Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020)
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
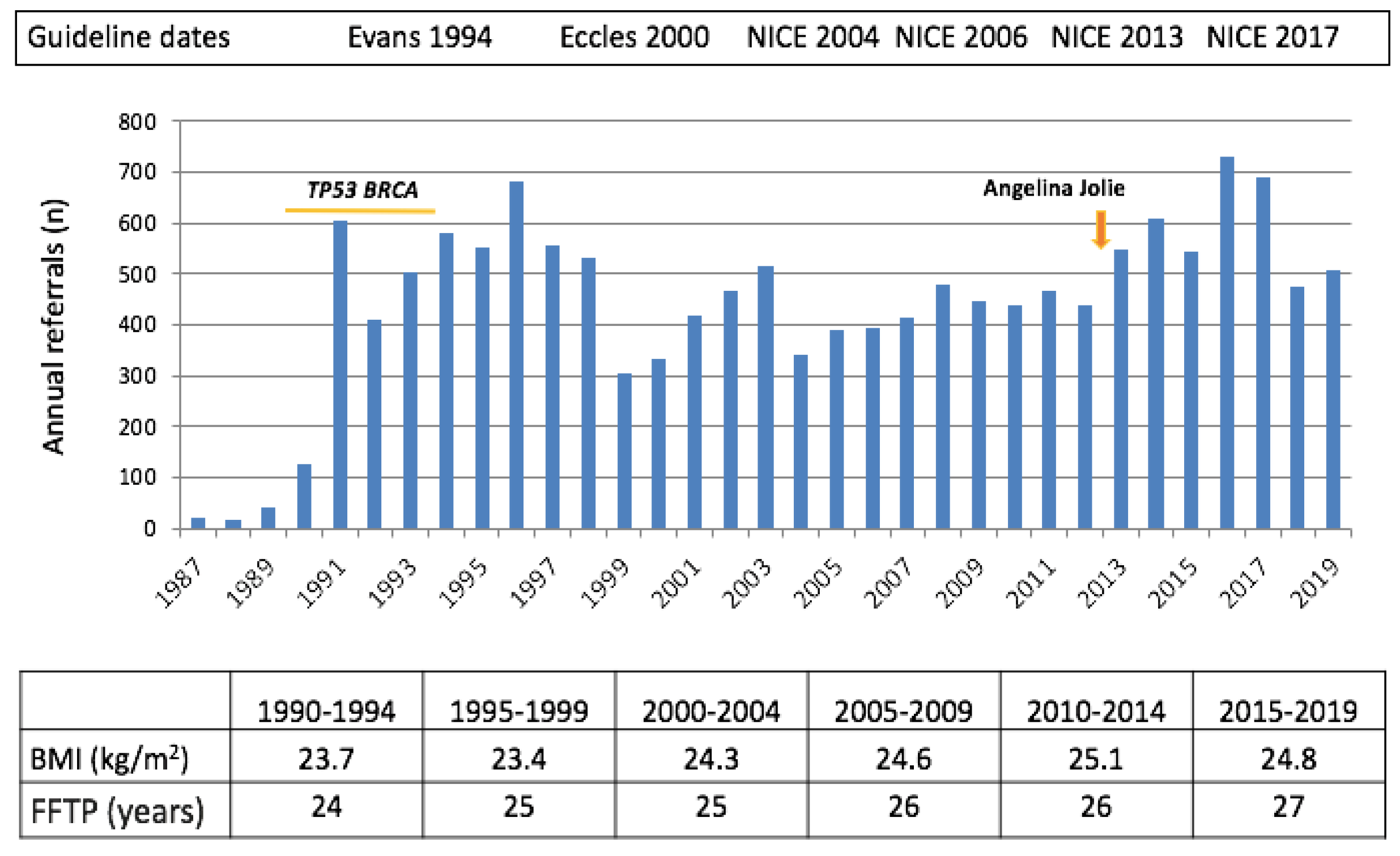
2.1. Referrals to the Clinic
2.2. Estimated Lifetime BC Risk
2.3. Risk Perception and Cancer Worry
2.4. Genetic Testing
2.5. Mammographic Screening
2.6. Lifestyle Prevention
2.7. Chemoprevention
2.8. Risk-Reducing Mastectomy
3. Discussion
3.1. Referrals
3.2. Risk Estimation
3.3. Genes
3.4. Breast Screening
3.5. Lifestyle Advice
3.6. Chemoprevention
3.7. Risk-Reducing Surgery
3.8. Summary
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, D.G.; Fentiman, I.S.; McPherson, K.; Asbury, D.; Ponder, B.A.; Howell, A. Fortnightly review: Familial breast cancer. BMJ 1994, 15, 183–187. [Google Scholar] [CrossRef]
- Eccles, D.M.; Evans, D.G.R.; Mackay, J. Guidelines for a genetic risk based approach to advising women with a family history of breast cancer. J. Med. Genet. 2000, 37, 203–209. [Google Scholar] [CrossRef]
- McIntosh, A.; Shaw, C.; Evans, G.; Turnbull, N.; Bahar, N.; Barclay, M.; Easton, D.; Emery, J.; Gray, J.; Halpin, J.; et al. (2004 updated 2006 and 2013) Clinical Guidelines and Evidence Review for The Classification and Care of Women at Risk of Familial Breast Cancer, London: National Collaborating Centre for Primary Care/University of Sheffield. NICE Guideline CG164. Available online: https://www.nice.org.uk/Guidance/CG164 (accessed on 15 November 2020).
