Living with Metastatic Cancer: A Roadmap for Future Research
Abstract
Simple Summary
Abstract
1. Introduction
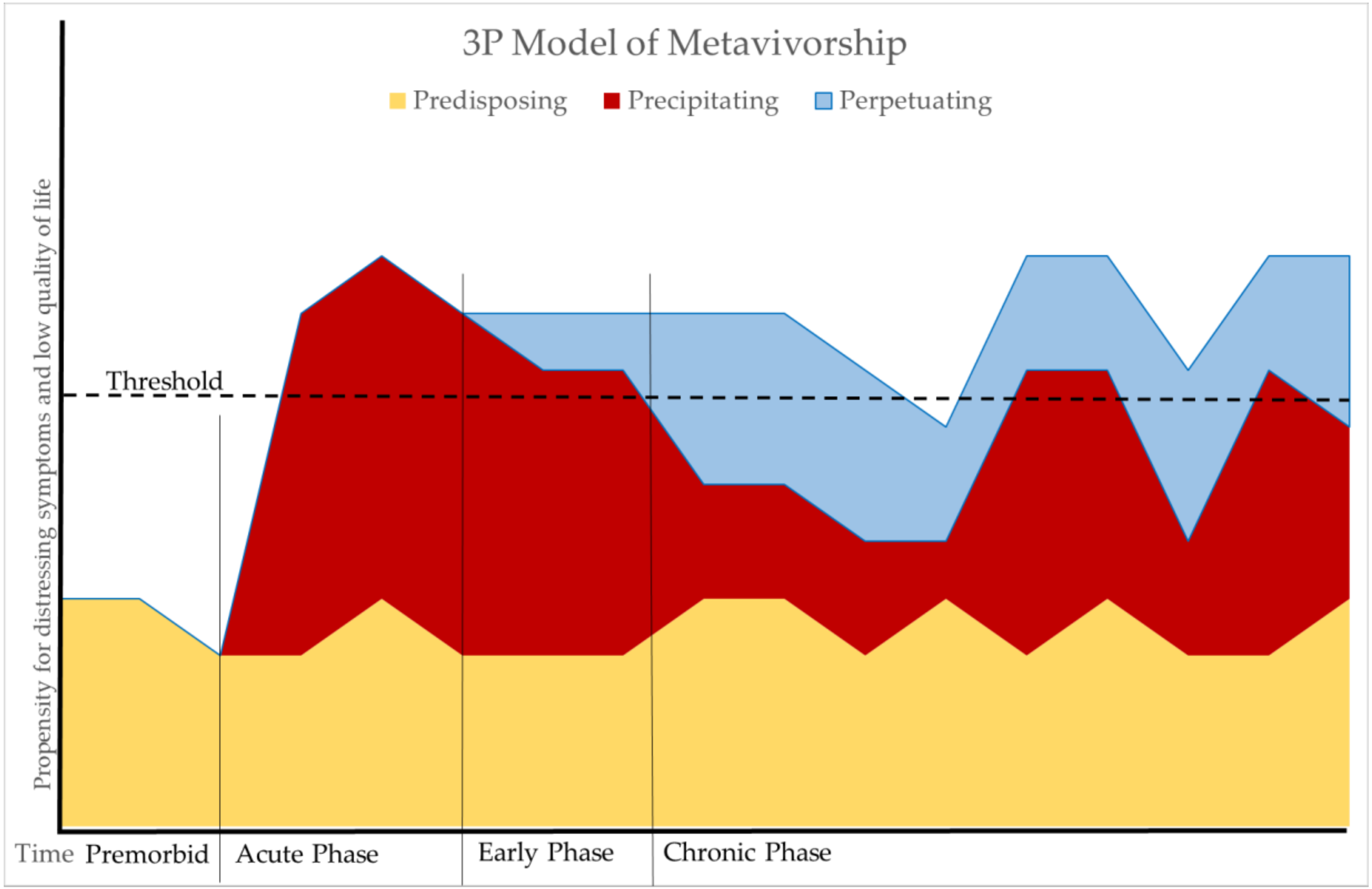
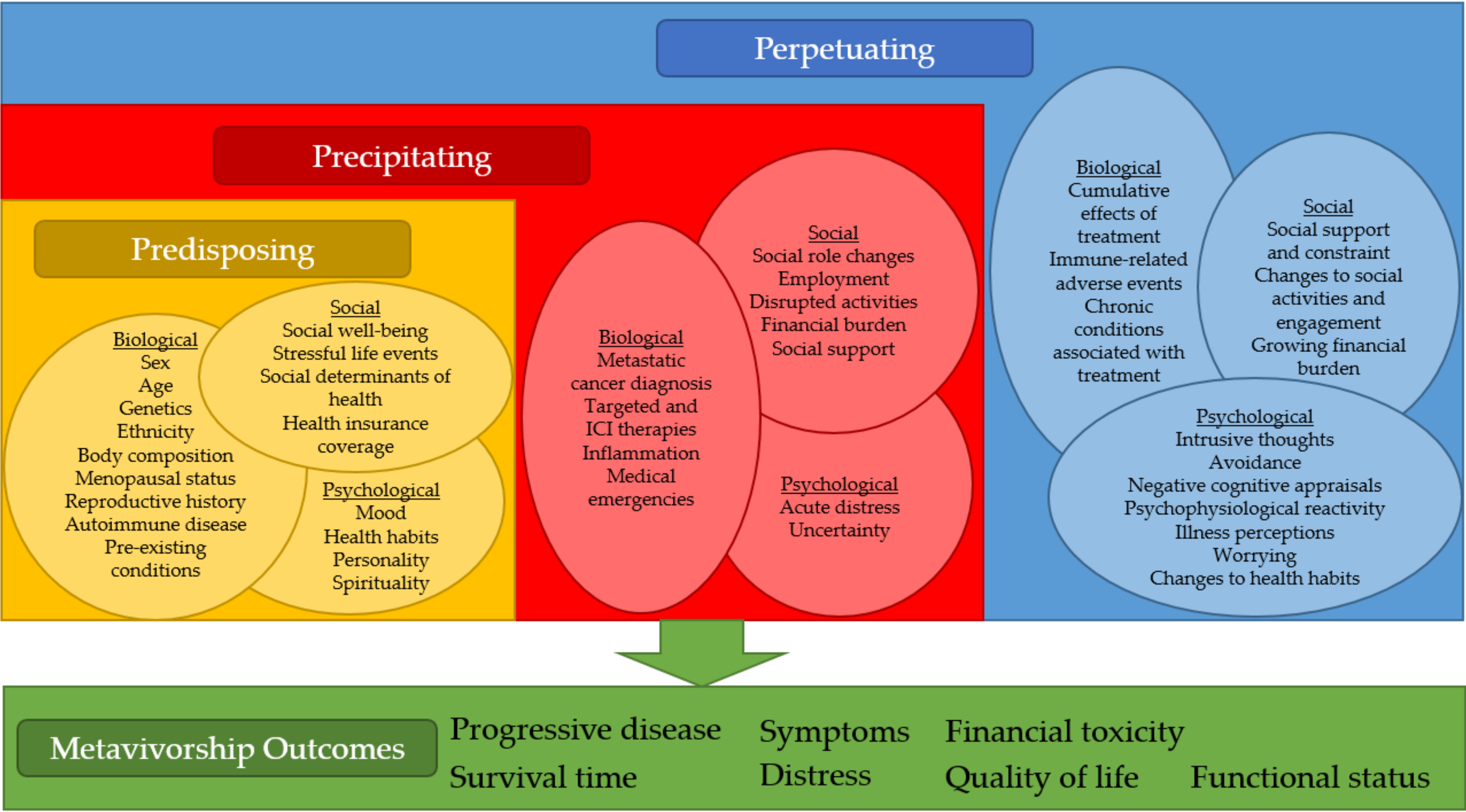
2. The Predisposing, Precipitating, and Perpetuating (3P) Model of Metavivorship
2.1. Predisposing Factors
2.2. Precipitating Factors
2.3. Perpetuating Factors
3. Implications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hewitt, M.; Greenfield, S.; Stovall, E. From Cancer Patient to Cancer Survivor: Lost in Transition; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Davis, K. Opportunities and barriers to high-quality care in metastatic breast cancer from a patient perspective. In Proceedings of the ASCO 2019, Chicago, IL, USA, 4 June 2019. [Google Scholar]
- Spoozak, L.; Wulff-Burchfield, E.; Brooks, J.V. Rallying cry from the place in between. JCO Oncol. Pract. 2020, 16, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Nipp, R.D.; Ganz, P.A. Addressing the survivorship care needs of patients receiving extended cancer treatment. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, M.; Brudin, L.; Tejler, G. Improved survival in metastatic breast cancer 1985–2016. Breast 2017, 31, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New systematic therapies and trends in cutaneous melanoma deaths among US whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- US. Department of Health and Human Services Food and Drug Administration. Patient-reported outcome measures: Use in medical product development to support labeling claims. In Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration: Silver Spring, MD, USA, 2009. [Google Scholar]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatr. Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Wright, C.D.; Tiani, A.G.; Billingsley, A.L.; Steinman, S.A.; Larkin, K.T.; McNeil, D.W. A framework for understanding the role of psychological processes in disease development, maintenance, and treatment: The 3P-disease model. Front. Psychol. 2019, 10. [Google Scholar] [CrossRef]
- Wacholder, S.; Hartge, P.; Prentice, R.; Garcia-Closas, M.; Feigelson, H.S.; Diver, W.R.; Thun, M.J.; Cox, D.G.; Hankinson, S.E.; Kraft, P.; et al. Performance of common genetic variants in breast-cancer risk models. N. Engl. J. Med. 2010, 362, 986–993. [Google Scholar] [CrossRef]
- Simapivapan, P.; Boltong, A.; Hodge, A. To what extent is alcohol consumption associated with breast cancer recurrence and second primary breast cancer?: A systematic review. Cancer Treat. Rev. 2016, 50, 155–167. [Google Scholar] [CrossRef]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Ando, S.; Catalano, S. The weight of obesity in breast cancer progression and metastasis: Clinical and molecular perspectives. Semin. Cancer Biol. 2020, 60, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hanson, H.A.; Smith, K.R.; Zimmer, Z. Reproductive history and later-life comorbidity trajectories: A medicare-lnked cohort study from the Utah population database. Demography 2015, 52, 2021–2049. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and risk factors of melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Jing, L.; Su, L.; Ring, B.Z. Ethnic background and genetic variation in the evaluation of cancer risk: A systematic review. PLoS ONE 2014, 9, e97522. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; Eiger, D.; Ameye, L.; Ponde, N.; Caparica, R.; De Angelis, C.; Brandao, M.; Desmedt, C.; Di Cosimo, S.; Kotecki, N.; et al. Clinical implications of body mass index in metastatic breast cancer patients treated with abemaciclib and endocrine therapy. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sattar, J.; Kartolo, A.; Hopman, W.M.; Lakoff, J.M.; Baetz, T. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J. Geriatr. Oncol. 2019, 10, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Crocetti, E.; Fancelli, L.; Manneschi, G.; Caldarella, A.; Pimpinelli, N.; Chiarugi, A.; Nardini, P.; Buzzoni, C. Melanoma survival: Sex does matter, but we do not know how. Eur. J. Cancer Prev. 2016, 25, 404–409. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Sauder, C.A.M.; Goldfarb, M.; Li, Q.; Wun, T.; Keegan, T.H.M. Disparities in the occurrence of late effects following treatment among adolescent and young adult melanoma survivors. Cancer Epidemiol. Biomark. Prev. 2020. [Google Scholar] [CrossRef]
- Chu, M.P.; Li, Y.; Ghosh, S.; Sass, S.; Smylie, M.; Walker, J.; Sawyer, M.B. Body composition is prognostic and predictive of ipilimumab activity in metastatic melanoma. J. Cachexia Sarcopenia Muscle 2020, 11, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.S.; Waikar, S.S.; Johnson, A.E.W.; Buchbinder, E.I.; Haq, R.; Hodi, F.S.; Schoenfeld, J.D.; Ott, P.A. Complex inter-relationship of body mass index, gender and serum creatinine on survival: Exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J. Immunother. Cancer 2019, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.; Verma, V.; Butler, E.B.; Teh, B.S. Racial and socioeconomic disparities in the delivery of immunotherapy for metastatic melanoma in the United States. J. Immunother. 2019, 42, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, H.; Jiang, P.; Jiang, X.; He, Z.; Guo, C.; Mou, Y. Trend and socioeconomic disparities in survival outcome of metastatic melanoma after approval of immune checkpoint inhibitors: A population-based study. Am. J. Transl. Res. 2020, 12, 3767–3779. [Google Scholar]
- Basch, E.; Jia, X.; Heller, G.; Barz, A.; Sit, L.; Fruscione, M.; Appawu, M.; Iasonos, A.; Atkinson, T.; Goldfarb, S.; et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J. Natl. Cancer Inst. 2009, 101, 1624–1632. [Google Scholar] [CrossRef]
- Petersen, M.A.; Larsen, H.; Pedersen, L.; Sonne, N.; Groenvold, M. Assessing health-related quality of life in palliative care: Comparing patient and physician assessments. Eur. J. Cancer 2006, 42, 1159–1166. [Google Scholar] [CrossRef]
