Unusual Faces of Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
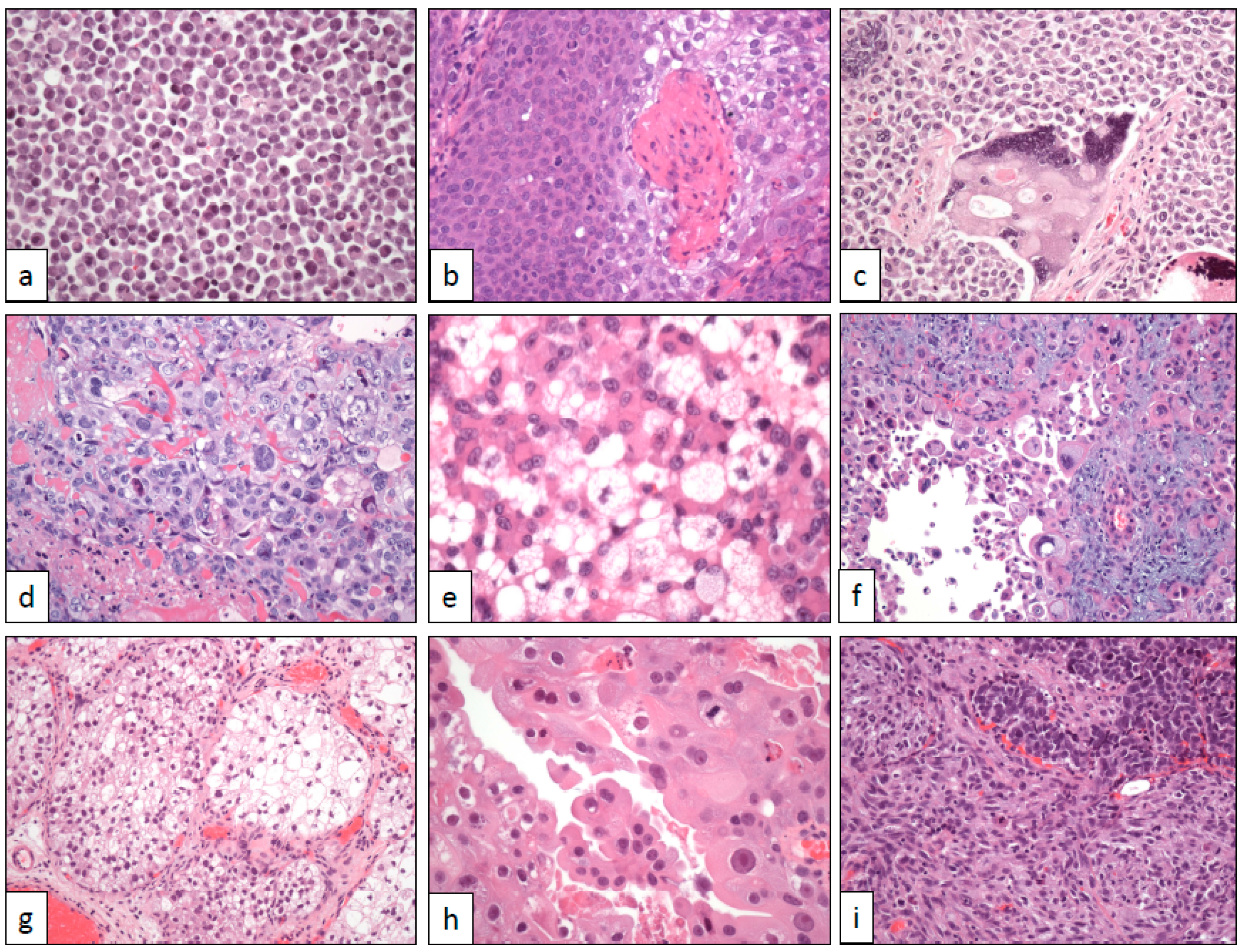
2. Architectural Changes
2.1. Nested and Large Nested Architecture
2.2. Micropapillary Architecture
2.3. Myxoid Stromal Change
2.4. Small Tubules and Nephrogenic Adenoma-Like Architecture
2.5. Microcystic Architecture
2.6. Verrucous Architecture
2.7. Diffuse Architecture with Lymphoepithelioma-Like Changes
3. Cytological Changes
3.1. Plasmacytoid Cells
3.2. Signet-Ring Cells
3.3. Basaloid-Squamous Cells
3.4. Yolk Sac Cells
3.5. Trophoblastic Cells
3.6. Rhabdoid Cells
3.7. Lipid/Lipoblast-Like Cells
3.8. Giant Cells
3.9. Clear Cells
3.10. Eosinophilic (Oncocytoid) Cells
3.11. Sarcomatoid Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.Y.; Huang, J.; Ko, W.Y.; Lok, V.; Choi, P.; Ng, C.F.; Sengupta, S.; Mostafid, H.; Kamat, A.M.; Black, P.C.; et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic products per capita. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Lobo, N.; Shariat, S.F.; Guo, C.C.; Fernandez, M.I.; Kassouf, W.; Choudhury, A.; Gao, J.; Williams, S.B.; Galsky, M.D.; Taylor III, J.A.; et al. What is the significance of variant histology in urothelial carcinoma? Eur. Urol. Focus. 2020, 6, 653–663. [Google Scholar] [CrossRef]
- Al-Ahmadie, H.; Netto, G.J. Updates on the genomics of bladder cancer and novel molecular taxonomy. Adv. Anat. Pathol. 2020, 27, 36–43. [Google Scholar] [CrossRef]
- Talbert, M.L.; Young, R.H. Carcinomas of the urinary bladder with deceptively benign-appearing foci. A report of three cases. Am. J. Surg. Pathol. 1989, 13, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.B. Unusual benign bladder tumor of von Brunn nest origin. Urology 1979, 14, 288–289. [Google Scholar] [CrossRef]
- Lin, O.; Cardillo, M.; Dalbagni, G.; Linkov, I.; Hutchinson, B.; Reuter, V.E. Nested variant of urothelial carcinoma: A clinicopathologic and immunohistochemical study of 12 cases. Mod. Pathol. 2003, 16, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.R.; Cheng, L. The expanding molecular and mutational landscape of nested variant of urothelial carcinoma. Histopathology 2020, 76, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Epstein, J.I. Large nested variant of urothelial carcinoma: 23 cases mimicking von Brunn nests and inverted growth pattern of noninvasive papillary urothelial carcinoma. Am. J. Surg. Pathol. 2011, 35, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; McKenney, J.K.; Hartmann, A.; Hes, O.; Bertz, S.; Varinot, J.; Brimo, F. Large nested variant of urothelial carcinoma: A clinicopathological study of 36 cases. Histopathology 2017, 71, 703–710. [Google Scholar] [CrossRef]
- Hacihasanoglu, E.; Behzatoglu, K. Large nested urothelial carcinoma: A clinicopathological study of 22 cases on transurethral resection materials. Ann. Diagn. Pathol. 2019, 42, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Weyerer, V.; Eckstein, M.; Compérat, E.; Juette, H.; Gaisa, N.T.; Allory, Y.; Stöhr, R.; Wullich, B.; Rouprêt, M.; Hartmann, A.; et al. Pure large nested variant of urothelial carcinoma (LNUC) is the prototype of an FGFR3 mutated aggressive urothelial carcinoma with luminal-papillary phenotype. Cancers 2020, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.S.; Kaag, M.; Raman, J.D.; Chan, W.; Tran, T.; Kunchala, S.; Shuman, L.; DeGraff, D.J.; Chen, G.; Warrick, J.I. Clinical significance of prominent retraction clefts in invasive urothelial carcinoma. Hum. Pathol. 