- Hoskins, K.F.; Stopfer, J.E.; Calzone, K.A.; Merajver, S.D.; Rebbeck, T.R.; Garber, J.E.; Weber, B.L. Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA 1995, 15, 577–585. [Google Scholar] [CrossRef]
- Merajver, S.D.; Milliron, K. Breast cancer risk assessment: A guide for clinicians using the NCCN Breast Cancer Risk Reduction Guidelines. J. Natl. Comp. Cancer Netw. 2003, 1, 297–301. [Google Scholar] [CrossRef]
- Evans, D.G.; Astley, S.; Stavrinos, P.; Harkness, E.; Donnelly, L.S.; Dawe, S.; Jacob, I.; Harvie, M.; Cuzick, J.; Brentnall, A.; et al. Improvement in Risk Prediction, Early Detection and Prevention of Breast Cancer in the NHS Breast Screening Programme and Family History Clinics: A Dual Cohort Study. Southampt. (UK) NIHR J. Libr. 2016. [Google Scholar] [CrossRef]
- Claus, E.B.; Risch, N.; Thompson, W.D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am. J. Hum. Genet. 1991, 48, 232–242. [Google Scholar]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Antoniou, A.C.; Pharoah, P.P.; Smith, P.; Easton, D.F. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br. J. Cancer 2004, 18, 1580–1590. [Google Scholar] [CrossRef]
- Amir, E.; Evans, D.G.; Shenton, A.; Lalloo, F.; Moran, A.; Boggis, C.; Wilson, M.; Howell, A. Evaluation of Breast Cancer Risk Assessment Packages in the Family History Evaluation and Screening Programme. J. Med. Genet. 2003, 40, 807–814. [Google Scholar] [CrossRef]
- Evans, D.G.; Ingham, S.; Dawe, S.; Roberts, L.; Lalloo, F.; Brentnall, A.R.; Stavrinos, P.; Howell, A. Breast cancer risk assessment in 8,824 women attending a family history evaluation and screening programme. FAM Cancer 2014, 13, 189–196. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Burnell, L.; Hopwood, P.; Howell, A. Perception of risk in women with a family history of breast cancer. Br. J. Cancer 1993, 67, 612–614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, D.G.R.; Blair, V.; Greenhalgh, R.; Hopwood, P.; Howell, A. The impact of genetic counselling on risk perception in women with a family history of breast cancer. Br. J Cancer 1994, 70, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, P.; Keeling, F.; Long, A.; Pool, C.; Evans, G.; Howell, A. Psychological support needs for women at high genetic risk of breast cancer: Some preliminary indicators. Psycho-Oncology 1998, 7, 402–412. [Google Scholar] [CrossRef]
- Hopwood, P.; Shenton, A.; Lalloo, F.; Evans, D.G.; Howell, A. Risk perception and cancer worry: An exploratory study of the impact of genetic risk counselling in women with a family history of breast cancer. J. Med. Genet. 2001, 38, 139. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, P.; Howell, A.; Lalloo, F.; Evans, G. Do women understand the odds? Risk perceptions and recall of risk information in women with a family history of breast cancer. Community Genet. 2003, 6, 214–223. [Google Scholar] [CrossRef]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F., Jr.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A.; et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990, 30, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef]
- Evans, D.G.; Eccles, D.M.; Rahman, N.; Young, K.; Bulman, M.; Amir, E.; Shenton, A.; Howell, A.; Lalloo, F. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J. Med. Genet. 2004, 41, 474–480. [Google Scholar] [CrossRef]
- Evans, D.G.; Harkness, E.F.; Plaskocinska, I.; Wallace, A.J.; Clancy, T.; Woodward, E.R.; Howell, A.; Tischkowitz, M.; Lalloo, F. Pathology update to the Manchester Scoring System based on testing in over 4000 families. J. Med. Genet. 2017, 54, 674–681. [Google Scholar] [CrossRef]
- Familial Breast Cancer: Classification, care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer Clinical Guideline Published: 25 June 2013. Available online: www.nice.org.uk/guidance/cg164 (accessed on 12 October 2020).
