Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patient Cohort and Genetic Testing
2.2. HDGC Criteria
2.3. Helicobacter pylori (HP) Infection
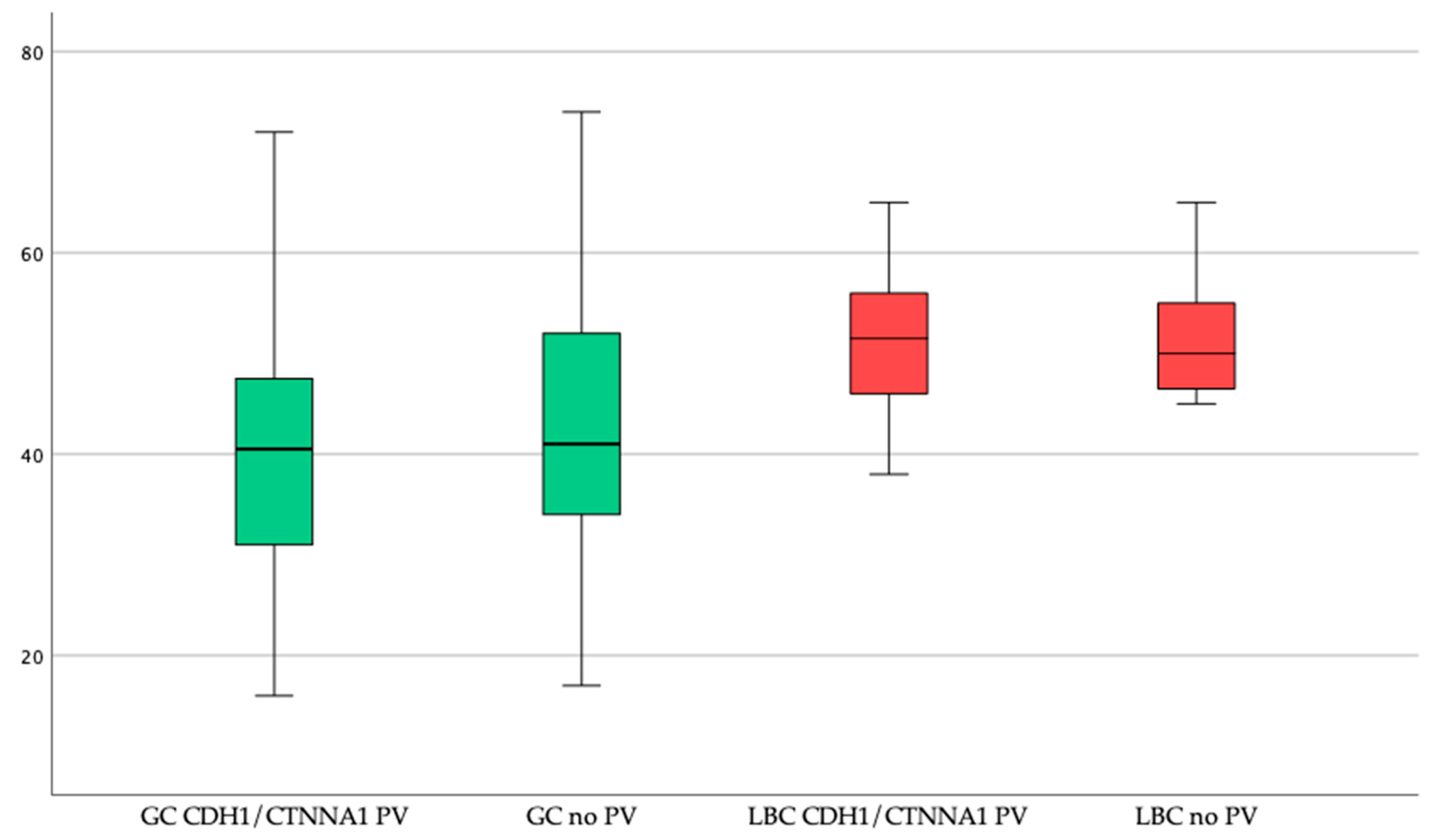
2.4. Patients with CDH1 or CTNNA1 PV
2.4.1. Gastric Cancer
2.4.2. Breast Cancer
2.5. Patients without a Proven CDH1 or CTNNA1 PV
2.5.1. Gastric Cancer
2.5.2. Breast Cancer
2.6. Comparison of PV Carriers with Patients without a PV
2.7. Patients with a CDH1 VUS
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Genetic Testing
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberts:, S.R.; Cervantes, A.; van de Velde, C.J.H. Gastric cancer: Epidemiology, pathology and treatment. Ann. Oncol. 2003, 4, ii31–ii36. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Seruca, R.; Carneiro, F. Genetics, pathology, and clinics of familial gastric cancer. Int. J. Surg. Pathol. 2006, 14, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Caldas, C.; Carneiro, F.; Lynch, H.T.; Yokota, J.; Wiesner, G.L.; Powell, S.M.; Lewis, F.R.; Huntsman, D.G.; Pharoah, P.D.P.; Jankowski, J.A.; et al. Familial gastric cancer: Overview and guidelines for management. J. Med. Genet. 1999, 36, 873–880. [Google Scholar] [PubMed]
- Fitzgerald, R.C.; Hardwick, R.; Huntsman, D.; Carneiro, F.; Guilford, P.; Blair, V.; Chung, D.C.; Norton, J.; Ragunath, K.; Van Krieken, J.H.; et al. Hereditary diffuse gastric cancer: Updated consensus guidelines for clinical management and directions for future research. J. Med. Genet. 2010, 47, 436–444. [Google Scholar] [CrossRef] [Green Version]
- van der Post, R.S.; Vogelaar, I.P.; Carneiro, F.; Guilford, P.; Huntsman, D.; Hoogerbrugge, N.; Caldas, C.; Chelcun Schreiber, K.E.; Hardwick, R.H.; Ausems, M.G.E.M.; et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J. Med. Genet. 2015, 52, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; Van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; Van Der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef]
- Van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef]
- Oliveira, C.; Sousa, S.; Pinheiro, H.; Karam, R.; Bordeira-Carriço, R.; Senz, J.; Kaurah, P.; Carvalho, J.; Pereira, R.; Gusmão, L.; et al. Quantification of Epigenetic and Genetic 2nd Hits in CDH1 During Hereditary Diffuse Gastric Cancer Syndrome Progression. Gastroenterology 2009, 136, 2137–2148. [Google Scholar] [CrossRef]
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; Schrader, K.A.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015, 1, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.E.; Ranola, J.M.O.; Marshall, M.L.; Susswein, L.R.; Graceffo, S.; Bohnert, K.; Tsai, G.; Klein, R.T.; Hruska, K.S.; Shirts, B.H. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol. 2019, 5, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Xicola, R.M.; Li, S.; Rodriguez, N.; Reinecke, P.; Karam, R.; Speare, V.; Black, M.H.; Laduca, H.; Llor, X. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J. Med. Genet. 