Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
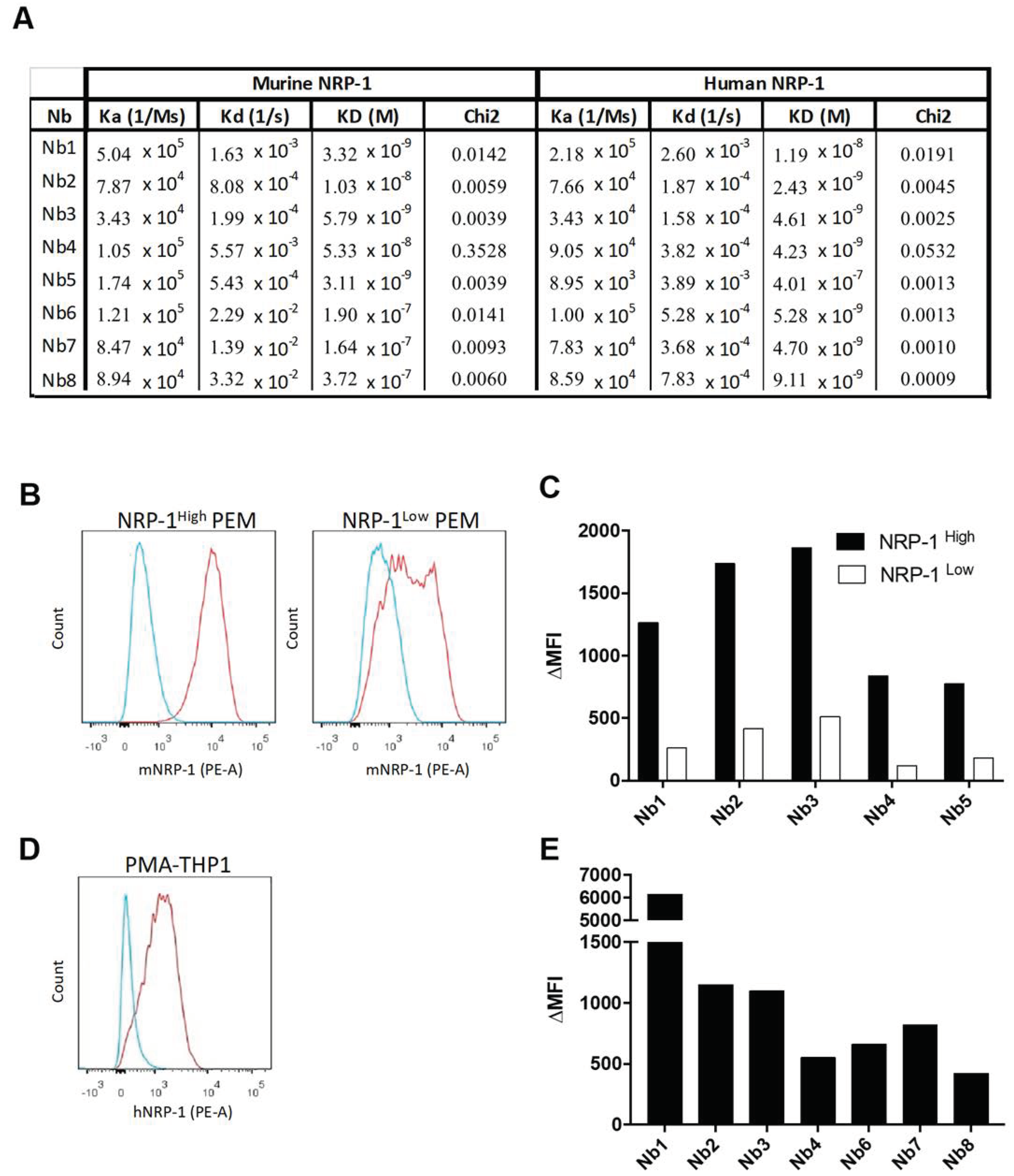