- Guan, M.; Gresham, G.; Shinde, A.; Lapite, I.; Gong, J.; Placencio-Hickok, V.R.; Forrest, C.B.; Hendifar, A.E. Priority rankings of patient-reported outcomes for pancreatic ductal adenocarcinoma: A comparison of patient and physician perspectives. J. Natl. Compr. Cancer Netw. 2020, 18, 1075–1083. [Google Scholar] [CrossRef]
- Pakhomov, S.V.; Jacobsen, S.J.; Chute, C.G.; Roger, V.L. Agreement between patient-reported symptoms and their documentation in the medical record. Am. J. Manag. Care 2008, 14, 530–539. [Google Scholar]
- Finn, R.S.; Crown, J.P.; Ettl, J.; Schmidt, M.; Bondarenko, I.M.; Lang, I.; Pinter, T.; Boer, K.; Patel, R.; Randolph, S.; et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016, 18, 67. [Google Scholar] [CrossRef]
- Kish, J.K.; Ward, M.A.; Garofalo, D.; Ahmed, H.V.; McRoy, L.; Laney, J.; Zanotti, G.; Braverman, J.; Yu, H.; Feinberg, B.A. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res. 2018, 20, 37. [Google Scholar] [CrossRef]
- Lee, K.W.C.; Lord, S.; Finn, R.S.; Lim, E.; Martin, A.; Loi, S.; Lynch, J.; Friedlander, M.; Lee, C.K. The impact of ethnicity on efficacy and toxicity of cyclin D kinase 4/6 inhibitors in advanced breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019, 174, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Turner, N.C.; Finn, R.S.; Joy, A.A.; Verma, S.; Harbeck, N.; Masuda, N.; Im, S.A.; Huang, X.; Kim, S.; et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: A pooled analysis of randomised PALOMA clinical studies. Eur. J. Cancer 2018, 101, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.; Wildiers, H.; Awada, A.; de Azambuja, E.; Deliens, C.; Lago, L.D. Targeted therapy for breast cancer in older patients. J. Geriatr. Oncol. 2020, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Omarini, C.; Piacentini, F.; Sperduti, I.; Barbolini, M.; Isca, C.; Toss, A.; Cortesi, L.; Barbieri, E.; Dominici, M.; Moscetti, L. Combined endocrine approaches vs endocrine therapy alone as first line treatment in elderly patients with hormone receptor-positive, HER2 negative, advanced breast cancer: To prescribe for the patient or the physician? A meta-analysis of phase II and III randomized clinical trials. BMC Cancer 2020, 20, 418. [Google Scholar] [CrossRef]
- Clifton, K.; Min, Y.; Kimmel, J.; Litton, J.; Tripathy, D.; Karuturi, M. Progression-free survival (PFS) and toxicities of palbociclib in a geriatric population. Breast Cancer Res. Treat. 2019, 175, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Coens, C.; Suciu, S.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; et al. Health-related quality of life with adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): Secondary outcomes of a multinational, randomised, double-blind, phase 3 trial. Lancet Oncol. 2017, 18, 393–403. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Ascierto, P.A.; Robert, C.; Hassel, J.C.; Rutkowski, P.; Savage, K.J.; Taylor, F.; Coon, C.; Gilloteau, I.; et al. Effect of nivolumab on health-related quality of life in patients with treatment-naive advanced melanoma: Results from the phase III CheckMate 066 study. Ann. Oncol. 2016, 27, 1940–1946. [Google Scholar] [CrossRef]
- Petrella, T.M.; Robert, C.; Richtig, E.; Miller, W.H., Jr.; Masucci, G.V.; Walpole, E.; Lebbe, C.; Steven, N.; Middleton, M.R.; Hille, D.; et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur. J. Cancer 2017, 86, 115–124. [Google Scholar] [CrossRef]
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015, 13, 211. [Google Scholar] [CrossRef]
- Weber, J.S.; Yang, J.C.; Atkins, M.B.; Disis, M.L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 2015, 33, 2092–2099. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.P.; Song, H.; Ye, F.; Moslehi, J.J.; Balko, J.M.; Salem, J.E.; Johnson, D.B. Demographic factors associated with toxicity in patients treated with anti-programmed cell death-1 therapy. Cancer Immunol. Res. 2020, 8, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: Are we all equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.E.; Power, D.G.; O’Reilly, A.; Donnellan, P.; Cushen, S.J.; O’Sullivan, K.; Twomey, M.; Woodlock, D.P.; Redmond, H.P.; Ryan, A.M. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer. 2017, 116, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018, 25, e403–e410. [Google Scholar] [CrossRef]
- Kahler, K.C.; Eigentler, T.K.; Gesierich, A.; Heinzerling, L.; Loquai, C.; Meier, F.; Meiss, F.; Pfohler, C.; Schlaak, M.; Terheyden, P.; et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol. Immunother. 2018, 67, 825–834. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, H.C. Increased risk of cancer subsequent to severe depression—A nationwide population-based study. J. Affect. Disord. 2011, 131, 200–206. [Google Scholar] [CrossRef]
- Goldacre, M.J.; Wotton, C.J.; Yeates, D.; Seagroatt, V.; Flint, J. Cancer in people with depression or anxiety: Record-linkage study. Soc. Psychiatry Psychiatr. Epidemiol. 2007, 42, 683–689. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2019, 25, 1487–1499. [Google Scholar] [CrossRef]
- Jia, Y.; Li, F.; Liu, Y.F.; Zhao, J.P.; Leng, M.M.; Chen, L. Depression and cancer risk: A systematic review and meta-analysis. Public Health 2017, 149, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.L.; Gallo, J.J.; Eaton, W.W. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control 2010, 21, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.J.; Lee, Y.J.; Yang, Y.R.; Park, S.; Suh, P.G.; Follo, M.Y.; Cocco, L.; Ryu, S.H. Molecular mechanisms underlying psychological stress and cancer. Curr. Pharm. Des. 2016, 22, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Giese-Davis, J.; Collie, K.; Rancourt, K.M.; Neri, E.; Kraemer, H.C.; Spiegel, D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J. Clin. Oncol. 2011, 29, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, C.; Hoerger, M.; Turiano, N.A.; Duberstein, P. Big Five personality and health in adults with and without cancer. J. Health Psychol. 2019, 24, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Aschwanden, D.; Gerend, M.A.; Luchetti, M.; Stephan, Y.; Sutin, A.R.; Terracciano, A. Personality traits and preventive cancer screenings in the Health Retirement Study. Prev. Med. 2019, 126, 105763. [Google Scholar] [CrossRef]
