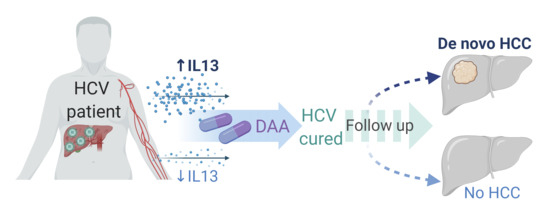
Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Multiplex Assay
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. WJG 2016, 22, 7824–7840. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, V.; Craxi, A. Hepatocellular carcinoma and direct-acting antivirals: A never ending story? Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37, 812–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahon, P.; Layese, R.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; de Ledinghen, V.; Ouzan, D.; et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in Surveillance Programs. Gastroenterology 2018, 155, 1436–1450.e6. [Google Scholar] [CrossRef] [Green Version]
- Macek Jilkova, Z.; Afzal, S.; Marche, H.; Decaens, T.; Sturm, N.; Jouvin-Marche, E.; Huard, B.; Marche, P.N. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17(+) neutrophils. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 1116–1124. [Google Scholar] [CrossRef]
- Jouvin-Marche, E.; Macek Jilkova, Z.; Thelu, M.A.; Marche, H.; Fugier, E.; Van Campenhout, N.; Hoang, X.S.; Marlu, A.; Sturm, N.; Callanan, M.; et al. Lymphocytes degranulation in liver in hepatitis C virus carriers is associated with IFNL4 polymorphisms and ALT levels. J. Infect. Dis. 2014, 209, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Fugier, E.; Marche, H.; Thelu, M.A.; Macek Jilkova, Z.; Van Campenhout, N.; Dufeu-Duchesne, T.; Leroy, V.; Zarski, J.P.; Sturm, N.; Marche, P.N.; et al. Functions of liver natural killer cells are dependent on the severity of liver inflammation and fibrosis in chronic hepatitis C. PLoS ONE 2014, 9, e95614. [Google Scholar] [CrossRef]
- Strunz, B.; Hengst, J.; Deterding, K.; Manns, M.P.; Cornberg, M.; Ljunggren, H.G.; Wedemeyer, H.; Bjorkstrom, N.K. Chronic hepatitis C virus infection irreversibly impacts human natural killer cell repertoire diversity. Nat. Commun. 2018, 9, 2275. [Google Scholar] [CrossRef]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.R.; Li, J.H.; Zhang, R.; Chen, R.X.; Wang, Y.H. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J. Surg. Oncol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budhu, A.; Wang, X.W. The role of cytokines in hepatocellular carcinoma. J. Leukoc. Biol. 2006, 80, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, J.; Liu, H.; Wan, L.; Zhang, H.; Huang, Q.; Xu, E.; Lai, M. IL-13/STAT6 signaling plays a critical role in the epithelial-mesenchymal transition of colorectal cancer cells. Oncotarget 2016, 7, 61183–61198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef] [PubMed]
- El-Derany, M.O. Polymorphisms in Interleukin 13 signaling and interacting genes predict advanced fibrosis and hepatocellular carcinoma development in non-alcoholic steatohepatitis. Biology 2020, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Yamashita, T.; Terashima, T.; Suda, T.; Okada, H.; Asahina, Y.; Hayashi, T.; Hara, Y.; Nio, K.; Sunagozaka, H.; et al. Serum cytokine profiles predict survival benefits in patients with advanced hepatocellular carcinoma treated with sorafenib: A retrospective cohort study. BMC Cancer 2017, 17, 870. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.D.; Park, S.; Jeong, S.; Lee, Y.J.; Lee, H.; Kim, C.G.; Kim, K.H.; Hong, S.M.; Lee, J.Y.; Kim, S.; et al. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8(+) T Cells in hepatocellular carcinoma. Hepatology 2020, 71, 955–971. [Google Scholar] [CrossRef] [Green Version]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a key predictor of de novo or recurrent hepatocellular cancer after hepatitis C Virus direct-acting antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef] [Green Version]