2.1. Generation and Characterization of NRP-1-Specific Nanobodies
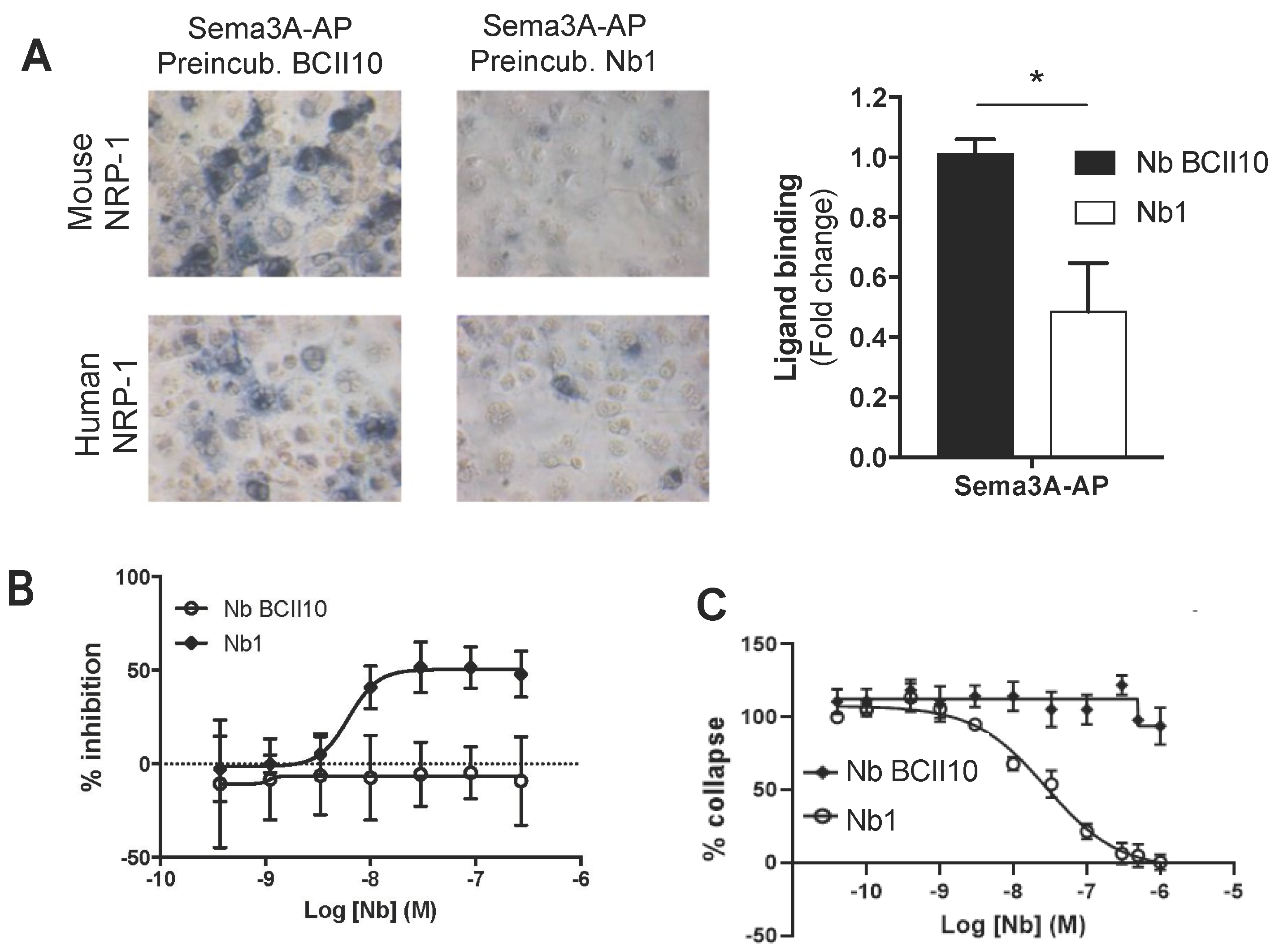