- NHS England. National Genomic Test Directory 2020. Available online: https://www.england.nhs.uk/publication/national-genomic-test-directories/ (accessed on 12 October 2020).
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C. Breast cancer risk genes: Association analysis of rare coding variants in 34 genes in 60,466 cases and 53,461 controls. N. Engl. J. Med. 2020, 383. in press. [Google Scholar]
- Lalloo, F.; Boggis, C.R.; Evans, D.G.; Shenton, A.; Threlfall, A.G.; Howell, A. Screening by mammography, women with a family history of breast cancer. Eur. J. Cancer 1998, 34, 937–940. [Google Scholar] [CrossRef]
- Leach, M.O.; Boggis, C.R.; Dixon, A.K.; Easton, D.F.; Eeles, R.A.; Evans, D.G.; Gilbert, F.J.; Griebsch, I.; Hoff, R.J.; Kessar, P.; et al. MARIBS study group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS). Lancet 2005, 365, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Pegington, M.; Evans, D.G.; Howell, A.; Donnelly, L.S.; Wiseman, J.; Cuzick, J.M.; Harvie, M.N. Lifestyle behaviours and health measures of women at increased risk of breast cancer taking chemoprevention. Eur. J. Cancer Prev. 2019, 28, 500–506. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Breast Cancer 2018. Available online: https://www.wcrf.org/sites/default/files/Breast-cancer-report.pdf (accessed on 12 October 2020).
- Harvie, M.; Howell, A.; Vierkant, R.A.; Kumar, N.; Cerhan, J.R.; Kelemen, L.E.; Folsom, A.R.; Sellers, T.A. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol. Biomark. Prev. 2005, 14, 656–661. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. (Lond.) 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef]
- Harvie, M.; French, D.P.; Pegington, M.; Evans, D.G.R. Howell. Family History Lifestyle Study. ISRCTN Regist. 2020. serial online. [Google Scholar]
- Cuzick, J.; Forbes, J.; Edwards, R.; Baum, M.; Cawthorn, S.; Coates, A.; Hamed, A.; Howell, A.; Powles, T. IBIS investigators. First results from the International Breast Cancer Intervention Study (IBIS-I): A randomised prevention trial. Lancet 2002, 360, 817–824. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Knox, J.; Cawthorn, S.; Saunders, C.; Roche, N.; Mansel, R.E.; von Minckwitz, G.; et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 2014, 383, 1041–1048. [Google Scholar] [CrossRef]
- Lalloo, F.; Baildam ABrain, A.; Hopwood, P.; Evans, D.G.; Howell, A. A protocol for preventative mastectomy in women with an increased lifetime risk of breast cancer. Eur. J. Surg. Oncol. 2000, 26, 711–713. [Google Scholar] [CrossRef]
- Gandhi, A.; Duxbury, P.; Murphy, J.; Foden, P.; Lalloo, F.; Clancy, T.; Wisely, J.; Howell, A.; Evans, D.G. Patient Reported Outcome Measures in a Cohort of Patients at High Risk of Breast Cancer Treated by Bilateral Risk Reducing Mastectomy and Breast Reconstruction. Plast. Reconstr. Surg. in press.
- Hopwood, P.; Lee, A.; Shenton, A.; Baildam, A.; Brain, A.; Lalloo, F.; Evans, G.; Howell, A. Clinical follow-up after bilateral risk reducing (‘prophylactic’) mastectomy: Mental health and body image outcomes. Psychooncology 2000, 9, 462–472. [Google Scholar] [CrossRef]
- Hatcher, M.B.; Fallowfield, L.; A’Hern, R. The psychosocial impact of bilateral prophylactic mastectomy: Prospective study using questionnaires and semistructured interviews. BMJ 2001, 322, 76. [Google Scholar] [CrossRef] [PubMed]
- Tyrer-Cuzick (IBIS) Risk Evaluation Tool. Available online: riskevaluator@ems-trials.org (accessed on 20 October 2020).