- Hulbert-Williams, N.; Neal, R.; Morrison, V.; Hood, K.; Wilkinson, C. Anxiety, depression and quality of life after cancer diagnosis: What psychosocial variables best predict how patients adjust? Psycho-Oncol. 2012, 21, 857–867. [Google Scholar] [CrossRef]
- Morgan, S.; Cooper, B.; Paul, S.; Hammer, M.J.; Conley, Y.P.; Levine, J.D.; Miaskowski, C.; Dunn, L.B. Association of personality profiles with depressive, anxiety, and cancer-related symptoms in patients undergoing chemotherapy. Personal. Individ. Differ. 2017, 117, 130–138. [Google Scholar] [CrossRef]
- Puchalski, C.M. Spirituality in the cancer trajectory. Ann. Oncol. 2012, 23 (Suppl. S3), 49–55. [Google Scholar] [CrossRef]
- Gall, T.L.; Kristjansson, E.; Charbonneau, C.; Florack, P. A longitudinal study on the role of spirituality in response to the diagnosis and treatment of breast cancer. J. Behav. Med. 2009, 32, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Jutagir, D.R.; Blomberg, B.B.; Carver, C.S.; Lechner, S.C.; Timpano, K.R.; Bouchard, L.C.; Gudenkauf, L.M.; Jacobs, J.M.; Diaz, A.; Lutgendorf, S.K.; et al. Social well-being is associated with less pro-inflammatory and pro-metastatic leukocyte gene expression in women after surgery for breast cancer. Breast Cancer Res. Treat. 2017, 165, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Butler, L.D.; Koopman, C.; Giese-Davis, J.; Carlson, R.; Spiegel, D. Stress history and breast cancer recurrence. J. Psychosom. Res. 2007, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.J.; Riba, M.B.; Spiegel, D. Post-traumatic stress disorder and cancer. Lancet Psychiatry 2017, 4, 330–338. [Google Scholar] [CrossRef]
- Langford, D.J.; Cooper, B.; Paul, S.; Humphreys, J.; Keagy, C.; Conley, Y.P.; Hammer, M.J.; Levine, J.D.; Wright, F.; Melisko, M.; et al. Evaluation of coping as a mediator of the relationship between stressful life events and cancer-related distress. Health Psychol. 2017, 36, 1147–1160. [Google Scholar] [CrossRef]
- Lentz, R.; Benson, A.B., 3rd; Kircher, S. Financial toxicity in cancer care: Prevalence, causes, consequences, and reduction strategies. J. Surg. Oncol. 2019, 120, 85–92. [Google Scholar] [CrossRef]
- Carrera, P.M.; Kantarjian, H.M.; Blinder, V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef]
- Gordon, L.G.; Merollini, K.M.D.; Lowe, A.; Chan, R.J. A systematic review of financial toxicity among cancer survivors: We can’t pay the co-pay. Patient 2017, 10, 295–309. [Google Scholar] [CrossRef]
- Hastert, T.A.; Banegas, M.P.; Hamel, L.M.; Reed, A.R.; Baird, T.; Beebe-Dimmer, J.L.; Schwartz, A.G. Race, financial hardship, and limiting care due to cost in a diverse cohort of cancer survivors. J. Cancer Surviv. 2019, 13, 429–437. [Google Scholar] [CrossRef]
- de Souza, J.A.; Yap, B.J.; Wroblewski, K.; Blinder, V.; Araújo, F.S.; Hlubocky, F.J.; Nicholas, L.H.; O’Connor, J.M.; Brockstein, B.; Ratain, M.J.; et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 2017, 123, 476–484. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ferrara, N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol. Res. 2016, 4, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, A.E.; Dowling, R.J.O.; Ennis, M.; Amir, E.; Elser, C.; Brezden-Masley, C.; Vandenberg, T.; Lee, E.; Fazaee, K.; Stambolic, V.; et al. Association of metabolic, inflammatory, and tumor markers with circulating tumor cells in metastatic breast cancer. JNCI Cancer Spectr. 2018, 2, pky028. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol. Immunother. 2017, 66, 1015–1023. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kitano, S.; Takahashi, A.; Tsutsumida, A.; Namikawa, K.; Tanese, K.; Abe, T.; Funakoshi, T.; Yamamoto, N.; Amagai, M.; et al. Nivolumab for advanced melanoma: Pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 2016, 7, 77404–77415. [Google Scholar] [CrossRef]
- Bjoern, J.; Juul Nitschke, N.; Zeeberg Iversen, T.; Schmidt, H.; Fode, K.; Svane, I.M. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology 2016, 5, e1100788. [Google Scholar] [CrossRef]
- Yamazaki, N.; Kiyohara, Y.; Uhara, H.; Iizuka, H.; Uehara, J.; Otsuka, F.; Fujisawa, Y.; Takenouchi, T.; Isei, T.; Iwatsuki, K.; et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017, 108, 1022–1031. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Weinrib, A.Z.; Penedo, F.; Russell, D.; DeGeest, K.; Costanzo, E.S.; Henderson, P.J.; Sephton, S.E.; Rohleder, N.; Lucci, J.A., 3rd; et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J. Clin. Oncol. 2008, 26, 4820–4827. [Google Scholar] [CrossRef]
- Lacourt, T.E.; Vichaya, E.G.; Chiu, G.S.; Dantzer, R.; Heijnen, C.J. The high costs of low-grade inflammation: Persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front. Behav. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef]
- Lacourt, T.E.; Vichaya, E.G.; Escalante, C.; Manzullo, E.F.; Gunn, B.; Hess, K.R.; Heijnen, C.J.; Dantzer, R. An effort expenditure perspective on cancer-related fatigue. Psychoneuroendocrinology 2018, 96, 109–117. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Vichaya, E.G.; Gross, P.S.; Ford, B.G.; Scott, K.A.; Estrada, D.; Vermeer, D.W.; Vermeer, P.; Dantzer, R. Interleukin 6-independent metabolic reprogramming as a driver of cancer-related fatigue. Brain Behav. Immun. 2020, 88, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Vichaya, E.G.; Christian, D.L.; Molkentine, J.M.; Vermeer, D.W.; Gross, P.S.; Vermeer, P.D.; Lee, J.H.; Dantzer, R. Tumor-associated fatigue in cancer patients develops independently of IL1 signaling. Cancer Res. 2018, 78, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Weinrib, A.Z.; Sephton, S.E.; Degeest, K.; Penedo, F.; Bender, D.; Zimmerman, B.; Kirschbaum, C.; Sood, A.K.; Lubaroff, D.M.; Lutgendorf, S.K. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer 2010, 116, 4410–4419. [Google Scholar] [CrossRef]
