A Comparison of p53 Isoform Profiles and Apoptosis Induced by Camptothecin or a Herbal Khat Extract (Catha Edulis (Vahl) Forssk. ex Endl.) in Leukemic Cell Lines: Exploring Cellular Responses in Therapy Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
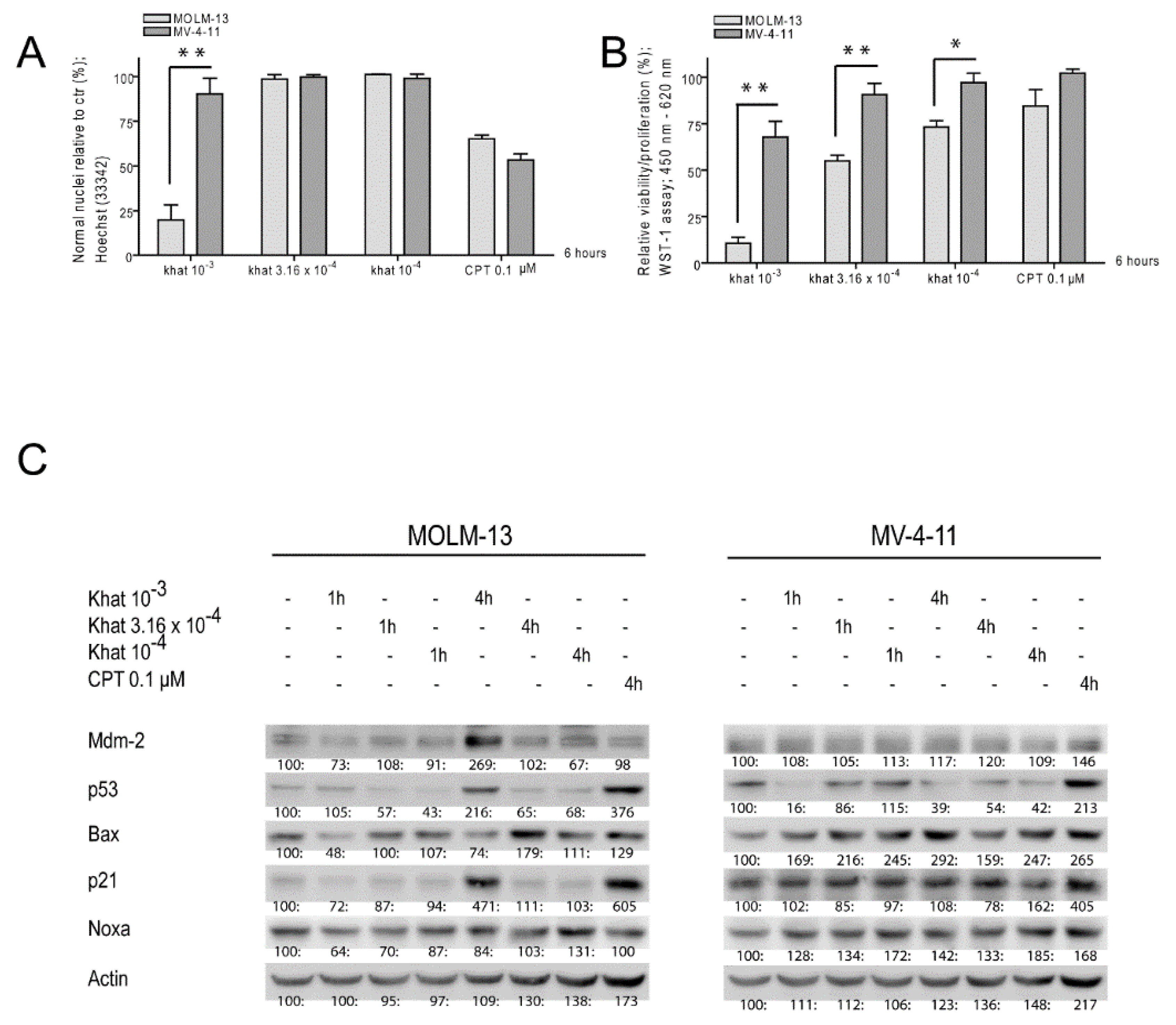
2.1. Khat Reduced MOLM-13 Proliferation/Viability in a Concentration Dependent Manner
2.2. Khat Induced p53 Protein in MOLM-13 but Temporary Attenuated p53 in MV-4-11
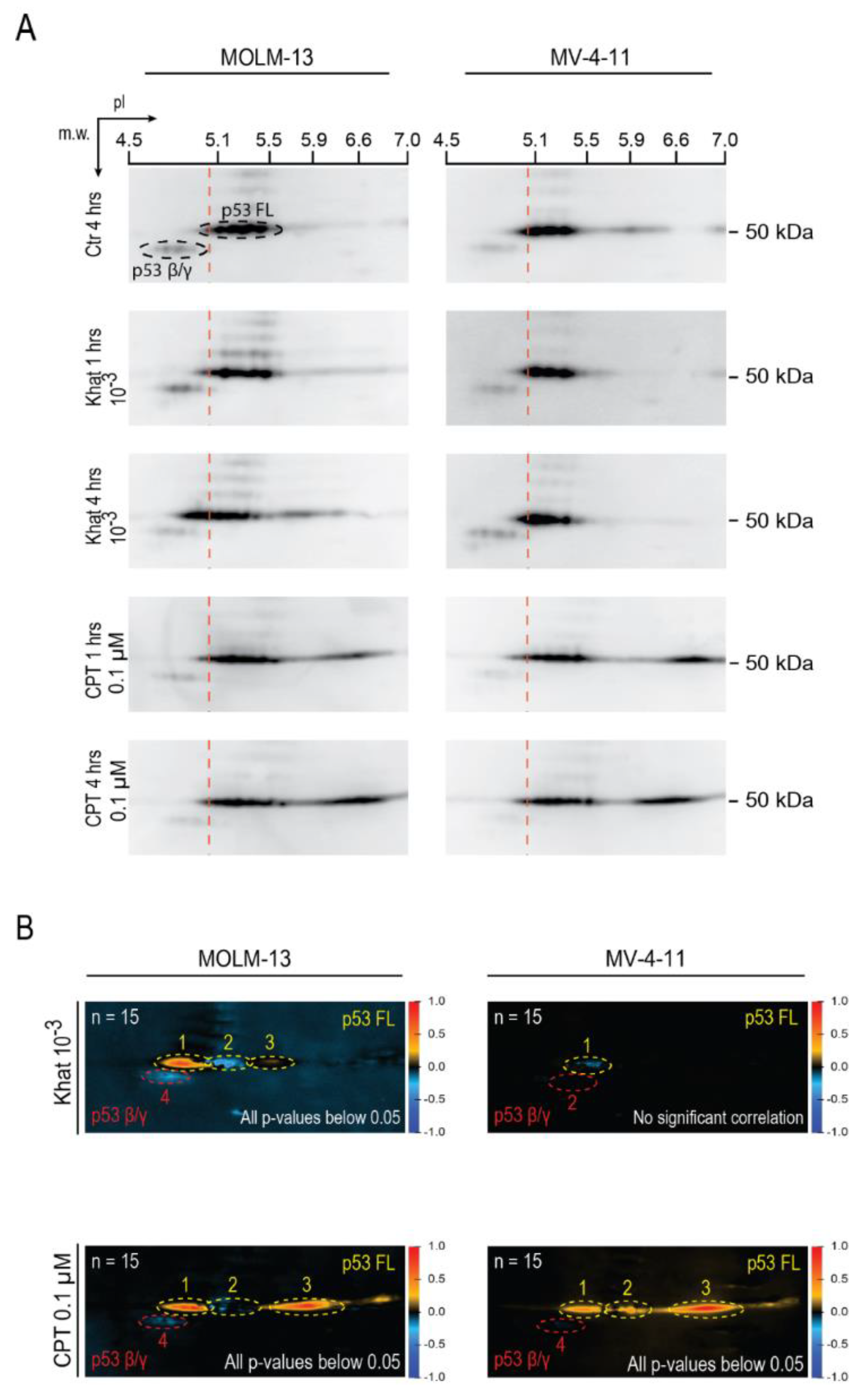
2.3. Khat Altered p53 Isoform Distribution and Induced Post-Translational Modifications
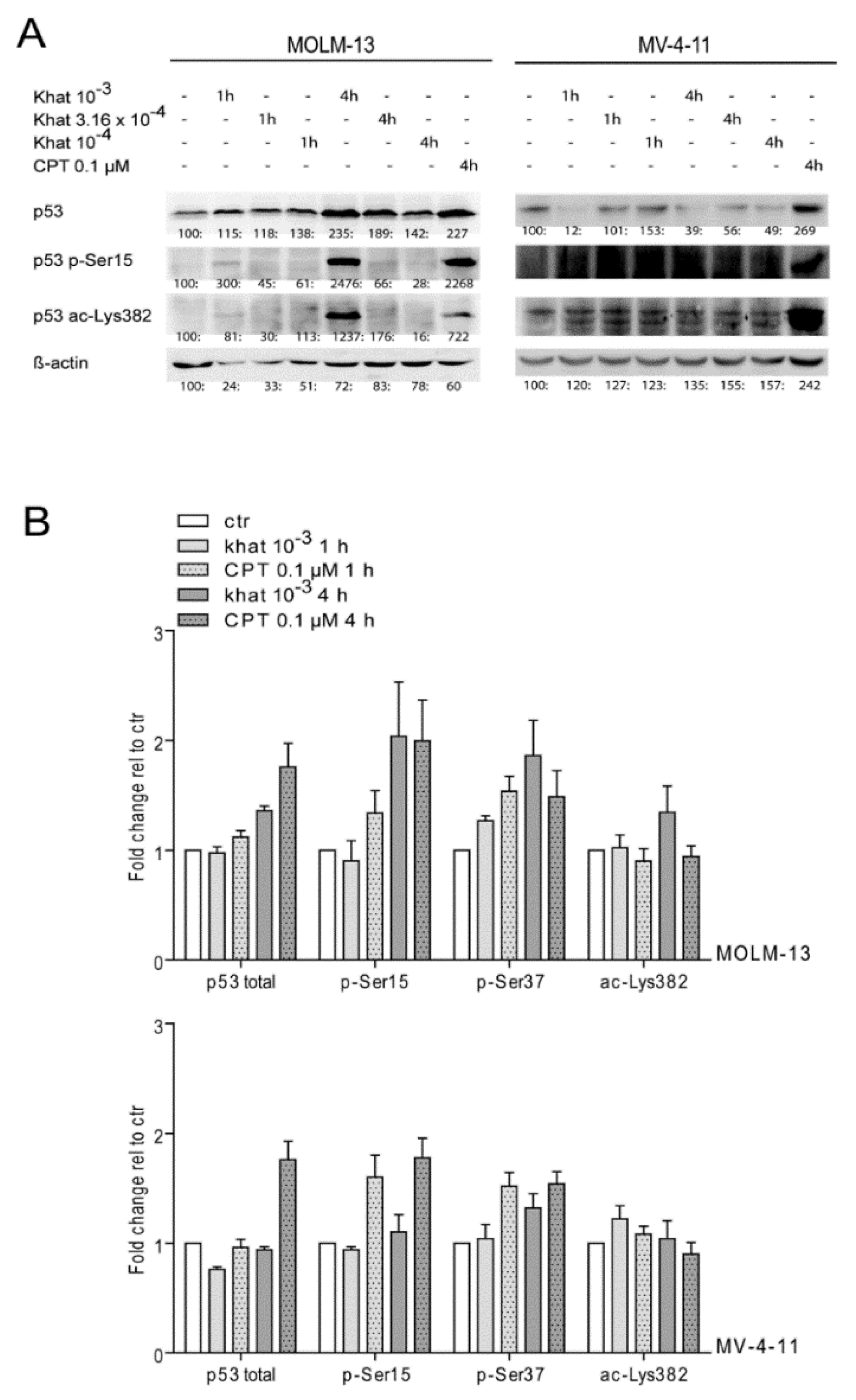
2.4. Khat Induced Phosphorylation and Acetylation of p53 in MOLM-13 and MV-4-11
2.5. p38 Inhibitors Sensitized Resistant MV-4-11 Cells to Khat
2.6. Functional p53 Protein Is Not Essential for Khat-Mediated Cell Death
3. Discussion
4. Materials and Methods
4.1. Khat (Catha edulis (Vahl) Forssk. ex Endl.) Extract
4.2. Chemicals
4.3. Cell Lines and Cultivation
4.4. Cell Death Determination Based on Nuclear Morphology
4.5. Cell Viability/Proliferation Assay
4.6. Animal Experiments
4.7. Measurement of Cell Death in Primary Mouse Bone Marrow Cells
4.8. Flow Cytometry
4.9. One-Dimensional Polyacrylamide Gel Electrophoresis (1D-PAGE) and Western Blotting
4.10. Two-Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, A.J. p53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef]
- Sane, S.; Rezvani, K. Essential Roles of E3 Ubiquitin Ligases in p53 Regulation. Int. J. Mol. Sci. 2017, 18, 442. [Google Scholar] [CrossRef]