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. Version 2.BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Evans, D.G.; Howell, A.; et al. Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet 2020, 395, 117–122. [Google Scholar] [CrossRef]
- Evans, D.G.; Lalloo, F.; Ashcroft, L.; Shenton, A.; Clancy, T.; Baildam, A.D.; Brain, A.; Hopwood, P.; Howell, A. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2318–2324. [Google Scholar] [CrossRef]
- Evans, D.G.; Brentnall, A.R.; Harvie, M.; Dawe, S.; Sergeant, J.C.; Stavrinos, P.; Astley, S.; Wilson, M.; Ainsworth, J.; Cuzick, J.; et al. Breast cancer risk in young women in the national breast screening programme: Implications for applying NICE guidelines for additional screening and chemoprevention. Cancer Prev. Res. (Phila.) 2014, 7, 993–1001. [Google Scholar] [CrossRef]
- Evans, D.G.; Barwell, J.; Eccles, D.M.; Collins, A.; Izatt, L.; Jacobs, C.; Donaldson, A.; Brady, A.F.; Cuthbert, A.; Harrison, R.; et al. The Angelina Jolie effect: How high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 2014, 16, 442. [Google Scholar] [CrossRef]
- Evans, D.G.; Wisely, J.; Clancy, T.; Lalloo, F.; Wilson, M.; Johnson, R.; Duncan, J.; Barr, L.; Gandhi, A.; Howell, A. Longer term effects of the Angelina Jolie effect: Increased risk-reducing mastectomy rates in BRCA carriers and other high-risk women. Breast Cancer Res. 2015, 17, 143. [Google Scholar] [CrossRef]
- Evans, D.G.; Donnelly, L.S.; Harkness, E.F.; Astley, S.M.; Stavrinos, P.; Dawe, S.; Watterson, D.; Fox, L.; Sergeant, J.C.; Ingham, S.; et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br. J. Cancer 2016, 114, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Pankratz, V.S.; Scott, C.G.; Haeberle, L.; Ziv, E.; Jensen, M.R.; Brandt, K.R.; Whaley, D.H.; Olson, J.E.; Heusinger, K.; et al. The contributions of breast density and common genetic variation to breast cancer risk. J. Natl. Cancer Inst. 2015, 107, dju397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rice, M.; Tworoger, S.S.; Rosner, B.A.; Eliassen, A.H.; Tamimi, R.M.; Joshi, A.D.; Lindstrom, S.; Qian, J. Addition of a polygenic risk score, mammographic density, and endogenous hormones to existing breast cancer risk prediction models: A nested case-control study. PLoS Med. 2018, 15, e1002644. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Liao, Y.; Whittemore, A.S.; Leoce, N.; Buchsbaum, R.; Zeinomar, N.; Dite, G.S.; Chung, W.K.; Knight, J.A.; Southey, M.C.; et al. 10-year performance of four models of breast cancer risk: A validation study. Lancet Oncol. 2019, 20, 504–517. [Google Scholar] [CrossRef]
- Pal, C.P.; Wilcox, A.N.; Brook, M.N.; Zhang, Y.; Ahearn, T.; Orr, N.; Coulson, P.; Schoemaker, M.J.; Jones, M.E.; Gail, M.H.; et al. Comparative Validation of Breast Cancer Risk Prediction Models and Projections for Future Risk Stratification. J. Natl. Cancer Inst. 2020, 112, 278–285. [Google Scholar] [CrossRef]
- Easton, D.F.; Pooley, K.A.; Dunning, A.M.; Pharoah, P.D.; Thompson, D.; Ballinger, D.G.; Struewing, J.P.; Morrison, J.; Field, H.; Luben, R.; et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007, 447, 1087–1093. [Google Scholar] [CrossRef]
- Turnbull, C.; Ahmed, S.; Morrison, J.; Pernet, D.; Renwick, A.; Maranian, M.; Seal, S.; Ghoussaini, M.; Hines, S.; Healey, C.S.; et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat. Genet. 2010, 42, 504–507. [Google Scholar] [CrossRef]
- Evans, D.G.; Brentnall, A.; Byers, H.; Harkness, E.; Stavrinos, P.; Howell, A.; FH-risk study Group; Newman, W.G.; Cuzick, J. The impact of a panel of 18 SNPs on breast cancer risk in women attending a UK familial screening clinic: A case-control study. J. Med. Genet. 2017, 54, 111–113. [Google Scholar] [CrossRef]
- Van Veen, E.M.; Brentnall, A.R.; Byers, H.; Harkness, E.F.; Astley, S.M.; Sampson, S.; Howell, A.; Newman, W.G.; Cuzick, J.; Evans, D.G.R. Use of Single-Nucleotide Polymorphisms and Mammographic Density Plus Classic Risk Factors for Breast Cancer Risk Prediction. JAMA Oncol. 2018, 4, 476–482. [Google Scholar] [CrossRef]
- Brentnall, A.R.; van Veen, E.M.; Harkness, E.F.; Rafiq, S.; Byers, H.; Astley, S.M.; Sampson, S.; Howell, A.; Newman, W.G.; Cuzick, J.; et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int. J. Cancer 2020, 146, 2122–2129. [Google Scholar] [CrossRef]