- Kettner, N.M.; Bui, T.; Chen, X.; Hunt, K.K.; Tripathy, D.; Keyomarsi, K. Role of IL-6 in promoting endocrine therapy and palbociclib resistance estrogen receptor positive breast cancer cells. In Proceedings of the Cancer Research Symposium, San Antonio, TX, USA, 10 December 2019. [Google Scholar]
- Martinez Bueno, A.; Bertran Aramillo, J.; Garcia Mosquera, J.J.; Gimenez Capitan, A.; Codony-Servat, J.; Aguilar, A.; Garcia Roman, S.; Mayo de las Casas, C.; Viteri, S.; Molina, M.A. Palbociclib-induced senescence upregulates the expression of IL-8 and may enhance the response to immunotherapy. Ann. Oncol. 2019, 30, III15–III16. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Tredan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Dulos, J.; Carven, G.J.; van Boxtel, S.J.; Evers, S.; Driessen-Engels, L.J.; Hobo, W.; Gorecka, M.A.; de Haan, A.F.; Mulders, P.; Punt, C.J.; et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 2012, 35, 169–178. [Google Scholar] [CrossRef]
- Yoshino, K.; Nakayama, T.; Ito, A.; Sato, E.; Kitano, S. Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: Potential role of Th1-dominant immune response in immune-related adverse events: Two case reports. BMC Cancer 2019, 19, 1019. [Google Scholar] [CrossRef]
- Tanaka, R.; Okiyama, N.; Okune, M.; Ishitsuka, Y.; Watanabe, R.; Furuta, J.; Ohtsuka, M.; Otsuka, A.; Maruyama, H.; Fujisawa, Y.; et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-alpha is a biomarker of nivolumab recativity. J. Dermatol. Sci. 2017, 86, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Saito, A.; Nakamura, Y.; Ishitsuka, Y.; Watanabe, R.; Fujisawa, Y.; Kanzaki, M.; Mizuno, S.; et al. Activation of CD8 T cells accelerates anti-PD-1 antibody-induced psoriasis-like dermatitis through IL-6. Commun. Biol. 2020, 3, 571. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Lee, J.H.; Gide, T.N.; Menzies, A.M.; Guminski, A.; Carlino, M.S.; Breen, E.J.; Yang, J.Y.H.; Ghazanfar, S.; Kefford, R.F.; et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving Anti-PD-1-based immunotherapy. Clin. Cancer Res. 2019, 25, 1557–1563. [Google Scholar] [CrossRef]
- Andrykowski, M.A. Kangas, M. Posttraumatic stress disorder associated with cancer diagnosis and treatment. In Psycho-Oncology, 2nd ed.; Holland, J.C., Ed.; Oxford University Press: Oxford, UK, 2010; pp. 348–357. [Google Scholar]
- Kähler, K.C.; Hassel, J.C.; Heinzerling, L.; Loquai, C.; Thoms, K.-M.; Ugurel, S.; Zimmer, L.; Gutzmer, R. Side effect management during immune checkpoint blockade using CTLA-4 and PD-1 antibodies for metastatic melanoma—An update. J. Dtsch. Dermatol. Ges. 2020, 18, 582–609. [Google Scholar] [CrossRef]
- Kangas, M.; Henry, J.L.; Bryant, R.A. Posttraumatic stress disorder following cancer: A conceptual and empirical review. Clin. Psychol. Rev. 2002, 22, 499–524. [Google Scholar] [CrossRef]
- Temel, J.S.; Shaw, A.T.; Greer, J.A. Challenge of prognostic uncertainty in the modern era of cancer therapeutics. J. Clin. Oncol. 2016, 34, 3605–3608. [Google Scholar] [CrossRef]
- Krigel, S.; Myers, J.; Befort, C.; Krebill, H.; Klemp, J. Cancer changes everything!’ Exploring the lived experiences of women with metastatic breast cancer. Int. J. Palliat. Nurs. 2014, 20, 334–342. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Steffens, R.F.; Bush, H.M.; Tucker, T.C. Lung cancer diagnosis and treatment as a traumatic stressor in DSM-IV and DSM-5: Prevalence and relationship to mental health outcomes. J. Trauma. Stress 2015, 28, 206–213. [Google Scholar] [CrossRef]
- Lillis, T.A.; Gerhart, J.; Bouchard, L.C.; Cvengros, J.; O’Mahony, S.; Kopkash, K.; Kabaker, K.B.; Burns, J. Sleep disturbance mediates the association of post-traumatic stress disorder symptoms and pain in patients with cancer. Am. J. Hosp. Palliat. Care 2018, 35, 788–793. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Lee, J.-W.; Terhaar, A.; Sesto, M.E.; Smith, M.L.; Cleeland, C.S.; Fisch, M.J. Working after a metastatic cancer diagnosis: Factors affecting employment in the metastatic setting from ECOG-ACRIN’s Symptom Outcomes and Practice Patterns study. Cancer 2016, 122, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, L.; Monfredo, M.; Citterio, C. Job loss and return to work of patients with cancer. A prospective observational study on 416 cancer patients. Recenti Prog. Med. 2019, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Rotter, J.; Spencer, J.C.; Wheeler, S.B. Financial toxicity in advanced and metastatic cancer: Overburdened and underprepared. J. Oncol. Pract. 2019, 15, e300–e307. [Google Scholar] [CrossRef] [PubMed]
- Low, C.A. Stanton, A.L. Activity disruption and depressive symptoms in women living with metastatic breast cancer. Health Psychol. 2015, 34, 89–92. [Google Scholar] [CrossRef]
- Cardoso, F.; Harbeck, N.; Mertz, S.; Fenech, D. Evolving psychosocial, emotional, functional, and support needs of women with advanced breast cancer: Results from the Count Us, Know Us, Join Us and Here & Now surveys. Breast 2016, 28, 5–12. [Google Scholar] [CrossRef]
- Maunsell, E.; Brisson, J.; Deschênes, L. Social support and survival among women with breast cancer. Cancer 1995, 76, 631–637. [Google Scholar] [CrossRef]
- Koopman, C.; Hermanson, K.; Diamond, S.; Angell, K.; Spiegel, D. Social support, life stress, pain and emotional adjustment to advanced breast cancer. Psychooncology 1998, 7, 101–111. [Google Scholar] [CrossRef]