2.2. Anti-NRP-1 Nb1 Inhibits the NRP-1/Sema3A Interaction
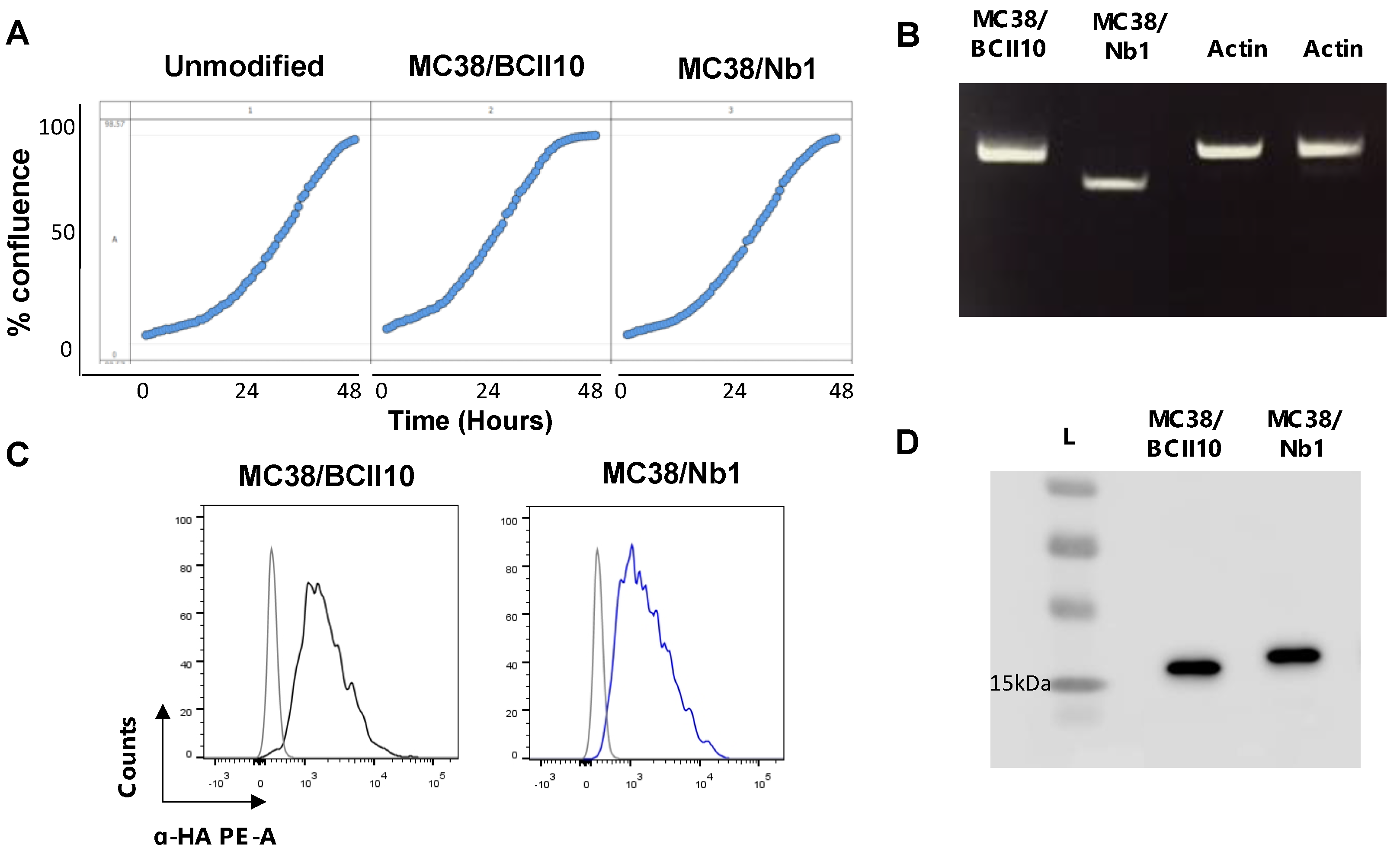