- Maurice, A.R.; Evans, D.G.R.; Shenton, A.; Ashcroft, L.; Baildam, A.; Barr, L.; Byrne, G.; Bundred, N.; Boggis, C.; Wilson, M.; et al. Howell A Screening younger women with a family history of breast cancer-does early detection improve outcome? Eur. J. Cancer 2006, 42, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W. FH01 collaborative teams. Mammographic surveillance in women younger than 50 years who have a family history of breast cancer: Tumour characteristics and projected effect on mortality in the prospective, single-arm, FH01 study. Lancet Oncol. 2010, 11, 1127–1134. [Google Scholar] [CrossRef]
- Evans, D.G.; Thomas, S.; Caunt, J.; Burch, A.; Brentnall, A.R.; Roberts, L.; Howell, A.; Wilson, M.; Fox, R.; Hillier, S.; et al. Final Results of the Prospective FH02 Mammographic Surveillance Study of Women Aged 35-39 at Increased Familial Risk of Breast Cancer. EClinicalMedicine 2019, 7, 39–46. [Google Scholar] [CrossRef]
- Van den Ende, C.; Oordt-Speets, A.M.; Vroling, H.; van Agt, H.M.E. Benefits and harms of breast cancer screening with mammography in women aged 40-49 years: A systematic review. Int. J. Cancer 2017, 141, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Vulkan, D.; Cuckle, H.; Parmar, D.; Sheikh, S.; Smith, R.A.; Evans, A.; Blyuss, O.; Johns, L.; Ellis, I.O.; et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): Final results of a randomised, controlled trial. Lancet Oncol. 2020, 21, 1165–1172. [Google Scholar] [CrossRef]
- Shieh, Y.; Eklund, M.; Madlensky, L.; Sawyer, S.D.; Thompson, C.K.; Stover Fiscalini, A.; Ziv, E.; Van’t Veer, L.J.; Esserman, L.J.; Tice, J.A.; et al. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- UNICANCER (2018) My Personalized Breast Screening (myPeBS). Available online: https://clinicaltrials.gov/ct2/show/NCT0367233 (accessed on 12 October 2020).
- Williams, M.; Woof, V.G.; Donnelly, L.S.; Howell, A.; Evans, D.G.; French, D.P. Risk stratified breast cancer screening: UK healthcare policy decision-making stakeholders’ views on a low-risk breast screening pathway. BMC Cancer 2020, 20, 680. [Google Scholar] [CrossRef]
- Ionescu, G.V.; Fergie, M.; Berks, M.; Harkness, E.F.; Hulleman, J.; Brentnall, A.R.; Cuzick, J.; Evans, D.G.; Astley, S.M. Prediction of reader estimates of mammographic density using convolutional neural networks. J. Med. Imaging (Bellingham) 2019, 6, 031405. [Google Scholar] [CrossRef]
- Hopper, J.L.; Nguyen, T.L.; Schmidt, D.F.; Makalic, E.; Song, Y.M.; Sung, J.; Dite, G.S.; Dowty, J.G.; Li, S. Going Beyond Conventional Mammographic Density to Discover Novel Mammogram-Based Predictors of Breast Cancer Risk. J. Clin. Med. 2020, 9, 627. [Google Scholar] [CrossRef]
- Evans, D.G.; Kesavan, N.; Lim, Y.; Gadde, S.; Hurley, E.; Massat, N.J.; Maxwell, A.J.; Ingham, S.; Eeles, R.; Leach, M.O.; et al. MRI breast screening in high-risk women: Cancer detection and survival analysis. Breast Cancer Res. Treat. 2014, 145, 663–672. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Brekelmans, C.T.; Boetes, C.; Besnard, P.E.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Peterse, H.; Tilanus-Linthorst, M.M.; et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Plewes, D.B.; Hill, K.A.; Causer, P.A.; Zubovits, J.T.; Jong, R.A.; Cutrara, M.R.; DeBoer, G.; Yaffe, M.J.; Messner, S.J.; et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004, 292, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Harkness, E.; Howell, A.; Wilson, M.; Hurley, E.; Lim, Y.; Maxwell, A.; Moller, P. Intensive breast screening in BRCA2 mutation carriers is associated with reduced breast cancer specific and all cause mortality. Hered. Cancer Clin. Pract. 2016, 14, 8. [Google Scholar] [CrossRef]
- Hopper, J.L.; Dite, G.S.; MacInnis, R.J. Age-specific breast cancer risk by body mass index and familial risk: Prospective family study cohort (ProF-SC). Breast Cancer Res. 2018, 20, 132. [Google Scholar] [CrossRef]
- Arthur, R.S.; Wang, T.; Xue, X.; Kamensky, V.; Rohan, T.E. Genetic factors, adherence to healthy lifestyle behavior, and risk of invasive breast cancer among women in the UK Biobank. J. Natl. Cancer Inst 2020, 893–901. [Google Scholar] [CrossRef]
- Maas, P.; Barrdahl, M.; Joshi, A.D. Breast Cancer Risk from Modifiable and Nonmodifiable Risk Factors among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- Behaviour Change Guidance PH6. 2007. Available online: https://www.nice.org.uk/Guidance/PH6 (accessed on 12 October 2020).