- Dinh, K.T.; Aizer, A.A.; Muralidhar, V.; Mahal, B.A.; Chen, Y.W.; Beard, C.J.; Choueiri, T.K.; Hoffman, K.E.; Hu, J.C.; Martin, N.E.; et al. Increased vulnerability to poorer cancer-specific outcomes following recent divorce. Am. J. Med. 2018, 131, 517–523. [Google Scholar] [CrossRef]
- Pyter, L.M.; Suarez-Kelly, L.P.; Carson, W.E., 3rd; Kaur, J.; Bellisario, J.; Bever, S.R. Novel rodent model of breast cancer survival with persistent anxiety-like behavior and inflammation. Behav. Brain Res. 2017, 330, 108–117. [Google Scholar] [CrossRef]
- Khoja, L.; Atenafu, E.G.; Ye, Q.; Gedye, C.; Chappell, M.; Hogg, D.; Butler, M.O.; Joshua, A.M. Real-world efficacy, toxicity and clinical management of ipilimumab treatment in metastatic melanoma. Oncol. Lett. 2016, 11, 1581–1585. [Google Scholar] [CrossRef][Green Version]
- Simeone, E.; Grimaldi, A.M.; Esposito, A.; Curvietto, M.; Palla, M.; Paone, M.; Mozzillo, N.; Ascierto, P.A. Serious haematological toxicity during and after ipilimumab treatment: A case series. J. Med. Case Rep. 2014, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Couey, M.A.; Bell, R.B.; Patel, A.A.; Romba, M.C.; Crittenden, M.R.; Curti, B.D.; Urba, W.J.; Leidner, R.S. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: Diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Thanarajasingam, G.; Atherton, P.J.; Novotny, P.J.; Loprinzi, C.L.; Sloan, J.A.; Grothey, A. Longitudinal adverse event assessment in oncology clinical trials: The Toxicity over Time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol. 2016, 17, 663–670. [Google Scholar] [CrossRef]
- Struzik, L.; Vermani, M.; Duffin, J.; Katzman, M.A. Anxiety sensitivity as a predictor of panic attacks. Psychiatry Res. 2004, 129, 273–278. [Google Scholar] [CrossRef]
- Rasic, D.T.; Belik, S.L.; Bolton, J.M.; Chochinov, H.M.; Sareen, J. Cancer, mental disorders, suicidal ideation and attempts in a large community sample. Psychooncology 2008, 17, 660–667. [Google Scholar] [CrossRef]
- 121. PDQ Supportive and Palliative Care Editorial Board, PDQ Cancer-Related Post-Traumatic Stress. National Cancer Institute: Bethesda, MD, USA. Updated 10/30/2019. Available online: https://www.cancer.gov/about-cancer/coping/survivorship/new-normal/ptsd-hp-pdq (accessed on 15 October 2020). [PubMed]
- Butler, L.D.; Koopman, C.; Classen, C.; Spiegel, D. Traumatic stress, life events, and emotional support in women with metastatic breast cancer: Cancer-related traumatic stress symptoms associated with past and current stressors. Health Psychol. 1999, 18, 555–560. [Google Scholar] [CrossRef]
- Rogiers, A.; Leys, C.; Lauwyck, J.; Schembri, A.; Awada, G.; Schwarze, J.K.; De Cremer, J.; Theuns, P.; Maruff, P.; De Ridder, M.; et al. Neurocognitive function, psychosocial outcome, and health-related quality of life of the first-generation metastatic melanoma survivors treated with ipilimumab. J. Immunol. Res. 2020, 2020, 2192480. [Google Scholar] [CrossRef]
- Rogiers, A.; Leys, C.; De Cremer, J.; Awada, G.; Schembri, A.; Theuns, P.; De Ridder, M.; Neyns, B. Health-related quality of life, emotional burden, and neurocognitive function in the first generatio of metastatic melanoma survivors treated with pembrolizumab: A longitudinal pilot study. Support. Care Cancer 2019, 28, 3267–3278. [Google Scholar] [CrossRef]
- Smith, S.K.; Zimmerman, S.; Williams, C.S.; Benecha, H.; Abernethy, A.P.; Mayer, D.K.; Edwards, L.J.; Ganz, P.A. Post-traumatic stress symptoms in long-term non-Hodgkin’s lymphoma survivors: Does time heal? J. Clin. Oncol. 2011, 29, 4526–4533. [Google Scholar] [CrossRef]
- Pérez, S.; Conchado, A.; Andreu, Y.; Galdón, M.J.; Cardeña, E.; Ibáñez, E.; Durá, E. Acute stress trajectories 1 year after a breast cancer diagnosis. Support. Care Cancer 2016, 24, 1671–1678. [Google Scholar] [CrossRef]
- Andersen, B.L.; Valentine, T.R.; Lo, S.B.; Carbone, D.P.; Presley, C.J.; Shields, P.G. Newly diagnosed patients with advanced non-small cell lung cancer: A clinical description of those with moderate to severe depressive symptoms. Lung Cancer 2020, 145, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, A.; Groarke, A.; Sweeney, K. Predicting general and cancer-related distress in women with newly diagnosed breast cancer. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Scheiber, C.; Kesler, S.; Mustian, K.; Koopman, C.; Schapira, L. Management of side effects during and post-treatment in breast cancer survivors. Breast J. 2018, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ebede, C.C.; Jang, Y.; Escalante, C.P. Cancer-related fatigue in cancer survivorship. Med. Clin. N. Am. 2017, 101, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Carson-Stevens, A.; Gillespie, D.; Edwards, A.G. Psychological interventions for women with metastatic breast cancer. Cochr. Database Syst. Rev. 2013, Cd004253. [Google Scholar] [CrossRef] [PubMed]
- Hasson-Ohayon, I.; Goldzweig, G.; Dorfman, C.; Uziely, B. Hope and social support utilisation among different age groups of women with breast cancer and their spouses. Psychol. Health 2014, 29, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Vilhauer, R.P. ‘Them’ and ‘us’: The experiences of women with metastatic disease in mixed-stage versus stage-specific breast cancer support groups. Psychol. Health 2011, 26, 781–797. [Google Scholar] [CrossRef]
- Verma, V.; Sprave, T.; Haque, W.; Simone, C.B., II; Chang, J.Y.; Welsh, J.W.; Thomas, C.R., Jr. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J. Immunother. Cancer 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Diaby, V.; Tawk, R.; Sanogo, V.; Xiao, H.; Montero, A.J. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res. Treat. 2015, 151, 27–40. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H.; Goetzinger, A.M.; Zhong, X.; Abernethy, A.P. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef]