2017, 61, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H. Carcinomas with micropapillary morphology. Clinical significance and current concepts. Adv. Anat. Pathol. 2004, 11, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Jin, K.; Qiu, S.; Zhou, X.; Yuan, Q.; Yang, L.; Wei, Q. Prognostic values of the clinicopathological characteristics and survival outcomes in micropapillary urothelial carcinoma of the bladder: A SEER database analysis. Cancer Med. 2020, 9, 4897–4906. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Middleton, L.P.; Tamboli, P.; Troncoso, P.; Silva, E.G.; Ayala, A.G. Invasive micropapillary carcinoma of the breast metastatic to the urinary bladder and endometrium: Diagnostic pitfalls and review of the literature of tumors with micropapillary features. Ann. Diagn. Pathol. 2003, 7, 112–119. [Google Scholar] [CrossRef]
- Amin, M.B.; Ro, J.Y.; El-Sharkawy, T.; Lee, K.M.; Troncoso, P.; Silva, E.G.; Ordóñez, N.G.; Ayala, A.G. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am. J. Surg. Pathol. 1994, 18, 1224–1232. [Google Scholar] [CrossRef]
- Holmäng, S.; Thomsen, J.; Johansson, S.L. Micropapillary carcinoma of the renal pelvis and ureter. J. Urol. 2006, 175, 463–466. [Google Scholar] [CrossRef]
- Perez-Montiel, D.; Hes, O.; Michal, M.; Suster, S. Micropapillary urothelial carcinoma of the upper urinary tract: Clinicopathologic study of five cases. Am. J. Clin. Pathol. 2006, 126, 86–92. [Google Scholar] [CrossRef]
- Guo, C.C.; Dadhania, V.; Zhang, L.; Majewski, T.; Bondaruk, J.; Sykulski, M.; Wronowska, W.; Gambin, A.; Wang, Y.; Zhang, S.; et al. Gene Expression Profile of the Clinically Aggressive Micropapillary Variant of Bladder Cancer. Eur. Urol. 2016, 70, 211–220. [Google Scholar] [CrossRef]
- Zinnall, U.; Weyerer, V.; Compérat, E.; Camparo, P.; Gaisa, N.T.; Knuechel-Clarke, R.; Perren, A.; Lugli, A.; Toma, M.; Baretton, G.; et al. Micropapillary urothelial carcinoma: Evaluation of HER2 status and immunohistochemical characterization of the molecular subtype. Hum. Pathol. 2018, 80, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kaimakliotis, H.Z.; Williamson, S.R.; Koch, M.O.; Huang, K.; Barboza, M.P.; Zhang, S.; Wang, M.; Idrees, M.T.; Grignon, D.J.; et al. Micropapillary urothelial carcinoma of urinary bladder displays immunophenotypic features of luminal and p53-like subtypes and is not a variant of adenocarcinoma. Urol. Oncol. 2020, 38, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.L.; Borghede, G.; Holmäng, S. Micropapillary bladder carcinoma: A clinicopathological study of 20 cases. J. Urol. 1999, 161, 1798–1802. [Google Scholar] [CrossRef]