- Bang, S.; Kaur, S.; Kurokawa, M. Regulation of the p53 Family Proteins by the Ubiquitin Proteasomal Pathway. Int. J. Mol. Sci. 2019, 21, 261. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tavana, O.; Gu, W. p53 modifications: Exquisite decorations of the powerful guardian. J. Mol. Cell Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Bourdon, J.-C. p53 isoforms change p53 paradigm. Mol. Cell. Oncol. 2014, 1, e969136. [Google Scholar] [CrossRef]
- Bourdon, J.-C.; Fernandes, K.; Murray-Zmijewski, F.; Liu, G.; Diot, A.; Xirodimas, D.P.; Saville, M.K.; Lane, D.P. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005, 19, 2122–2137. [Google Scholar] [CrossRef] [Green Version]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.A.; Green, D.R. Direct Activation of Bax by p53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [Green Version]
- Engidawork, E. Pharmacological and Toxicological Effects ofCatha edulisF. (Khat). Phytotherapy Res. 2017, 31, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Wabe, N.T. Chemistry, Pharmacology, and Toxicology of Khat (Catha Edulis Forsk): A Review. Addict. Health 2014, 3, 137–149. [Google Scholar]
- Bredholt, T.; Dimba, E.O.; Hagland, H.R.; Wergeland, L.; Skavland, J.; Fossan, K.O.; Tronstad, K.J.; Johannessen, A.C.; Vintermyr, O.K.; Gjertsen, B.T. Camptothecin and khat (Catha edulis Forsk.) induced distinct cell death phenotypes involving modulation of c-FLIPL, Mcl-1, procaspase-8 and mitochondrial function in acute myeloid leukemia cell lines. Mol. Cancer 2009, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimba, E.A.O.; Gjertsen, B.T.; Bredholt, T.; Fossan, K.O.; Costea, D.; Francis, G.W.; Johannessen, A.C.; Vintermyr, O.K. Khat (Catha edulis)-induced apoptosis is inhibited by antagonists of caspase-1 and -8 in human leukaemia cells. Br. J. Cancer 2004, 91, 1726–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukandu, O.M.; Costea, D.; Dimba, E.A.; Neppelberg, E.; Bredholt, T.; Gjertsen, B.T.; Vintermyr, O.K.; Johannessen, A.C. Khat