- Wright, C.E.; Harvie, M.; Howell, A.; Evans, D.G.; Hulbert-Williams, N.; Donnelly, L.S. Beliefs about weight and breast cancer: An interview study with high risk women following a 12 month weight loss intervention. Hered Cancer Clin. Pract 2015, 13, 1. [Google Scholar] [CrossRef][Green Version]
- French, D.P.; Cameron, E.; Benton, J.S.; Deaton, C.; Harvie, M. Can Communicating Personalised Disease Risk Promote Healthy Behaviour Change? A Systematic Review of Systematic Reviews. Ann. Behav. Med. 2017, 51, 718–729. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Bonanni, B.; Costantino, J.P.; Cummings, S.; DeCensi, A.; Dowsett, M.; Forbes, J.F.; Ford, L.; LaCroix, A.Z.; et al. Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet 2013, 381, 1827–1834. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J.F.; IBIS-I Investigators. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Aragaki, A.K.; Manson, J.E.; Stefanick, M.L.; Pan, K.; Barrington, W.; Kuller, L.H.; Simon, M.S.; Lane, D.; et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.S.; Evans, D.G.; Wiseman, J.; Fox, J.; Greenhalgh, R.; Affen, J.; Juraskova, I.; Stavrinos, P.; Dawe, S.; Cuzick, J.; et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br. J. Cancer 2014, 110, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Sestak, I.; Forster, A.; Partridge, A.; Side, L.; Wolf, M.S. Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 575–590. [Google Scholar] [CrossRef]
- Fallowfield, L.; Fleissig, A.; Edwards, R.; West, A.; Powles, T.J.; Howell, A.; Cuzick, J. Tamoxifen for the prevention of breast cancer: Psychosocial impact on women participating in two randomized controlled trials. J. Clin. Oncol. 2001, 19, 1885–1892. [Google Scholar] [CrossRef]
- Smith, S.G.; Sestak, I.; Howell, A.; Forbes, J.; Cuzick, J. Participant-Reported Symptoms and Their Effect on Long-Term Adherence in the International Breast Cancer Intervention Study I (IBIS I). J. Clin. Oncol. 2017, 35, 2666–2673. [Google Scholar] [CrossRef]
- Sestak, I.; Smith, S.G.; Howell, A.; Forbes, J.F.; Cuzick, J. Early participant-reported symptoms as predictors of adherence to anastrozole in the International Breast Cancer Intervention Studies II. Ann. Oncol. 2018, 29, 504–509. [Google Scholar] [CrossRef]
- DeCensi, A.; Puntoni, M.; Guerrieri-Gonzaga, A.; Caviglia, S.; Avino, F.; Cortesi, L.; Taverniti, C.; Pacquola, M.G.; Falcini, F.; Gulisano, M.; et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J. Clin. Oncol. 2019, 37, 1629–1637. [Google Scholar] [CrossRef]
- Robertson, J.F.; Willsher, P.C.; Winterbottom, L.; Blamey, R.W.; Thorpe, S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur. J. Cancer 1999, 35, 214–218. [Google Scholar] [CrossRef]
- Nolan, E.; Vaillant, F.; Branstetter, D.; Pal, B.; Giner, G.; Whitehead, L.; Lok, S.W.; Mann, G.B.; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab); Rohrbach, K.; et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 2016, 22, 933–939. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Schaid, D.J.; Woods, J.E.; Crotty, T.P.; Myers, J.L.; Arnold, P.G.; Petty, P.M.; Sellers, T.A.; Johnson, J.L.; McDonnell, S.K.; et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N. Engl. J. Med. 1999, 340, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Razdan, S.N.; Patel, V.; Jewell, S.; McCarthy, C.M. Quality of life among patients after bilateral prophylactic mastectomy: A systematic review of patient-reported outcomes. Qual. Life Res. 2016, 25, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Jakub, J.W.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Shay, P.; Jacobs, J. Autologous reconstruction following nipple sparing mastectomy: A comprehensive review of the current literature. Gland Surg. 2018, 7, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.V.; Vucicevic Al Highton, L.; Harvey, J.R.; Johnson, R.; Kirwan, C.C.; Murphy, J. Medium term outcomes following immediate prepectoral implant based breast reconstruction using acellular dermal matrix. Br. J. Surg. 2020. [Google Scholar] [CrossRef]