- Loscalzo, M.; Clark, K.; Pal, S.; Pirl, W.F. Role of biopsychosocial screening in cancer care. Cancer J. 2013, 19, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Chen, M.H.; McCarthy, E.P.; Mendu, M.L.; Koo, S.; Wilhite, T.J.; Graham, P.L.; Choueiri, T.K.; Hoffman, K.E.; Martin, N.E.; et al. Marital status and survival in patients with cancer. J. Clin. Oncol. 2013, 31, 3869–3876. [Google Scholar] [CrossRef] [PubMed]
- Pirl, W.F.; Fann, J.R.; Greer, J.A.; Braun, I.; Deshields, T.; Fulcher, C.; Harvey, E.; Holland, J.; Kennedy, V.; Lazenby, M.; et al. Recommendations for the Implementation of Distress Screening Programs in Cancer Centers Report From the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) Joint Task Force. Cancer 2014, 120, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Penedo, F.J.; Oswald, L.B.; Kronenfeld, J.P.; Garcia, S.F.; Cella, D.; Yanez, B. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020, 21, e240–e251. [Google Scholar] [CrossRef]
- Jim, H.S.L.; Hoogland, A.I.; Brownstein, N.C.; Barata, A.; Dicker, A.P.; Knoop, H.; Gonzalez, B.D.; Perkins, R.; Rollison, D.; Gilbert, S.M.; et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J. Clin. 2020, 70, 182–199. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef]
- Denis, F.; Lethrosne, C.; Pourel, N.; Molinier, O.; Pointreau, Y.; Domont, J.; Bourgeois, H.; Senellart, H.; Tremolieres, P.; Lizee, T.; et al. Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients. J. Natl. Cancer Inst. 2017, 109, 1. [Google Scholar] [CrossRef]
- Yanez, B.; Bouchard, L.C.; Cella, D.; Sosman, J.A.; Kircher, S.M.; Mohindra, N.A.; Cristofanilli, M.; Penedo, F.J. Patient-centered engagement and symptom/toxicity monitoring in the new era of tumor next-generation sequencing and immunotherapy: The OncoTool and OncoPRO platforms. Cancer 2019, 125, 2338–2344. [Google Scholar] [CrossRef]
- Wagner, L.I.; Schink, J.; Bass, M.; Patel, S.; Diaz, M.V.; Rothrock, N.; Pearman, T.; Gershon, R.; Penedo, F.J.; Rosen, S.; et al. Bringing PROMIS to practice: Brief and precise symptom screening in ambulatory cancer care. Cancer 2015, 121, 927–934. [Google Scholar] [CrossRef]
- Garcia, S.F.; Wortman, K.; Cella, D.; Wagner, L.I.; Bass, M.; Kircher, S.; Pearman, T.; Penedo, F.J. Implementing electronic health record-integrated screening of patient-reported symptoms and supportive care needs in a comprehensive cancer center. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Stover, A.M.; Wagner, L.I.; LeBlanc, T.W.; Topalaglu, U.; Zafar, Y.; Zullig, L.L.; Smeltzer, P.; Basch, E.M. Harmonization of patient-reported outcomes into EHRs at four cancer hospital outpatient clinics for patient care and quality assessment. J. Clin. Oncol. 2017, 35, 1. [Google Scholar] [CrossRef]
- Teo, I.; Krishnan, A.; Lee, G.L. Psychosocial interventions for advanced cancer patients: A systematic review. Psychooncology 2019, 28, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.M.; Butow, P.N.; Tattersall, M.H.; Devine, R.J.; Simpson, J.M.; Aggarwal, G.; Clark, K.J.; Currow, D.C.; Elliott, L.M.; Lacey, J.; et al. Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J. Clin. Oncol. 2007, 25, 715–723. [Google Scholar] [CrossRef]
- Epstein, R.M.; Duberstein, P.R.; Fenton, J.J.; Fiscella, K.; Hoerger, M.; Tancredi, D.J.; Xing, G.; Gramling, R.; Mohile, S.; Franks, P.; et al. Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol. 2017, 3, 92–100. [Google Scholar] [CrossRef]
- Shirai, Y.; Fujimori, M.; Ogawa, A.; Yamada, Y.; Nishiwaki, Y.; Ohtsu, A.; Uchitomi, Y. Patients’ perception of the usefulness of a question prompt sheet for advanced cancer patients when deciding the initial treatment: A randomized, controlled trial. Psycho-Oncol. 2012, 21, 706–713. [Google Scholar] [CrossRef]
- Meropol, N.J.; Egleston, B.L.; Buzaglo, J.S.; Balshem, A.; Benson, A.B., 3rd; Cegala, D.J.; Cohen, R.B.; Collins, M.; Diefenbach, M.A.; Miller, S.M.; et al. A Web-based communication aid for patients with cancer: The CONNECT Study. Cancer 2013, 119, 1437–1445. [Google Scholar] [CrossRef]
- Walczak, A.; Butow, P.N.; Tattersall, M.H.; Davidson, P.M.; Young, J.; Epstein, R.M.; Costa, D.S.; Clayton, J.M. Encouraging early discussion of life expectancy and end-of-life care: A randomised controlled trial of a nurse-led communication support program for patients and caregivers. Int. J. Nurs. Stud. 2017, 67, 31–40. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Podgurski, L.M.; Eichler, A.F.; Plotkin, S.R.; Temel, J.S.; Mitchell, S.L.; Chang, Y.C.; Barry, M.J.; Volandes, A.E. Use of Video to Facilitate End-of-Life Discussions With Patients With Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2010, 28, 305–310. [Google Scholar] [CrossRef]
- Leighl, N.B.; Shepherd, H.L.; Butow, P.N.; Clarke, S.J.; McJannett, M.; Beale, P.J.; Wilcken, N.R.; Moore, M.J.; Chen, E.X.; Goldstein, D.; et al. Supporting treatment decision making in advanced cancer: A randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J. Clin. Oncol. 2011, 29, 2077–2084. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, S.J.; Kim, S.C.; An, S.; Rha, S.Y.; Ahn, J.B.; Cho, B.C.; Choi, H.J.; Sohn, J.H.; Kim, H.S.; et al. Randomized controlled trial of standardized education and telemonitoring for pain in outpatients with advanced solid tumors. Support. Care Cancer 2013, 21, 1751–1759. [Google Scholar] [CrossRef]
- Kaasa, S.; Loge, J.H.; Aapro, M.; Albreht, T.; Anderson, R.; Bruera, E.; Brunelli, C.; Caraceni, A.; Cervantes, A.; Currow, D.C.; et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018, 19, e588–e653. [Google Scholar] [CrossRef]
- Vanbutsele, G.; Pardon, K.; Van Belle, S.; Surmont, V.; De Laat, M.; Colman, R.; Eecloo, K.; Cocquyt, V.; Geboes, K.; Deliens, L. Effect of early and systematic integration of palliative care in patients with advanced cancer: A randomised controlled trial. Lancet Oncol. 2018, 19, 394–404. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Palliative Care. Version 1.2020. Updated 020/7/2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf (accessed on 2 December 2020).