2019, 56, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, J.; Melo, S.; Carneiro, P.; Moreira, A.M.; Fernandes, M.S.; Ribeiro, A.S.; Guilford, P.; Paredes, J.; Seruca, R. Clinical spectrum and pleiotropic nature of CDH1 germline mutations. J. Med. Genet. 2019, 56, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kievit, A.; Tessadori, F.; Douben, H.; Jordens, I.; Maurice, M.; Hoogeboom, J.; Hennekam, R.; Nampoothiri, S.; Kayserili, H.; Castori, M.; et al. Variants in members of the cadherin-catenin complex, CDH1 and CTNND1, cause blepharocheilodontic syndrome /692/699 /631/208/1516 article. Eur. J. Hum. Genet. 2018, 26, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Guilford, P.J.; Hopkins, J.B.W.; Grady, W.M.; Markowitz, S.D.; Willis, J.; Lynch, H.; Rajput, A.; Wiesner, G.L.; Lindor, N.M.; Burgart, L.J.; et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum. Mutat. 1999, 14, 249–255. [Google Scholar] [CrossRef]
- Norton, J.A.; Ham, C.M.; Dam, J.; Van Jeffrey, R.B.; Longacre, T.A.; Huntsman, D.G.; Chun, N.; Kurian, A.W.; Ford, J.M. CDH1 truncating mutations in the E-cadherin gene: An indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann. Surg. 2007, 245, 873–879. [Google Scholar] [CrossRef]
- Hebbard, P.C.; MacMillan, A.; Huntsman, D.; Kaurah, P.; Carneiro, F.; Wen, X.; Kwan, A.; Boone, D.; Bursey, F.; Green, J.; et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): The Newfoundland experience with 23 patients. Ann. Surg. Oncol. 2009, 16, 1890–1895. [Google Scholar] [CrossRef]
- van der Post, R.S.; Oliveira, C.; Guilford, P.; Carneiro, F. Hereditary gastric cancer: What’s new? Update 2013–2018. Fam. Cancer 2019, 18, 363–367. [Google Scholar] [CrossRef]
- Kumar, S.; Katona, B.W.; Long, J.M.; Domchek, S.; Rustgi, A.K.; Roses, R.; Ginsberg, G.G. Endoscopic Ultrasound Has Limited Utility in Diagnosis of Gastric Cancer in Carriers of CDH1 Mutations. Clin. Gastroenterol. Hepatol. 2020, 18, 505–508.e1. [Google Scholar] [CrossRef]
- Ruff, S.; Curtin, B.; Quezado, M.; Heller, T.; Koh, C.; Steinberg, S.M.; Connolly, M.; Hernandez, J.M.; Davis, J.L. Evaluation of confocal endoscopic microscopy for detection of early-stage gastric cancer in hereditary diffuse gastric cancer (HDGC) syndrome. J. Gastrointest. Oncol. 2019, 10, 407–411. [Google Scholar] [CrossRef]
- Hüneburg, R.; Marwitz, T.; van Heteren, P.; Weismüller, T.; Trebicka, J.; Adam, R.; Aretz, S.; Perez Bouza, A.; Pantelis, D.; Kalff, J.; et al. Chromoendoscopy in combination with random biopsies does not improve detection of gastric cancer foci in CDH1 mutation positive patients. Endosc. Int. Open 2016, 4, E1305–E1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Long, J.M.; Ginsberg, G.G.; Katona, B.W. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J. Gastroenterol. 2019, 25, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Di Pietro, M.; O’Donovan, M.; Richardson, S.; Debiram, I.; Dwerryhouse, S.; Hardwick, R.H.; Tischkowitz, M.; Caldas, C.; Ragunath, K.; et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest. Endosc. 