induces G1-phase arrest and increased expression of stress-sensitive p53 and p16 proteins in normal human oral keratinocytes and fibroblasts. Eur. J. Oral Sci. 2008, 116, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Øyan, A.M.; Anensen, N.; Bø, T.H.; Stordrange, L.; Jonassen, I.; Bruserud, Ø.; Kalland, K.-H.; Gjertsen, B.T. Genes of cell-cell interactions, chemotherapy detoxification and apoptosis are induced during chemotherapy of acute myeloid leukemia. BMC Cancer 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar]
- Li, T.-K.; Liu, L.F. Tumorcelldeathinduced Bytopoisomerase-Targetingdrugs. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 53–77. [Google Scholar] [CrossRef]
- Slater, T.F.; Sawyer, B.; Straeuli, U. Studies on Succinate-Tetrazolium Reductase Systems. Iii. Points of Coupling of Four Different Tetrazolium Salts. Biochim. Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef]
- O’Farrell, P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975, 250, 4007–4021. [Google Scholar]
- Anensen, N.; Øyan, A.M.; Bourdon, J.-C.; Kalland, K.-H.; Bruserud, Ø.; Gjertsen, B.T. A Distinct p53 Protein Isoform Signature Reflects the Onset of Induction Chemotherapy for Acute Myeloid Leukemia. Clin. Cancer Res. 2006, 12, 3985–3992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Belle, W.; Anensen, N.; Haaland, I.; Bruserud, Ø.; Høgda, K.A.; Gjertsen, B.T. Correlation analysis of two-dimensional gel electrophoretic protein patterns and biological variables. BMC Bioinform. 2006, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukandu, O.; Neppelberg, E.; Vintermyr, O.; Johannessen, A.; Costea, D. Khat Alters the Phenotype of in vitro-reconstructed Human Oral Mucosa. J. Dent. Res. 2009, 89, 270–275. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Weinberg, R.A. Ras oncogenes: Split personalities. Nat. Rev. Mol. Cell Biol. 2008, 9, 517–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasdemir, E.; Maiuri, M.C.; Galluzzi, L.; Vitale, I.; Djavaheri-Mergny, M.; D’Amelio, M.; Criollo, A.; Morselli, E.; Zhu, C.; Harper, F.; et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008, 10, 676–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, S.; Tariq, M.; Parmar, N.S.; Al-Meshal, I.A. Cytological Effects of Khat (Catha Edulis) in Somatic and Male Germ Cells of Mice. Drug Chem. Toxicol. 1988, 11, 151–165. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, M.J.; Lee, S.Y.; Lee, Y.-N.; Chi, G.Y.; Eom, H.S.; Kim, N.D.; Choi, B.T. Phosphorylation of p53, induction of Bax and activation of caspases during beta-lapachone-mediated apoptosis in human prostate epithelial cells. Int. J. Oncol. 2002, 21, 1293–1299. [Google Scholar]
- Morgan, M.A.; Dolp, O.; Reuter, C.W. Cell-cycle-dependent activation of mitogen-activated protein kinase kinase (MEK-1/2) in myeloid leukemia cell lines and induction of growth inhibition and apoptosis by inhibitors of RAS signaling. Blood 2001, 97, 1823–1834. [Google Scholar] [CrossRef] [Green Version]
- Arakia, T.; Hayashi, M.; Watanabea, N.; Kanukab, H.; Yoshinoa, J.; Miurab, M.; Sarutaa, T. Down-Regulation of Mcl-1 by Inhibition of the PI3-K/Akt Pathway Is Required for Cell Shrinkage-Dependent Cell Death. Biochem. Biophys. Res. Commun. 2002, 290, 1275–1281. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, S.-J.; Jung, Y.S.; Xu, Y.; Kang, H.S.; Ha, N.-C.; Park, B.-J. Blocking of p53-Snail Binding, Promoted by Oncogenic K-Ras, Recovers p53 Expression and function. Neoplasia 2009, 11, 22-IN6. [Google Scholar] [CrossRef] [Green Version]