- Tavora, F.; Epstein, J.I. Urothelial carcinoma with abundant myxoid stroma. Hum. Pathol. 2009, 40, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Gilg, M.M.; Wimmer, B.; Ott, A.; Langner, C. Urothelial carcinoma with abundant myxoid stroma. Evidence for mucinous production by cancer cells. Virchows Arch. 2012, 461, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Li, C.; Cao, Y.; Liu, K.; Du, J.; Gu, Y.; Wang, X.; Li, Y.; Zhang, S. CK7 expression associates with the location, differentiation, lymph node metastasis, and the Dukes’ stage of primary colorectal cancers. J. Cancer 2019, 10, 2510–2519. [Google Scholar] [CrossRef]
- Young, R.H.; Oliva, E. Transitional cell carcinomas of the urinary bladder that may be underdiagnosed. A report of four invasive cases exemplifying the homology between neoplastic and non-neoplastic transitional cell lesions. Am. J. Surg. Pathol. 1996, 20, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Santi, R.; Angulo, J.C.; Nesi, G.; de Petris, G.; Kuroda, N.; Hes, O.; López, J.I. Common and uncommon features of nephrogenic adenoma revisited. Pathol. Res. Pract. 2019, 215, 152561. [Google Scholar] [CrossRef]
- Sharifai, N.; Abro, B.; Chen, J.F.; Zhao, M.; He, H.; Cao, D. Napsin A is a highly sensitive marker for nephrogenic adenoma: An immunohistochemical study with a specificity test in genitourinary tumors. Hum. Pathol. 2020, 102, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltrán, A.; Montironi, R.; Cheng, L. Microcystic urothelial carcinoma: Morphology, immunohistochemistry and clinical behaviour. Histopathology 2014, 64, 872–879. [Google Scholar] [CrossRef]
- Park, S.; Reuter, V.E.; Hansel, D.E. Non-urothelial carcinomas of the bladder. Histopathology 2019, 74, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.R.; Ruiz, M.R.; Florian, R.E.; De Leon, W.; Jose, L.S. Pan-urothelial verrucous carcinoma unrelated to schistosomiasis. BMJ Case Rep. 2009, 2009, bcr08.2008.0787. [Google Scholar] [CrossRef] [PubMed]
- Holmäng, S.; Borghede, G.; Johansson, S.L. Bladder carcinoma with lymphoepithelioma-like differentiation: A report of 9 cases. J. Urol. 1998, 159, 779–782. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Luque, R.J.; Vicioso, L.; Anglada, F.; Requena, M.J.; Quintero, A.; Montironi, R. Lymphoepithelioma-like carcinoma of the urinary bladder: A clinicopathologic study of 13 cases. Virchows Arch. 2001, 438, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Tamas, E.F.; Nielsen, M.E.; Schoenberg, M.P.; Epstein, J.I. Lymphoepithelioma-like carcinoma of the urinary tract: A clinicopathological study of 30 pure and mixed cases. Mod. Pathol. 2007, 20, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Zhang, S.; Lopez-Beltran, A.; Shah, R.B.; Montironi, R.; Tan, P.H.; Wang, M.; Baldridge, L.A.; MacLennan, G.T.; Cheng, L. Lymphoepithelioma-like carcinoma of the urinary bladder: Clinicopathologic, immunohistochemical and molecular features. Am. J. Surg. Pathol. 2011, 35, 474–483. [Google Scholar] [CrossRef] [PubMed]
- López-Beltrán, A.; Paner, G.; Blanca, A.; Montironi, R.; Tsuzuki, T.; Nagashima, Y.; Chuang, S.S.; Win, K.T.; Madruga, L.; Raspollini, M.R.; et al. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Arch. 2017, 470, 703–709. [Google Scholar] [CrossRef]
- Park, S.; Cho, M.S.; Kim, K.H. A case report of urothelial carcinoma with combined micropapillary and plasmacytoid morphology in the urinary bladder. Diagn. Cytopathol. 2016, 44, 124–127. [Google Scholar] [CrossRef]