2014, 80, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Lobo, J.; Pita, I.; Videira, F.; Pedro-Afonso, L.; Dinis-Ribeiro, M.; Brandão, C. Random biopsies in patients harboring a CDH1 mutation: Time to change the approach? Rev. Esp. Enferm. Dig. 2020, 112. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Guidelines for Men and Women with CDH1 Mutations. Available online: https://www.facingourrisk.org/understanding-brca-and-hboc/information/risk-management/introduction/basics/guidelines_for_cdh1.php (accessed on 16 August 2020).
- Majewski, I.J.; Kluijt, I.; Cats, A.; Scerri, T.S.; De Jong, D.; Kluin, R.J.C.; Hansford, S.; Hogervorst, F.B.L.; Bosma, A.J.; Hofland, I.; et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J. Pathol. 2013, 229, 621–629. [Google Scholar] [CrossRef]
- Weren, R.D.A.; Van Der Post, R.S.; Vogelaar, I.P.; Han Van Krieken, J.; Spruijt, L.; Lubinski, J.; Jakubowska, A.; Teodorczyk, U.; Aalfs, C.M.; Van Hest, L.P.; et al. Role of germline aberrations affecting CTNNA1, MAP3K6 and MYD88 in gastric cancer susceptibility. J. Med. Genet. 2018, 55, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.F.; Michalski, S.T.; Tondon, R.; Nehoray, B.; Ebrahimzadeh, J.; Hughes, S.K.; Soper, E.R.; Domchek, S.M.; Rustgi, A.K.; Pineda-Alvarez, D.; et al. Loss-of-function variants in CTNNA1 detected on multigene panel testing in individuals with gastric or breast cancer. Genet. Med. 2020, 22, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Benusiglio, P.R.; Colas, C.; Guillerm, E.; Canard, A.; Delhomelle, H.; Warcoin, M.; Bellanger, J.; Eyries, M.; Zizi, M.; Netter, J.; et al. Clinical implications of CTNNA1 germline mutations in asymptomatic carriers. Gastric Cancer 2019, 22, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Lowstuter, K.; Espenschied, C.R.; Sturgeon, D.; Ricker, C.; Karam, R.; LaDuca, H.; Culver, J.O.; Dolinsky, J.S.; Chao, E.; Sturgeon, J.; et al. Unexpected CDH1 Mutations Identified on Multigene Panels Pose Clinical Management Challenges. JCO Precis. Oncol. 2017, 1–12. [Google Scholar] [CrossRef]
- Benusiglio, P.R.; Colas, C.; Rouleau, E.; Uhrhammer, N.; Romero, P.; Remenieras, A.; Moretta, J.; Wang, Q.; De Pauw, A.; Buecher, B.; et al. Hereditary diffuse gastric cancer syndrome: Improved performances of the 2015 testing criteria for the identification of probands with a CDH1 germline mutation. J. Med. Genet. 2015, 52, 563–565. [Google Scholar] [CrossRef]
- Van Der Post, R.S.; Vogelaar, I.P.; Manders, P.; Van Der Kolk, L.E.; Cats, A.; Van Hest, L.P.; Sijmons, R.; Aalfs, C.M.; Ausems, M.G.E.M.; Gómez García, E.B.; et al. Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1. Gastroenterology 2015, 149, 897–906.e19. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Krempely, K.; Roberts, M.E.; Anderson, M.J.; Carneiro, F.; Chao, E.; Dixon, K.; Figueiredo, J.; Ghosh, R.; Huntsman, D.; et al. Specifications of the ACMG/AMP variant curation guidelines for the analysis of germline CDH1 sequence variants. Hum. Mutat. 