 125 (80.65%) | 17 (85.00%) | |
| Alcohol drinking | 0.47 | |||
| No | 42 (24.00%) | 39 (25.16%) | 3 (15.00%) | |
| Yes (Former/Current) | 133 (76.00%) | 116 (74.84%) | 17 (85.00%) | |
| Alcohol flush | 0.77 | |||
| No | 64 (36.57%) | 58 (37.42%) | 6 (30.00%) | |
| Yes | 108 (61.71%) | 95 (61.29%) | 13 (65.00%) | |
| Missing | 3 (1.71%) | 2 (1.29%) | 1 (5.00%) | |
| Stage | 0.53 | |||
| I | 35 (20.00%) | 31 (20.00%) | 4 (20.00%) | |
| II | 22 (12.57%) | 21 (13.55%) | 1 (5.00%) | |
| III | 36 (20.57%) | 33 (21.29%) | 3 (15.00%) | |
| IV | 82 (46.86%) | 70 (45.16%) | 12 (60.00%) | |
| Location | 0.15 | |||
| Oral | 115 (65.71%) | 106 (68.39%) | 9 (45.00%) | |
| Pharyngeal | 32 (18.29%) | 26 (16.77%) | 6 (30.00%) | |
| Hypopharyngeal | 25 (14.29%) | 20 (12.90%) | 5 (25.00%) | |
| Laryngeal | 3 (1.71%) | 3 (1.94%) | (0.00%) | |
| LVL classification | 0.001 | |||
| A + B | 134 (76.57%) | 125 (80.65%) | 9 (45.00%) | |
| C + D | 41 (23.43%) | 30 (19.35%) | 11 (55.00%) |
| Items | Total | No Dysplasia | Metachronous ESCN | Logistic Regression | ||
|---|---|---|---|---|---|---|
| (n = 155) | (n = 20) | |||||
| No. (%) | No. (%) | No. (%) | cOR (95% CI) | aOR1 (95% CI) | p Value | |
| Cigarette smoking | ||||||
| No | 25 (14.29%) | 21 (13.55%) | 4 (20.00%) | 1 | 1 | |
| Yes (Former/Current) | 150 (85.71%) | 134 (86.45%) | 16 (80.00%) | 0.63 (0.19, 2.06) | 0.24 (0.05, 1.06) | 0.06 |
| Betel nut chewing | ||||||
| No | 33 (18.86%) | 30 (19.35%) | 3 (15.00%) | 1 | 1 | |
| Yes (Former/Current) | 142 (81.14%) | 125 (80.65%) | 17 (85.00%) | 1.36 (0.37, 4.94) | 1.61 (0.35, 7.42) | 0.54 |
| Alcohol drinking | ||||||
| No | 42 (24.00%) | 39 (25.16%) | 3 (15.00%) | 1 | 1 | |
| Yes (Former/Current) | 133 (76.00%) | 116 (74.84%) | 17 (85.00%) | 1.91 (0.53, 6.85) | 0.98 (0.22, 4.39) | 0.98 |
| Alcohol flush | ||||||
| No | 64 (36.57%) | 58 (37.42%) | 6 (30.00%) | 1 | 1 | |
| Yes | 108 (61.71%) | 95 (61.29%) | 13 (65.00%) | 1.32 (0.48, 3.67) | 1.66 (0.52, 5.27) | 0.39 |
| Missing | 3 (1.71%) | 2 (1.29%) | 1 (5.00%) | - | ||
| Stage | ||||||
| I | 35 (20.00%) | 31 (20.00%) | 4 (20.00%) | 1 | 1 | |
| II | 22 (12.57%) | 21 (13.55%) | 1 (5.00%) | 0.37 (0.04, 3.54) | 0.25 (0.02, 3.06) | 0.28 |
| III | 36 (20.57%) | 33 (21.29%) | 3 (15.00%) | 0.71 (0.15, 3.40) | 0.38 (0.06, 2.34) | 0.3 |
| IV | 82 (46.86%) | 70 (45.16%) | 12 (60.00%) | 1.33 (0.40, 4.45) | 0.81 (0.19, 3.49) | 0.78 |
| Location | ||||||
| Oral | 115 (65.71%) | 106 (68.39%) | 9 (45.00%) | 1 | 1 | |
| Pharyngeal | 32 (18.29%) | 26 (16.77%) | 6 (30.00%) | 2.72 (0.89, 8.32) | 2.25 (0.57, 8.90) | 0.25 |
| Hypopharyngeal | 25 (14.29%) | 20 (12.90%) | 5 (25.00%) | 2.94 (0.89, 9.71) | 2.41 (0.54, 10.69) | 0.25 |