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Good, P.; Haywood, A.; Gogna, G.; Martin, J.; Yates, P.; Greer, R.; Hardy, J. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: A double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat. Care 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Traeger, L.; Bemis, H.; Solis, J.; Hendriksen, E.S.; Park, E.R.; Pirl, W.F.; Temel, J.S.; Prigerson, H.G.; Safren, S.A. A pilot randomized controlled trial of brief cognitive-behavioral therapy for anxiety in patients with terminal cancer. Oncologist 2012, 17, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.; Smith, C.B.; Goldstein, N.E.; Gomez, J.E.; Redd, W.H. Dyadic psychosocial intervention for advanced lung cancer patients and their family caregivers: Results of a randomized pilot trial. Cancer 2015, 121, 150–158. [Google Scholar] [CrossRef]
- Yanez, B.; McGinty, H.L.; Mohr, D.C.; Begale, M.J.; Dahn, J.R.; Flury, S.C.; Perry, K.T.; Penedo, F.J. Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer 2015, 121, 4407–4415. [Google Scholar] [CrossRef]
- Chambers, S.K.; Occhipinti, S.; Foley, E.; Clutton, S.; Legg, M.; Berry, M.; Stockler, M.R.; Frydenberg, M.; Gardiner, R.A.; Lepore, S.J.; et al. Mindfulness-Based Cognitive Therapy in Advanced Prostate Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 291–297. [Google Scholar] [CrossRef]
- Cheung, E.O.; Cohn, M.A.; Dunn, L.B.; Melisko, M.E.; Morgan, S.; Penedo, F.J.; Salsman, J.M.; Shumay, D.M.; Moskowitz, J.T. A randomized pilot trial of a positive affect skill intervention (lessons in linking affect and coping) for women with metastatic breast cancer. Psychooncology 2017, 26, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, T.M.; Paiva, B.S.R.; de Oliveira, C.Z.; Nascimento, M.S.A.; Paiva, C.E. The feasibility and benefit of a brief psychosocial intervention in addition to early palliative care in patients with advanced cancer to reduce depressive symptoms: A pilot randomized controlled clinical trial. BMC Cancer 2017, 17, 564. [Google Scholar] [CrossRef] [PubMed]
- Lapid, M.I.; Rummans, T.A.; Brown, P.D.; Frost, M.H.; Johnson, M.E.; Huschka, M.M.; Sloan, J.A.; Richardson, J.W.; Hanson, J.M.; Clark, M.M. Improving the quality of life of geriatric cancer patients with a structured multidisciplinary intervention: A randomized controlled trial. Palliat. Support. Care 2007, 5, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.M.; Rummans, T.A.; Atherton, P.J.; Cheville, A.L.; Johnson, M.E.; Frost, M.H.; Miller, J.J.; Sloan, J.A.; Graszer, K.M.; Haas, J.G.; et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer 2013, 119, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Gaston-Johansson, F.; Fall-Dickson, J.M.; Nanda, J.P.; Sarenmalm, E.K.; Browall, M.; Goldstein, N. Long-term effect of the self-management comprehensive coping strategy program on quality of life in patients with breast cancer treated with high-dose chemotherapy. Psychooncology 2013, 22, 530–539. [Google Scholar] [CrossRef]
- Northouse, L.L.; Mood, D.W.; Schafenacker, A.; Kalemkerian, G.; Zalupski, M.; LoRusso, P.; Hayes, D.F.; Hussain, M.; Ruckdeschel, J.; Fendrick, A.M.; et al. Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family caregivers. Psychooncology 2013, 22, 555–563. [Google Scholar] [CrossRef]
- Mosher, C.E.; Secinti, E.; Johns, S.A.; O’Neil, B.H.; Helft, P.R.; Shahda, S.; Jalal, S.I.; Champion, V.L. Examining the effect of peer helping in a coping skills intervention: A randomized controlled trial for advanced gastrointestinal cancer patients and their family caregivers. Qual. Life Res. 2018, 27, 515–528. [Google Scholar] [CrossRef]
- Penedo, F.J.; Fox, R.S.; Oswald, L.B.; Moreno, P.I.; Boland, C.L.; Estabrook, R.; McGinty, H.L.; Mohr, D.C.; Begale, M.J.; Dahn, J.R.; et al. Technology-Based Psychosocial Intervention to Improve Quality of Life and Reduce Symptom Burden in Men with Advanced Prostate Cancer: Results from a Randomized Controlled Trial. Int. J. Behav. Med. 2020. [Google Scholar] [CrossRef]
- Ward, S.; Donovan, H.; Gunnarsdottir, S.; Serlin, R.C.; Shapiro, G.R.; Hughes, S. A randomized trial of a representational intervention to decrease cancer pain (RIDcancerPain). Health Psychol. 2008, 27, 59–67. [Google Scholar] [CrossRef]
- Chan, C.W.; Richardson, A.; Richardson, J. Managing symptoms in patients with advanced lung cancer during radiotherapy: Results of a psychoeducational randomized controlled trial. J. Pain Symptom Manag. 2011, 41, 347–357. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L.; Abbott-Anderson, K.; Cherwin, C.; Roiland, R.; Serlin, R.C.; Ward, S.E. Pilot randomized controlled trial of a patient-controlled cognitive-behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancer. J. Pain Symptom Manag. 2012, 44, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Guisado, H.; Pautex, S. Promoting sleep for hospitalized patients with advanced cancer with relaxation therapy: Experience of a randomized study. Am. J. Hosp. Palliat. Care 2013, 30, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.C.; Prevost, A.T.; McCrone, P.; Brafman-Price, B.; Bentley, A.; Higginson, I.J.; Todd, C.; Booth, S. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Castro, M.J.; Soto-Perez-de-Celis, E.; Covarrubias-Gómez, A.; Sánchez-Román, S.; Quiróz-Friedman, P.; Navarro-Lara, Á.; Ramos-Lopez, W.A.; Moreno-García, M.L.; Contreras-Garduño, S.; Perez-Montessoro, V.; et al. Symptom assessment and early access to supportive and palliative care for patients with advanced solid tumors in Mexico. J. Palliat. Care 2020, 35, 40–45. [Google Scholar] [CrossRef]
- Wright, A.A.; Zhang, B.; Ray, A.; Mack, J.W.; Trice, E.; Balboni, T.; Mitchell, S.L.; Jackson, V.A.; Block, S.D.; Maciejewski, P.K.; et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008, 300, 1665–1673. [Google Scholar] [CrossRef]