2018, 39, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Frebourg, T.; Oliveira, C.; Hochain, P.; Karam, R.; Manouvrier, S.; Graziadio, C.; Vekemans, M.; Hartmann, A.; Baert-Desurmont, S.; Alexandre, C.; et al. Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J. Med. Genet. 2006, 43, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Yelskaya, Z.; Bacares, R.; Salo-Mullen, E.; Somar, J.; Lehrich, D.A.; Fasaye, G.A.; Coit, D.G.; Tang, L.H.; Stadler, Z.K.; Zhang, L. CDH1 missense variant c.1679C>G (p. T560R) completely disrupts normal splicing through creation of a novel 5′ splice site. PLoS ONE 2016, 11, e0165654. [Google Scholar] [CrossRef] [Green Version]
- Vécsey-Semjén, B.; Becker, K.F.; Sinski, A.; Blennow, E.; Vietor, I.; Zatloukal, K.; Beug, H.; Wagner, E.; Huber, L.A. Novel colon cancer cell lines leading to better understanding of the diversity of respective primary cancers. Oncogene 2002, 21, 4646–4662. [Google Scholar] [CrossRef] [Green Version]
- Kaurah, P.; MacMillan, A.; Boyd, N.; Senz, J.; De Luca, A.; Chun, N.; Suriano, G.; Zaor, S.; Van Manen, L.; Gilpin, C.; et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. J. Am. Med. Assoc. 2007, 297, 2360–2372. [Google Scholar] [CrossRef] [Green Version]
- Aronson, M.; Swallow, C.; Govindarajan, A.; Semotiuk, K.; Cohen, Z.; Kaurah, P.; Velsher, L.; Ambus, I.; Buckley, K.; Forster-Gibson, C.; et al. Germline variants and phenotypic spectrum in a canadian cohort of individuals with diffuse gastric cancer. Curr. Oncol. 2020, 27, e182–e190. [Google Scholar] [CrossRef]
- Pharoah, P.D.P.; Guilford, P.; Caldas, C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001, 121, 1348–1353. [Google Scholar] [CrossRef]
- Suriano, G.; Oliveira, C.; Ferreira, P.; Machado, J.C.; Bordin, M.C.; De Wever, O.; Bruyneel, E.A.; Moguilevsky, N.; Grehan, N.; Porter, T.R.; et al. Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum. Mol. Genet. 2003, 12, 575–582. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori Infection and the Development of Gastric Cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Solcia, E.; Fiocca, R.; Luinetti, O.; Villani, L.; Padovan, L.; Calistri, D.; Ranzani, G.N.; Chiaravalli, A.; Capella, C. Intestinal and diffuse gastric cancers arise in a different background of Helicobacter pylori gastritis through different gene involvement. Am. J. Surg. Pathol. 1996, 20, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Koshida, Y.; Koizumi, W.; Sasabe, M.; Katoh, Y.; Okayasu, I. Association of Helicobacter pylori-dependent gastritis with gastric carcinomas in young Japanese patients: Histopathological comparison of diffuse and intestinal type cancer cases. Histopathology 2000, 37, 124–130. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Helal, T.E.A.; El-Ghazawy, I.M.; Samaan, G.F.; Galal el-Din, M.M.; Abdellateif, M.S.; Desouky, E.; Zekri, A.R.N. The role of E-cadherin and Runx3 in Helicobacter Pylori–Associated gastric carcinoma is achieved through regulating P21waf and P27 expression. Cancer Genet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Palanduz, A.; Erdem, L.; Cetin, B.D.; Ozcan, N.G. Helicobacter pylori infection in family members of patients with gastroduodenal symptoms. A cross-sectional analytical study. Sao Paulo Med. J. 2018, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Drumm, B.; Perez-Perez, G.I.; Blaser, M.J.; Sherman, P.M. Intrafamilial clustering of Helicobacter pylori infection. N. Engl. J. Med. 1990, 322, 359–363. [Google Scholar] [CrossRef]
- Lim, S.H.; Kwon, J.-W.; Kim, N.; Kim, G.H.; Kang, J.M.; Park, M.J.; Yim, J.Y.; Kim, H.U.; Baik, G.H.; Seo, G.S.; et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: Nationwide multicenter study over 13 years. BMC Gastroenterol. 2013, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Mi, E.Z.; Mi, E.Z.; di Pietro, M.; O’Donovan, M.; Hardwick, R.H.; Richardson, S.; Ziauddeen, H.; Fletcher, P.C.; Caldas, C.; Tischkowitz, M.; et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest. Endosc. 2018, 87, 408–418. [Google Scholar] [CrossRef] [Green Version]
- van der Post, R.S.; Carneiro, F. Emerging Concepts in Gastric Neoplasia: Heritable Gastric Cancers and Polyposis Disorders. Surg. Pathol. Clin. 2017, 10, 931–945. [Google Scholar] [CrossRef]
- van der Post, R.S.; Gullo, I.; Oliveira, C.; Tang, L.H.; Grabsch, H.I.; O’Donovan, M.; Fitzgerald, R.C.; van Krieken, H.; Carneiro, F. Histopathological, molecular, and genetic profile of hereditary diffuse gastric cancer: Current knowledge and challenges for the future. Adv. Exp. Med. Biol. 2016, 908, 371–391. [Google Scholar] [CrossRef]
- Garziera, M.; Canzonieri, V.; Cannizzaro, R.; Geremia, S.; Caggiari, L.; De Zorzi, M.; Maiero, S.; Orzes, E.; Perin, T.; Zanussi, S.; et al. Identification and characterization of CDH1 germline variants in sporadic gastric cancer patients and in individuals at risk of gastric cancer. PLoS ONE 2013, 8, e77035. [Google Scholar] [CrossRef]
- Figueiredo, J.; Söderberg, O.; Simões-Correia, J.; Grannas, K.; Suriano, G.; Seruca, R. The importance of E-cadherin binding partners to evaluate the pathogenicity of E-cadherin missense mutations associated to HDGC. Eur. J. Hum. Genet. 2013, 21, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, P.S.; Teixeira, A.; Pinho, S.; Ferreira, P.; Fernandes, J.; Oliveira, C.; Seruca, R.; Suriano, G.; Casares, F. E-cadherin missense mutations, associated with hereditary diffuse gastric cancer (HDGC) syndrome, display distinct invasive behaviors and genetic interactions with the Wnt and Notch pathways in Drosophila epithelia. Hum. Mol. Genet. 2006, 15, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]

| HDGC Testing Criteria from 2015 [6] | HDGC Testing Criteria from 2020 [7] |
|---|---|
| Established testing criteria | Family criteria |
|
|
| Extended criteria (testing could be considered) | Individual criteria |
|
|
| Baseline and Clinical Characteristics | CDH1/CTNNA1 PV | CDH1 VUS | No PV |
|---|---|---|---|
| Number of patients | 62 | 5 | 140 |