- Gandhi, A.; Barr, L.; Johnson, R. Bioprosthetics: Changing the landscape for breast reconstruction. Eur. J. Surg. Oncol. 2013, 39, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.W.; Negenborn, V.L.; Twisk, D.J.W.R.; Rizopoulos, D.; Ket, J.C.F.; Smit, J.M.; Mullender, M.G. Autologous fat grafting in onco-plastic breast reconstruction: A systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 742–764. [Google Scholar] [CrossRef]
- Ingham, S.L.; Sperrin, M.; Baildam, A.; Ross, G.L.; Clayton, R.; Lalloo, F.; Buchan, I.; Howell, A.; Evans, D.G. Risk-reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Res. Treat. 2013, 142, 611–618. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef]
- Evans, D.G.; Howell, S.J.; Howell, A. Should unaffected female BRCA2 pathogenic variant carriers be told there is little or no advantage from risk reducing mastectomy? Fam. Cancer 2019, 18, 377–379. [Google Scholar] [CrossRef]
- Braude, L.; Kirsten, L.; Gilchrist, J.; Juraskova, I. A systematic review of women’s satisfaction and regret following risk-reducing mastectomy. Patient Educ. Couns. 2017, 100, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- French, D.P.; Astley, S.; Brentnall, A.R.; Cuzick, J.; Dobrashian, R.; Duffy, S.W.; Gorman, L.S.; Harkness, E.F.; Harrison, F.; Harvie, M.; et al. What are the benefits and harms of risk stratified screening as part of the NHS Breast Screening Programme? Study protocol for a multi-site non-randomised comparison of BC-Predict versus usual screening. BMC Cancer 2020, 20, 570. [Google Scholar] [CrossRef] [PubMed]
- French, D.P.; Southworth, J.; Howell, A.; Harvie, M.; Stavrinos, P.; Watterson, D.; Sampson, S.; Evans, D.G.; Donnely, L.S. Psychological impact of providing women with personalized ten-year breast cancer risk estimates. Br. J. Cancer 2018, 118, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, S.A.; McCarthy, E.P.; Phillips, R.S.; Burns, R.B. Breast cancer risk assessment and management in primary care: Provider attitudes, practices, and barriers. Cancer Detect Prev. 2007, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Keogh, L.A.; Steel, E.; Weideman, P.; Butow, P.; Collins, I.M.; Emery, J.D.; Mann, G.B.; Bickerstaffe, A.; Trainer, A.H.; Hopper, L.J.; et al. Consumer and clinician perspectives on personalising breast cancer prevention information. Breast 2019, 43, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Babb de Villiers, C.; Scheibl, F.; Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Easton, D.F.; McIntosh, J.G.; Emery, J.; et al. Evaluating clinician acceptability of the prototype CanRisk tool for predicting risk of breast and ovarian cancer: A multi-methods study. PLoS ONE 2020, 15, e0229999. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Development and validation of QMortality risk prediction algorithm to estimate short term risk of death and assess frailty: Cohort study. BMJ 2017, 358, j4208. [Google Scholar] [CrossRef]
- Lo, L.L.; Collins, I.M.; Bressel, M.; Butow, P.; Emery, J.; Keogh, L.; Weideman, P.; Steel, E.; Hopper, J.L.; Trainer, A.H.; et al. The iPrevent Online Breast Cancer Risk Assessment and Risk Management Tool: Usability and Acceptability Testing. JMIR Form Res. 2018, 2, e24. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.; Duffy, S.W.; Evans, D.G.; Garcia–Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk Determination and Prevention of Breast Cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