| Laryngeal | 3 (1.71%) | 3 (1.94%) | 0 (0.00%) | - | - | |
| LVL classification | ||||||
| A + B | 134 (76.57%) | 125 (80.65%) | 9 (45.00%) | 1 | 1 | |
| C + D | 41 (23.43%) | 30 (19.35%) | 11 (55.00%) | 5.09 (1.94, 13.39) | 5.03 (1.52, 16.67) | 0.0083 |
| Age (Years) | HNSCC Location | LVL Classification | Metachronous ESCN | Interval (Months) |
|---|---|---|---|---|
| 43 | Oropharynx | A | Low-grade dysplasia | 33 |
| 57 | Oral cavity | A | Low-grade dysplasia | 10 |
| 67 | Hypopharynx | A | Low-grade dysplasia | 44 |
| 51 | Hypopharynx | A | Low-grade dysplasia | 36 |
| 53 | Oral cavity | B | Low-grade dysplasia | 16 |
| 80 | Oral cavity | B | Low-grade dysplasia | 26 |
| 53 | Oral cavity | B | Low-grade dysplasia | 20 |
| 49 | Hypopharynx | B | Low-grade dysplasia | 22 |
| 55 | Oral cavity | B | Low-grade dysplasia | 10 |
| 55 | Oral cavity | C | Low-grade dysplasia | 92 |
| 56 | Oral cavity | C | Low-grade dysplasia | 73 |
| 46 | Oropharynx | C | Low-grade dysplasia | 53 |
| 50 | Oral cavity | C | Low-grade dysplasia | 54 |
| 63 | Oropharynx | C | Low-grade dysplasia | 11 |
| 71 | Hypopharynx | C | SCC | 53 |
| 52 | Hypopharynx | D | Low-grade dysplasia | 53 |
| 52 | Oropharynx | D | Low-grade dysplasia | 31 |
| 55 | Oropharynx | D | Low-grade dysplasia | 35 |
| 39 | Oropharynx | D | SCC | 27 |
| 61 | Oral cavity | D | SCC | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Wang, Y.-K.; Chuang, Y.-S.; Hsu, W.-H.; Kuo, C.-H.; Wu, C.-W.; Chan, L.-P.; Wu, M.-T.; Wu, I.-C. Endoscopic Surveillance for Metachronous Esophageal Squamous Cell Neoplasms among Head and Neck Cancer Patients. Cancers 2020, 12, 3832. https://doi.org/10.3390/cancers12123832
Chen Y-H, Wang Y-K, Chuang Y-S, Hsu W-H, Kuo C-H, Wu C-W, Chan L-P, Wu M-T, Wu I-C. Endoscopic Surveillance for Metachronous Esophageal Squamous Cell Neoplasms among Head and Neck Cancer Patients. Cancers. 2020; 12(12):3832. https://doi.org/10.3390/cancers12123832
Chicago/Turabian StyleChen, Yi-Hsun, Yao-Kuang Wang, Yun-Shiuan Chuang, Wen-Hung Hsu, Chao-Hung Kuo, Che-Wei Wu, Leong-Perng Chan, Ming-Tsang Wu, and I-Chen Wu. 2020. "Endoscopic Surveillance for Metachronous Esophageal Squamous Cell Neoplasms among Head and Neck Cancer Patients" Cancers 12, no. 12: 3832. https://doi.org/10.3390/cancers12123832
APA StyleChen, Y.-H., Wang, Y.-K., Chuang, Y.-S., Hsu, W.-H., Kuo, C.-H., Wu, C.-W., Chan, L.-P., Wu, M.-T., & Wu, I.-C. (2020). Endoscopic Surveillance for Metachronous Esophageal Squamous Cell Neoplasms among Head and Neck Cancer Patients. Cancers, 12(12), 3832. https://doi.org/10.3390/cancers12123832