| Gender | 22/62 (35%) male 40/62 (65%) female | 3/5 (60%) male 2/5 (40%) female | 41/140 (29%) male 99/140 (71%) female |
| Number of families | 29 | 3 | 129 |
| Age at study inclusion (range) | 42 ± 14 years (16–70) | 49 ± 8 years (39–62) | 49 ± 15 years (18–87) |
| GC prevalence | 38/62 (61%) | 2/5 (40%) | 102/140 (73%) |
| Age at GC diagnosis (range) | 40 ± 13 years (16–72) | 41 ± 6 years (37–45) | 44 ± 12 years (17–74) |
| LBC prevalence in women | 8/40 (20%) | 0 | 10/99 (10%) |
| Age at LBC diagnosis (range) | 51 ± 8 years (38–65) | – | 52 ± 7 years (45–65) |
| Number of gastrectomies (PTG *) | 40 32 * | 1 0 * | 27 0 * |
| Yield of GC in gastrectomies (PTG *) | 31/40 (78%) 23/32 (72%) * | 1/1 (100%) | 27/27 (100%) |
| Cancer staging in gastrectomy <pT2 ≥pT2 | 23/31 (74%) 8/31 (26%) | 0/1 (0%) 1/1 (100%) | 5/27 (19%) 22/27 (81%) |
| Patients with multifocal GC in gastrectomy | 11/31 (35%) | 0/1 (0%) | 0/27 (0%) |
| 2015 HDGC Criteria | Index Patients | Index Patients with CDH1/CTNNA1 PV | Detection Rate |
|---|---|---|---|
| Criterion 1 (Two cases of gastric cancer regardless of age with at least one confirmed DGC) | 97 | 20 | 21% |
| Criterion 2 (One case of DGC before age 40) | 87 | 13 | 15% |
| Criterion 3 (Personal or family history of DGC and LBC with one diagnosed before age 50) | 40 | 11 | 28% |
| Patients fulfilling more than one criterion | 66 | 15 | 23% |
| Total (index patients) | 161 | 29 | 18% |
| Total (all patients) | 207 | 62 | 30% |
| Variant | Reported Variant Classification | Classification with CDH1-Specific Criteria [33] | Type of Variant | Number of Family Members Included | Reported GC in Family Pedigree | Reported BC in Family Pedigree | Mean Age at Diagnosis of Manifest Cancer * |
|---|---|---|---|---|---|---|---|
| (Likely) pathogenic variants | |||||||
| c.3G>A;p.? | pathogenic | pathogenic | start lost | 6 | 2 | 0 | 47 (46–47) |
| c.48+1G>A;p.? | pathogenic | pathogenic | splice | 3 | 4 | 0 | 52 (33–75) |
| c.86dupA;p.(His296Glnfs*5) | pathogenic | likely pathogenic | frameshift | 1 | 2 | 1 | 29 (26–35) |
| c.521delA;p.(Asn174Thrfs*41) mosaicism | pathogenic | likely pathogenic | frameshift | 1 | 1 | 1 | 41 (41) |
| c.1108G>A;p.(Asp370Asn) | likely pathogenic | VUS | missense | 5 | 4 | 0 | 48 (41–61) |
| c.1137G>A;p.? | pathogenic | pathogenic | splice [34] | 1 | 4 | 0 | 47 (44–50) |
| c.1416dupC;p.(Val473Argfs*10) | pathogenic | likely pathogenic | frameshift | 1 | 1 | 0 | 48 (48) |
| c.1565+1G>A;p.? | pathogenic | pathogenic | splice | 1 | 0 | 2 | 49 (38–60) |
| c.1565+1G>A;p.? | pathogenic | pathogenic | splice | 2 | 4 | 0 | 47 (32–64) |
| c.1565+1G>A;p.? | pathogenic | pathogenic | splice | 1 | 0 | 5 | 65 (65) |
| c.1565+2dupT;p.? | likely pathogenic | pathogenic | splice | 3 | 1 | 0 | 34 (34) |
| c.1565+2dupT;p.? | likely pathogenic | pathogenic | splice | 1 | 2 | 0 | 50 (50) |
| c.1651G>T;p.(Glu551*) | pathogenic | likely pathogenic | nonsense | 3 | 3 | 1 | 55 (40–76) |