- Tasdemir, E.; Maiuri, M.C.; Morselli, E.; Criollo, A.; D’Amelio, M.; Djavaheri-Mergny, M.; Cecconi, F.; Tavernarakis, N.; Kroemer, G. A dual role of p53 in the control of autophagy. Autophagy 2008, 4, 810–814. [Google Scholar] [CrossRef] [Green Version]
- Fleckenstein, D.S.; Uphoff, C.C.; Drexler, H.G.; Quentmeier, H. Detection of p53 gene mutations by single strand conformational polymorphism (SSCP) in human acute myeloid leukemia-derived cell lines. Leuk. Res. 2002, 26, 207–214. [Google Scholar] [CrossRef]
- Brummelkamp, T.R.; Bernards, R.; Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2002, 2, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Gjertsen, B.T.; Cressey, L.I.; Ruchaud, S.; Houge, G.; Lanotte, M.; Døskeland, S.O. Multiple apoptotic death types triggered through activation of separate pathways by cAMP and inhibitors of protein phosphatases in one (IPC leukemia) cell line. J. Cell Sci. 1994, 107, 107. [Google Scholar]
- Donehower, L.A.; Harvey, M.; Slagle, B.L.; McArthur, M.J., Jr.; Montgomery, C.A.; Butel, J.S.; Bradley, A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nat. Cell Biol. 1992, 356, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, J.F.; Bakken, A.M.; Bruserud, Ø.; Gjertsen, B.T. Flow cytometric measurement of apoptosis and necrosis in cryopreserved PBPC concentrates from patients with malignant diseases. Bone Marrow Transpl. 2002, 29, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Wergeland, L.; Sjøholt, G.; Haaland, I.; Hovland, R.; Bruserud, Ø.; Gjertsen, B.T. Pre-apoptotic response to therapeutic DNA damage involves protein modulation of Mcl-1, Hdm2 and Flt3 in acute myeloid leukemia cells. Mol. Cancer 2007, 6. [Google Scholar] [CrossRef] [Green Version]