- Turati, F.; Edefonti, V.; Talamini, R.; Ferraroni, M.; Malvezzi, M.; Bravi, F.; Franceschi, S.; Montella, M.; Polesel, J.; Zucchetto, A.; et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology 2012, 55, 1416–1425. [Google Scholar] [CrossRef]
- Lonardo, A.; Lugari, S.; Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Maurantonio, M. A round trip from nonalcoholic fatty liver disease to diabetes: Molecular targets to the rescue? Acta Diabetol. 2019, 56, 385–396. [Google Scholar] [CrossRef] [PubMed]

| At Start of Treatment | DAA-HCV→HCC (n = 11) | DAA-HCV (n = 18) | p Value |
|---|---|---|---|
| Age (years) | 57.6 ± 5.6 | 57.2 ± 4.8 | 0.8475 |
| Sex (number, % men) | 11, 100% | 18, 100% | >0.999 |
| Fibrosis stage F3–F4/F4 | 0/11 | 2/16 | 0.5123 |
| Platelet count * | 113 ± 50 | 121 ± 48 | 0.6487 |
| AFP (ng/mL) * | 14.7 ± 9.3; 11.5 (4–36) | 20.8 ± 20; 13.8 (7–94) | 0.3795 |
| OPN (ng/mL) * | 60.9 ± 51.9; 54 (24–211) | 47.5 ± 20.7; 44 (19–103) | 0.7993 |
| GGT (UL/mL) * | 200 ± 158; 151 (81–573) | 237 ± 207; 118 (60–646) | 0.5864 |
| AST (UL/mL) * | 112 ± 72; 101 (50–314) | 113 ± 58; 95 (31–236) | 0.8251 |
| ALT (UL/mL) * | 149 ± 150; 86 (50–577) | 124 ± 88; 100 (40–346) | 0.7996 |
| Body weight (kg) | 74.2 ± 9.8 | 80.1 ± 13.7 | 0.2241 |
| BMI | 24.5 ± 2.7 | 27.2 ± 4.5 | 0.0818 |
| History of diabetes (number, %) | 2, 18.2% | 4, 22.2% | >0.999 |
| Alcohol consumption (number, %) | 7, 63.6% | 9, 50% | 0.2430 |
| Tobacco smoking (number, %) | 7, 63.6% | 11, 61.1% | >0.999 |
| HCV Genotype 1/3/4 | 6/4/1 | 11/6/1 | >0.852 |
| HBV co-infection (number, %) | 6, 54.5% | 55.6% | >0.959 |
| Treatment outcome | |||
| SVR (number, %) | 11, 100% | 18, 100% | >0.999 |
| HCC development | |||
| Start of DAA treatment to HCC (months) * | 19.9 ± 10.3; 15.8 [13.6–40.5] | - | |
| Number of tumor 1/2 | 8/3 | - | |
| Size of biggest tumor (mm) * | 24.2 ± 13.6; 18 [12–52] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macek Jílková, Z.; Seigneurin, A.; Coppard, C.; Ouaguia, L.; Aspord, C.; Marche, P.N.; Leroy, V.; Decaens, T. Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals. Cancers 2020, 12, 3820. https://doi.org/10.3390/cancers12123820
Macek Jílková Z, Seigneurin A, Coppard C, Ouaguia L, Aspord C, Marche PN, Leroy V, Decaens T. Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals. Cancers. 2020; 12(12):3820. https://doi.org/10.3390/cancers12123820
Chicago/Turabian StyleMacek Jílková, Zuzana, Arnaud Seigneurin, Celine Coppard, Laurissa Ouaguia, Caroline Aspord, Patrice N. Marche, Vincent Leroy, and Thomas Decaens. 2020. "Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals" Cancers 12, no. 12: 3820. https://doi.org/10.3390/cancers12123820
APA StyleMacek Jílková, Z., Seigneurin, A., Coppard, C., Ouaguia, L., Aspord, C., Marche, P. N., Leroy, V., & Decaens, T. (2020). Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals. Cancers, 12(12), 3820. https://doi.org/10.3390/cancers12123820