| c.1679C>G;r.1680_1711del;p.(Tyr561Phefs*16) | pathogenic | pathogenic | splice [35] | 1 | 2 | 1 | 49 (47–50) |
| c.1679C>G;r.1680_1711del;p.(Tyr561Phefs*16) | pathogenic | pathogenic | splice [35] | 1 | 3 | 0 | unknown |
| c.1746_1747dup;p.(Leu583Argfs*2) | pathogenic | likely pathogenic | frameshift | 3 | 4 | 0 | unknown |
| c.1746_1747dup;p.(Leu583Argfs*2) | pathogenic | likely pathogenic | frameshift | 4 | 6 | 1 | unknown |
| c.1786G>T;p.(Glu596*) | pathogenic | likely pathogenic | nonsense | 1 | 2 | 0 | 46 (44–47) |
| c.1792C>T;p.(Arg598*) | pathogenic | pathogenic | nonsense | 1 | 3 | 0 | 30 (23–37) |
| c.1792C>T;p.(Arg598*) | pathogenic | pathogenic | nonsense | 2 | 4 | 1 | 51 (43–63) |
| c.1901C>T;r.1900_1936del; p.(Ala634Profs*7) | pathogenic | pathogenic | splice [36] | 2 | 3 | 2 | 16 (16) |
| c.2116C>T;p.(Gln706*) | pathogenic | likely pathogenic | nonsense | 4 | 3 | 2 | 53 (52–54) |
| c.2165-2A>C;p? | pathogenic | likely pathogenic | splice | 3 | 2 | 0 | 40 (32–48) |
| deletion exon 1–2 | pathogenic | pathogenic | large exonic deletion | 1 | 2 | 0 | 39 (33–45) |
| deletion exon 1–2 | pathogenic | pathogenic | large exonic deletion | 1 | 4 | 0 | 43 (32–49) |
| deletion exon 3–8 | pathogenic | likely pathogenic | large exonic deletion | 1 | 3 | 2 | 49 (38–58) |
| deletion exon 1–16 | pathogenic | pathogenic | large exonic deletion | 1 | 2 | 0 | 47 (37–56) |
| deletion exon 8–11 | pathogenic | likely pathogenic | large exonic deletion | 5 | 7 | 5 | 56 (48–72) |
| CTNNA1: c.1175delA;p.(Asp392Valfs*13) | pathogenic | likely pathogenic | frameshift | 2 | 2 | 0 | 44 (44) |
| Variants of uncertain significance (VUS) | |||||||
| c.659T>G;p.(Leu220Arg) | VUS | VUS | missense | 2 | 2 | 0 | 47 (45–49) |
| c.1466C>T;p.(Pro489Leu) | VUS | VUS | missense | 1 | 1 | 0 | 37 (37) |
| c.2629G>A; p.(Gly877Arg) | VUS | VUS | missense | 2 | 2 | 0 | 43 (32–54) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marwitz, T.; Hüneburg, R.; Spier, I.; Lau, J.-F.; Kristiansen, G.; Lingohr, P.; Kalff, J.C.; Aretz, S.; Nattermann, J.; Strassburg, C.P. Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status. Cancers 2020, 12, 3726. https://doi.org/10.3390/cancers12123726
Marwitz T, Hüneburg R, Spier I, Lau J-F, Kristiansen G, Lingohr P, Kalff JC, Aretz S, Nattermann J, Strassburg CP. Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status. Cancers. 2020; 12(12):3726. https://doi.org/10.3390/cancers12123726
Chicago/Turabian StyleMarwitz, Tim, Robert Hüneburg, Isabel Spier, Jan-Frederic Lau, Glen Kristiansen, Philipp Lingohr, Jörg C. Kalff, Stefan Aretz, Jacob Nattermann, and Christian P. Strassburg. 2020. "Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status" Cancers 12, no. 12: 3726. https://doi.org/10.3390/cancers12123726
APA StyleMarwitz, T., Hüneburg, R., Spier, I., Lau, J.-F., Kristiansen, G., Lingohr, P., Kalff, J. C., Aretz, S., Nattermann, J., & Strassburg, C. P. (2020). Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status. Cancers, 12(12), 3726. https://doi.org/10.3390/cancers12123726