- Irish, J.M.; Anensen, N.; Hovland, R.; Skavland, J.; Børresen-Dale, A.-L.; Bruserud, Ø.; Nolan, G.P.; Gjertsen, B.T.; Garand, R.; Lode, L.; et al. Flt3 Y591 duplication and Bcl-2 overexpression are detected in acute myeloid leukemia cells with high levels of phosphorylated wild-type p53. Blood 2006, 109, 2589–2596. [Google Scholar] [CrossRef] [Green Version]
- Øye, O.K.; Jørgensen, K.M.; Hjelle, S.M.; Sulen, A.; Ulvang, D.M.; Gjertsen, B.T. Gel2DE—A software tool for correlation analysis of 2D gel electrophoresis data. BMC Bioinform. 2013, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyango, T.B.; Hjelle, S.M.; Haaland, I.; Vintermyr, O.K.; Johannessen, A.C.; Gjertsen, B.T. A Comparison of p53 Isoform Profiles and Apoptosis Induced by Camptothecin or a Herbal Khat Extract (Catha Edulis (Vahl) Forssk. ex Endl.) in Leukemic Cell Lines: Exploring Cellular Responses in Therapy Development. Cancers 2020, 12, 3596. https://doi.org/10.3390/cancers12123596
Onyango TB, Hjelle SM, Haaland I, Vintermyr OK, Johannessen AC, Gjertsen BT. A Comparison of p53 Isoform Profiles and Apoptosis Induced by Camptothecin or a Herbal Khat Extract (Catha Edulis (Vahl) Forssk. ex Endl.) in Leukemic Cell Lines: Exploring Cellular Responses in Therapy Development. Cancers. 2020; 12(12):3596. https://doi.org/10.3390/cancers12123596
Chicago/Turabian StyleOnyango, Therese Bredholt, Sigrun M. Hjelle, Ingvild Haaland, Olav K. Vintermyr, Anne Christine Johannessen, and Bjørn Tore Gjertsen. 2020. "A Comparison of p53 Isoform Profiles and Apoptosis Induced by Camptothecin or a Herbal Khat Extract (Catha Edulis (Vahl) Forssk. ex Endl.) in Leukemic Cell Lines: Exploring Cellular Responses in Therapy Development" Cancers 12, no. 12: 3596. https://doi.org/10.3390/cancers12123596