- Sahin, A.A.; Myhre, M.; Ro, J.Y.; Sneige, N.; Dekmezian, R.H.; Ayala, A.G. Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol. 1991, 35, 277–280. [Google Scholar]
- Kim, D.K.; Kim, J.W.; Ro, J.Y.; Lee, H.S.; Park, J.Y.; Ahn, H.K.; Lee, J.Y.; Cho, K.S. Plasmacytoid variant of urothelial carcinoma of the bladder: A systematic review and meta-analysis of clinicopathological features and survival outcomes. J. Urol. 2020, 204, 215–223. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Sardi, I.; Giunti, L.; Di Lollo, S.; Baroni, G.; Stomaci, N.; Menghetti, I.; Franchi, A. Plasmacytoid urothelial carcinoma of the urinary bladder: Clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of a case series. Hum. Pathol. 2011, 42, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, G.; Song, B.; Lee, C.; Park, J.H.; Moon, K.C. HER2 protein overexpression and gene amplification in plasmacytoid urothelial carcinoma of the urinary bladder. Dis. Markers. 2016, 2016, 8463731. [Google Scholar] [CrossRef] [PubMed]
- Palsgrove, D.N.; Taheri, D.; Springer, S.U.; Cowan, M.; Guner, G.; Mendoza Rodriguez, M.A.; Rodriguez Pena, M.D.C.; Wang, Y.; Kinde, I.; Cunha, I.; et al. Targeted sequencing of plasmacytoid urothelial carcinoma reveals frequent TERT promoter mutations. Hum. Pathol. 2019, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmadie, H.A.; Iyer, G.; Lee, B.H.; Scott, S.N.; Mehra, R.; Bagrodia, A.; Jordan, E.J.; Gao, S.P.; Ramirez, R.; Cha, E.K.; et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid-variant bladder cancer. Nat. Genet. 2016, 48, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Qiu, S.; Jin, K.; Zhou, X.; Cao, Q.; Yang, L.; Wei, Q. Signet-Ring Cell Carcinoma as an Independent Prognostic Factor for Patients with Urinary Bladder Cancer: A Population-Based Study. Front. Oncol. 2020, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Ereño, C.; Gaafar, A.; Garmendia, M.; Etxezarraga, C.; Bilbao, F.J.; Lopez, J.I. Basaloid squamous cell carcinoma of the head and neck. A clinicopathological and follow-up study of 40 cases and review of the literature. Head Neck Pathol. 2008, 2, 83–91. [Google Scholar] [CrossRef]
- Graham, R.P.; Arnold, C.A.; Naini, B.V.; Lam-Himlin, D.M. Basaloid squamous cell carcinoma of the anus revisited. Am. J. Surg. Pathol. 2016, 40, 354–360. [Google Scholar] [CrossRef]
- Vakar-Lopez, F.; Abrams, J. Basaloid squamous cell carcinoma occurring in the urinary bladder. Arch. Pathol. Lab. Med. 2000, 124, 455–459. [Google Scholar]
- Hagemann, I.S.; Lu, J.; Lewis, J.S., Jr. Basaloid squamous cell carcinoma arising in [corrected] the renal pelvis. Int. J. Surg. Pathol. 2008, 16, 199–201. [Google Scholar] [CrossRef]
- Neves, T.R.; Soares, M.J.; Monteiro, P.G.; Lima, M.S.; Monteiro, H.G. Basaloid squamous cell carcinoma in the urinary bladder with small cell carcinoma. J. Clin. Oncol. 2011, 29, e440–e442. [Google Scholar] [CrossRef]