- Prater, L.C.; Wickizer, T.; Bower, J.K.; Bose-Brill, S. The impact of advance care planning on end-of-life care: Do the type and timing make a difference for patients with advanced cancer referred to hospice? Am. J. Hosp. Palliat. Care 2019, 36, 1089–1095. [Google Scholar] [CrossRef]
- Emiloju, O.E.; Djibo, D.A.M.; Ford, J.G. Association between the timing of goals-of-care discussion and hospitalization outcomes in patients with metastatic cancer. Am. J. Hosp. Palliat. Care 2020, 37, 433–438. [Google Scholar] [CrossRef]
- Starr, L.T.; Ulrich, C.M.; Corey, K.L.; Meghani, S.H. Associations among end-of-life discussions, health-care utilization, and costs in persons with advanced cancer: A systematic review. Am. J. Hosp. Palliat. Care 2019, 36, 913–926. [Google Scholar] [CrossRef]
- Agarwal, R. Epstein, A.S. Advance care planning and end-of-life decision making for patients with cancer. Semin. Oncol. Nurs. 2018, 34, 316–326. [Google Scholar] [CrossRef]
- LoPresti, M.A.; Dement, F.; Gold, H.T. End-of-life care for people with cancer from ethnic minority groups: A systematic review. Am. J. Hosp. Palliat. Care. 2016, 33, 291–305. [Google Scholar] [CrossRef]
- Choudry, M.; Latif, A.; Warburton, K.G. An overview of the spiritual importances of end-of-life care among the five major faiths of the United Kingdom. Clin. Med. (Lond.) 2018, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- PDQ Supportive and Palliative Care Editorial Board, PDQ Planning the Transition to End-of-Life Care in Advanced Cancer. Bethesda, MD: National Cancer Institute. Updated 06/25/2020. Available online: https://www.cancer.gov/about-cancer/advanced-cancer/planning/end-of-life-hp-pdq (accessed on 1 December 2020).
- Duncan, M.; Moschopoulou, E.; Herrington, E.; Deane, J.; Roylance, R.; Jones, L.; Bourke, L.; Morgan, A.; Chalder, T.; Thaha, M.A.; et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 2017, 7, e015860. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The effect of nutrition therapy and exercise on cancer-related fatigue and quality of life in men with prostate cancer: A systematic review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Frankl, V.; Lasch, I. Man’s Search for Meaning: An Introduction to Logotherapy; Beacon Press: Boston, MA, USA, 1992. [Google Scholar]
- Montross Thomas, L.P.; Meier, E.A.; Irwin, S.A. Meaning-centered psychotherapy: A form of psychotherapy for patients with cancer. Curr. Psychiatry Rep. 2014, 16, 1–6. [Google Scholar] [CrossRef]
- Hyland, K.; Eisel, S.; Hoogland, A.; Nelson, A.; Knoop, H.; Jacobsen, P.; Jim, H. Perpetuating and exacerbating factors as mediators of change in a CBT-based intervention for fatigue in patients with chronic myeloid leukemia. Psychooncology 2020, 29, 56. [Google Scholar]
- Buckley, J. Herth, K. Fostering hope in terminally ill patients. Nurs. Stand. 2004, 19, 33–41. [Google Scholar] [CrossRef]
- Esbensen, B.A.; Thomsen, T. Quality of life and hope in elderly people with cancer. Open J. Nurs. 2011, 1, 26–32. [Google Scholar] [CrossRef]
- Sanatani, M.; Schreier, G.; Stitt, L. Level and direction of hope in cancer patients: An exploratory longitudinal study. Support. Care Cancer 2008, 16, 493–499. [Google Scholar] [CrossRef]
- Gum, A.; Snyder, C.R. Coping with terminal illness: The role of hopeful thinking. J. Palliat. Med. 2002, 5, 883–894. [Google Scholar] [CrossRef]
- Leary, M.R.; Tate, E.B.; Adams, C.E.; Allen, A.B.; Hancock, J. Self-compassion and reactions to unpleasant self-relevant events: The implications of treating oneself kindly. J. Personal. Soc. Psychol. 2007, 92, 887–904. [Google Scholar] [CrossRef]
- Diedrich, A.; Burger, J.; Kirchner, M.; Berking, M. Adaptive emotion regulation mediates the relationship between self-compassion and depression in individuals with unipolar depression. Psychol. Psychother. 2017, 90, 247–263. [Google Scholar] [CrossRef]
- Sydenham, M.; Beardwood, J.; Rimes, K.A. Beliefs about emotions, depression, anxiety and fatigue: A mediational analysis. Behav. Cogn. Psychother. 2017, 45, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Alda, M.; Puebla-Guedea, M.; Rodero, B.; Demarzo, M.; Montero-Marin, J.; Roca, M.; Garcia-Campayo, J. Zen meditation, length of telomeres, and the role of experiential avoidance and compassion. Mindfulness (NY) 2016, 7, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.G.; Morris, K.J.; Juckett, M.B.; Coe, C.L.; Broman, A.T.; Costanzo, E.S. Mindfulness, experiential avoidance, and recovery from hematopoietic stem cell transplantation. Ann. Behav. Med. 2019, 53, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Hales, S.; Chiu, A.; Panday, T.; Malfitano, C.; Jung, J.; Rydall, A.; Li, M.; Nissim, R.; Zimmermann, C.; et al. Managing cancer and living meaningfully (CALM): Randomised feasibility trial in patients with advanced cancer. BMJ Support. Palliat. Care 2019, 9, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rodin, G.; An, E.; Shnall, J.; Malfitano, C. Psychological interventions for patients with advanced disease: Implications for oncology and palliative care. J. Clin. Oncol. 2020, 38, 885–904. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tometich, D.B.; Hyland, K.A.; Soliman, H.; Jim, H.S.L.; Oswald, L. Living with Metastatic Cancer: A Roadmap for Future Research. Cancers 2020, 12, 3684. https://doi.org/10.3390/cancers12123684
Tometich DB, Hyland KA, Soliman H, Jim HSL, Oswald L. Living with Metastatic Cancer: A Roadmap for Future Research. Cancers. 2020; 12(12):3684. https://doi.org/10.3390/cancers12123684
Chicago/Turabian StyleTometich, Danielle B., Kelly A. Hyland, Hatem Soliman, Heather S. L. Jim, and Laura Oswald. 2020. "Living with Metastatic Cancer: A Roadmap for Future Research" Cancers 12, no. 12: 3684. https://doi.org/10.3390/cancers12123684
APA StyleTometich, D. B., Hyland, K. A., Soliman, H., Jim, H. S. L., & Oswald, L. (2020). Living with Metastatic Cancer: A Roadmap for Future Research. Cancers, 12(12), 3684. https://doi.org/10.3390/cancers12123684