| Group | Number | % of All Referrals | Known Gene in Family | % | High Risk | % | Moderate | % | Average | % | BRCA +v at Last Follow Up | % | Number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GP | 8004 | 55.9% | 165 | 2.1% | 2646 | 33.1% | 3542 | 44.3% | 1651 | 20.6% | 222 | 2.77% | 8004 |
| Surgery | 2157 | 15.1% | 38 | 1.8% | 751 | 34.9% | 831 | 38.6% | 537 | 24.9% | 55 | 2.55% | 2157 |
| Genetics | 2868 | 20.0% | 662 | 23.5% | 1445 | 51.5% | 594 | 21.2% | 168 | 6.0% | 406 | 14.16% | 2868 |
| PROCAS | 448 | 3.1% | 2 | 0.4% | 311 | 69.4% | 124 | 27.7% | 11 | 2.5% | 0 | 0.00% | 448 |
| Other | 833 | 5.8% | 54 | 6.5% | 307 | 36.9% | 299 | 35.9% | 173 | 20.8% | 53 | 6.36% | 833 |
| Totals | 14,311 | 921 | 6.4% | 5460 | 38.2% | 5390 | 37.7% | 2540 | 17.7% | 736 | 5.14% |
| A | B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Number Seen | Randomised to IBIS I/II Trial | Number Invited to Trial | % Joined Trial | Seen Since 2013 | Prescribed Chemoprevention | % | Joined Chemoprevention Trial | % | Either Trial or CP | |
| Known gene in family | 921 | 11 | 554 | 1.99% | 364 | 2 | 0.55% | 2 | 0.55% | 4 | 1.10% |
| High | 5488 | 465 | 3178 | 14.63% | 2604 | 162 | 6.22% | 168 | 6.45% | 323 | 12.40% |
| Moderate | 5370 | 431 | 3200 | 13.47% | 2221 | 118 | 5.31% | 113 | 5.09% | 226 | 10.18% |
| Average | 2532 | 112 | 1188 | 9.43% | 609 | 0 | 0 | 0 | |||
| Total | 14,311 | 1019 | 8120 | 12.55% | 5798 | 282 | 4.86% | 283 | 4.88% | 553 | 9.54% |
| Intervention | Current Practice | Issues to Be Addressed |
|---|---|---|
| Referral | Referrals from primary & secondary care established | Only 20% of women with FH referred Very few with ‘other’ risk factors |
| Risk estimation | Evolved to include more risk factors eg. mammographic density & SNPs | Using all factors approximately 20% of population at moderate & high risk |
| Gene testing | BRCA1/2 & PALB2 available in NHS 10% threshold for PV used (NICE) | New data suggest panel of 9 genes be should be used |
| Screening | Annual mammography & MRI established | Breast density & SNPs being tested in trials of risk & density adapted screening |
| Lifestyle change | Observational studies suggest introduction would be valuable | Mechanisms for general application being tested |
| Chemoprevention | Longer term risk and benefits established | Application suboptimal—consider assessment at home and primary care |
| Risk reducing surgery | Offer at appropriate risk levels established | Continue improvements in psychological and surgical techniques |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, A.; Gandhi, A.; Howell, S.; Wilson, M.; Maxwell, A.; Astley, S.; Harvie, M.; Pegington, M.; Barr, L.; Baildam, A.; et al. Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020). Cancers 2020, 12, 3697. https://doi.org/10.3390/cancers12123697
Howell A, Gandhi A, Howell S, Wilson M, Maxwell A, Astley S, Harvie M, Pegington M, Barr L, Baildam A, et al. Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020). Cancers. 2020; 12(12):3697. https://doi.org/10.3390/cancers12123697
Chicago/Turabian StyleHowell, Anthony, Ashu Gandhi, Sacha Howell, Mary Wilson, Anthony Maxwell, Susan Astley, Michelle Harvie, Mary Pegington, Lester Barr, Andrew Baildam, and et al. 2020. "Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020)" Cancers 12, no. 12: 3697. https://doi.org/10.3390/cancers12123697
APA StyleHowell, A., Gandhi, A., Howell, S., Wilson, M., Maxwell, A., Astley, S., Harvie, M., Pegington, M., Barr, L., Baildam, A., Harkness, E., Hopwood, P., Wisely, J., Wilding, A., Greenhalgh, R., Affen, J., Maurice, A., Cole, S., Wiseman, J., ... Evans, D. G. (2020). Long-Term Evaluation of Women Referred to a Breast Cancer Family History Clinic (Manchester UK 1987–2020). Cancers, 12(12), 3697. https://doi.org/10.3390/cancers12123697