- Ginori, A.; Barone, A.; Santopietro, R.; Barbanti, G.; Cecconi, F.; Tripodi, S.A. Human papillomavirus-related basaloid squamous cell carcinoma of the bladder associated with genital tract human papillomavirus infection. Int. J. Urol. 2015, 22, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Samaratunga, H.; Samaratunga, D.; Dunglison, N.; Perry-Keene, J.; Nicklin, J.; Delahunt, B. Alpha-fetoprotein-producing carcinoma of the renal pelvis exhibiting hepatoid and urothelial differentiation. Anticancer Res. 2012, 32, 4987–4991. [Google Scholar] [PubMed]
- Ravishankar, S.; Malpica, A.; Ramalingam, P.; Euscher, E.D. Yolk sac tumor in extragonadal pelvic sites: Still a diagnostic challenge. Am. J. Surg. Pathol. 2017, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Melms, J.C.; Thummalapalli, R.; Shaw, K.; Ye, H.; Tsai, L.; Bhatt, R.S.; Izar, B. Alpha-fetoprotein (AFP) as a tumor marker in a patient with urothelial cancer with exceptional response to anti-PD-L1 therapy and an escape lesion mimic. J. Immunother. Cancer 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Herrera, N.; Condom-Mundó, E. Yolk sac tumor differentiation in urothelial carcinoma of the urinary bladder: A case report and differential diagnosis. Diagn. Pathol. 2020, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- McNamee, T.; Damato, S.; McCluggage, W.G. Yolk sac tumours of the female genital tract in older adults derive commonly from somatic epithelial neoplasms: Somatically derived yolk sac tumours. Histopathology 2016, 69, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Przybycin, C.G.; McKenney, J.K.; Nguyen, J.K.; Shah, R.B.; Umar, S.A.; Harik, L.; Shih, I.M.; Cox, R.M. Urothelial carcinomas with trophoblastic differentiation, including choriocarcinoma. Clinicopathologic series of 16 cases. Am. J. Surg. Pathol. 2020, 44, 1322–1330. [Google Scholar]
- Douglas, J.; Sharp, A.; Chau, C.; Head, J.; Drake, T.; Wheater, M.; Geldart, T.; Mead, G.; Crabb, S.J. Serum total hCGβ level is an independent prognostic factor in transitional cell carcinoma of the urothelial tract. Br. J. Cancer 2014, 110, 1759–1766. [Google Scholar] [CrossRef]
- Banet, N.; Gown, A.M.; Shih, I.M.; Li, Q.K.; Roden, R.B.S.; Nucci, M.R.; Cheng, L.; Przybycin, C.G.; Nasseri-Nik, N.; Wu, L.S.F.; et al. GATA-3 expression in trophoblastic tissues. An immunohistochemical study of 445 cases, including diagnostic utility. Am. J. Surg. Pathol. 2015, 39, 101–108. [Google Scholar] [CrossRef]
- Miettinen, M.; Wang, Z.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Biernat, W.; Lasota, J.; See, Y.S. SALL4 expression in germ cell and non-germ cell tumors. A systematic immunohistochemical study of 3215 cases. Am. J. Surg. Pathol. 2014, 38, 410–420. [Google Scholar] [CrossRef]
- Mao, T.L.; Kurman, R.J.; Jeng, Y.M.; Husng, W.; Shih, I.M. HSD3B1 as a novel trophoblast-associated marker that assists in the differential diagnosis of trophoblastic tumors and tumorlike lesions. Am. J. Surg. Pathol. 2008, 32, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R.; Ritter, J.H.; Dehner, L.P. Malignant rhabdoid tumors: A clinicopathologic review and conceptual discussion. Sem. Diagn. Pathol. 1995, 12, 233–248. [Google Scholar]
- Warren, K.S.; Oxley, J.; Koupparis, A. Pure malignant rhabdoid tumor of the bladder. Can. Urol. Assoc. J. 2014, 8, e260–e262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sterling, M.E.; Long, C.J.; Bosse, K.R.; Bagatell, R.; Shukla, A.R. A rapid progression of disease after surgical excision of a malignant rhabdoid tumor of the bladder. Urology 2015, 85, 664–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Assadi, A.; Alzubaidi, A.; Lesmana, H.; Brennan, R.C.; Ortiz-Hernandez, V.; Gleason, J.M. Pure bladder malingnant rhabdoid tumor successfully treated with partial cystectomy, radiation, and chemotherapy: A case report and review of the literature. J. Pediatr. Hematol. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Parwani, A.V.; Herawi, M.; Volmar, K.; Tsai, S.H.; Epstein, J.I. Urothelial carcinoma with rhabdoid features: Report of 6 cases. Hum. Pathol. 2006, 37, 168–172. [Google Scholar] [CrossRef]
- Fukumura, Y.; Fujii, H.; Mitani, K.; Sakamoto, Y.; Matsumoto, T.; Suda, K.; Yao, T. Urothelial carcinoma of the renal pelvis with rhabdoid features. Pathol. Int. 2009, 59, 322–325. [Google Scholar] [CrossRef]
- Tajima, S. Rhabdoid variant of urotelial carcinoma of the urinary bladder: A case report with emphasis on immunohistochemical analysis regarding the formation of rhabdoid morphology. Int. J. Clin. Exp. Pathol. 2015, 8, 9638–9642. [Google Scholar]
- Mostofi, F.K.; Davis, C.J.; Sesterhenn, I.A. World Health Organization International Histological Classification of Tumours: Histological Typing of Urinary Bladder Tumours, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Leroy, X.; Gonzalez, S.; Zini, L.; Aubert, S. Lipoid-cell variant of urothelial carcinoma: A clinicopathologic and immunohistochemical study of five cases. Am. J. Surg. Pathol. 2007, 31, 770–773. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Amin, M.B.; Oliveira, P.S.; Montironi, R.; Algaba, F.; McKenney, J.K.; de Torres, I.; Mazerolles, C.; Wang, M.; Cheng, L. Urothelial carcinoma of the bladder. Lipid cell variant: Clinicopathologic findings and LOH analysis. Am. J. Surg. Pathol. 2010, 34, 371–376. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Blanca, A.; Montironi, R.; Cheng, L.; Regueiro, J.C. Pleomorphic giant cell carcinoma of the urinary bladder. Hum. Pathol. 2009, 40, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Samatunga, H.; Delahunt, B.; Egevad, L.; Adamson, M.; Hussey, D.; Malone, G.; Hoyle, K.; Nathan, T.; Kerle, D.; Ferguson, P.; et al. Pleomorphic giant cells carcinoma of the urinary bladder: An extreme form of tumour de-differentiation. Histopathology 2016, 68, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Priore, S.F.; Schwartz, L.E.; Epstein, J.I. An expanded immunohistochemical profile of osteoclast-rich undifferentiated carcinoma of the urinary tract. Mod. Pathol. 2018, 31, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.T.; Baterman, J.; Djordjevic, B.; Flood, T.A.; Belanger, E.C. Clear cell urothelial carcinoma: A study of 10 cases and meta-analysis of the entity. Evidence of mesonephric differentiation. Int. J. Surg. Pathol. 2017, 25, 18–25. [Google Scholar] [CrossRef]
- Pérez-Montiel, D.; Wakely, P.E.; Hes, O.; Michal, M.; Suster, S. High-grade urothelial carcinoma of the renal pelvis: Clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod. Pathol. 2006, 19, 494–503. [Google Scholar] [CrossRef]
- Amin, M.B. Histological variants of urothelial carcinoma: Diagnostic, therapeutic and prognostic implications. Mod. Pathol. 2009, 24, 6–15. [Google Scholar] [CrossRef]
- Hayashi, H.; Mann, S.; Kao, C.S.; Grignon, D.; Idrees, M. Variant morphology in upper urinary tract urothelial carcinoma: A 14-year case series of biopsy and resection specimens. Hum. Pathol. 2017, 65, 209–216. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Henriques, V.; Montironi, R.; Cimadamore, A.; Raspollini, M.R.; Cheng, L. Variants and new entities of bladder cancer. Histopathology 2019, 74, 77–96. [Google Scholar] [CrossRef]
- Rolim, I.; Henriques, V.; Rolim, N.; Blanca, A.; Marques, R.C.; Volavsek, M.; Carvalho, I.; Montironi, R.; Cimadamore, A.; Raspollini, M.R.; et al. Clinicopathologic analysis of upper urinary tract carcinoma with variant histology. Virchows Arch. 2020, 477, 111–120. [Google Scholar] [CrossRef]
- Sim, S.J.; Ro, J.Y.; Ordonez, N.G.; Park, Y.W.; Kee, K.H.; Ayala, A.G. Metastatic renal cell carcinoma to the bladder: A clinicopathologic and immunohistochemical study. Mod. Pathol. 1999, 12, 351–355. [Google Scholar]
- Tajima, S. Urothelial carcinoma with oncocytic features: An extremely rare case presenting a diagnostic challenge in urine cytology. Int. J. Clin. Exp. Pathol. 2015, 8, 8591–8597. [Google Scholar] [PubMed]
- McCabe, J.E.; Das, S.; Dowling, P.; Hamid, B.N.; Pettersson, B.A. Oncocytic carcinoid tumour of the bladder. J. Clin. Pathol. 2005, 58, 446–447. [Google Scholar] [PubMed]
- Genitsch, V.; Kollár, A.; Vandekerkhove, G.; Blarer, J.; Furrer, M.; Annala, M.; Herberts, C.; Pycha, A.; de Jong, J.J.; Liu, Y.; et al. Morphologic and genomic characterization of urothelial to sarcomatoid transition in muscle-invasive bladder cancer. Urol. Oncol. 2019, 37, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.; Wang, J.F.; Trepeta, R.; Feng, A.; Wang, J. Sarcomatoid carcinoma of the urinary bladder. Clin. Genitourin. Cancer 2016, 14, 366–372. [Google Scholar] [CrossRef]

| Architectural Changes | Prognostic Profiles |
| Worse prognosis - Nested - Large nested - Micropapillary | |
| Not worse prognosis - Myxoid stromal change - Small tubules - Nephrogenic adenoma-like - Microcystic - Verrucous - Diffuse lymphoepithelioma-like | |
| Cytological changes | |
| Worse prognosis - Plasmacytoid - Signet-ring - Basaloid-squamous - Yolk-sac -Trophoblastic - Rhabdoid - Giant pleomorphic - Clear - Sarcomatoid | |
| Not worse prognosis - Lipid/lipoblast - Giant osteoclast-like - Eosinophilic (oncocytoid) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manini, C.; López, J.I. Unusual Faces of Bladder Cancer. Cancers 2020, 12, 3706. https://doi.org/10.3390/cancers12123706
Manini C, López JI. Unusual Faces of Bladder Cancer. Cancers. 2020; 12(12):3706. https://doi.org/10.3390/cancers12123706
Chicago/Turabian StyleManini, Claudia, and José I. López. 2020. "Unusual Faces of Bladder Cancer" Cancers 12, no. 12: 3706. https://doi.org/10.3390/cancers12123706
APA StyleManini, C., & López, J. I. (2020). Unusual Faces of Bladder Cancer. Cancers, 12(12), 3706. https://doi.org/10.3390/cancers12123706