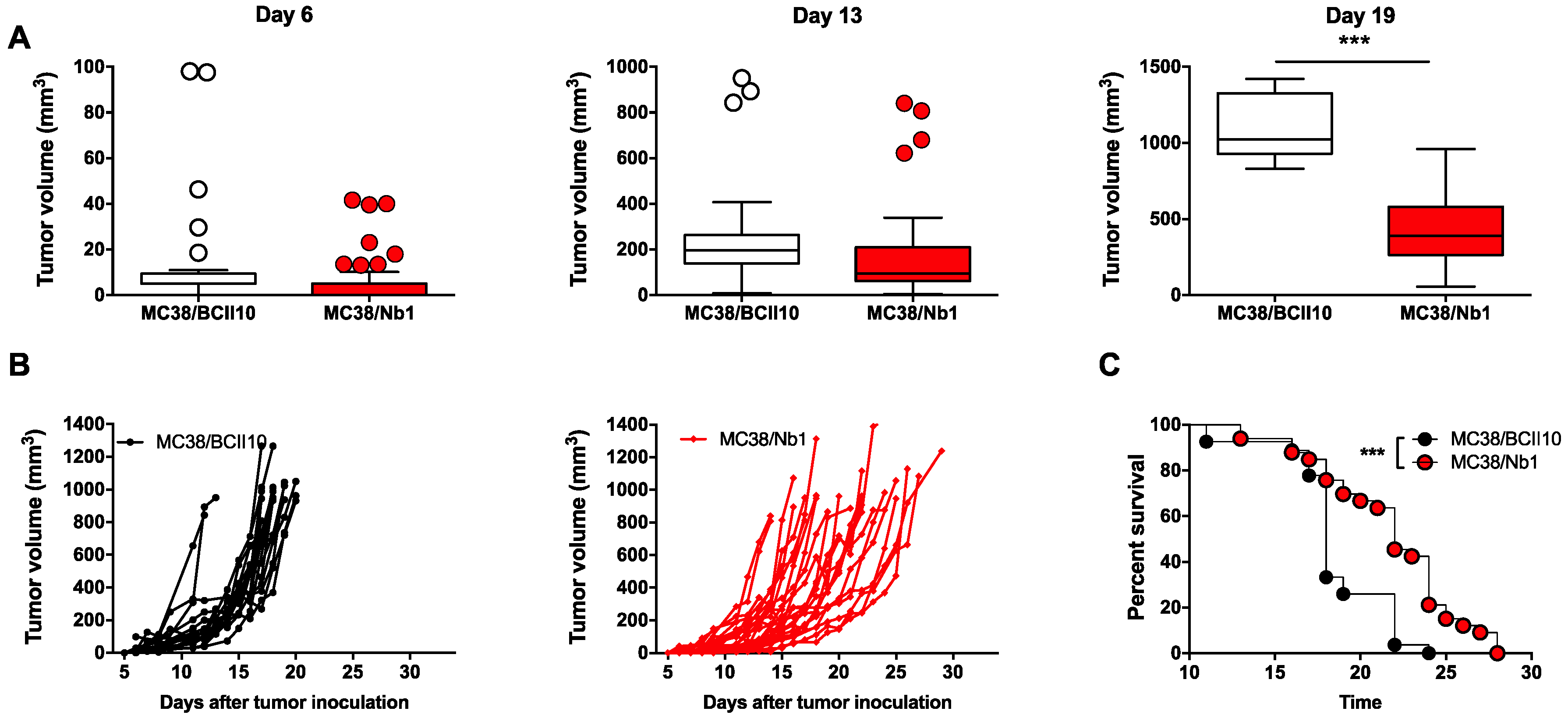
2.3. Anti-NRP-1 Nb1 Delays the Outgrowth of CRC Cells
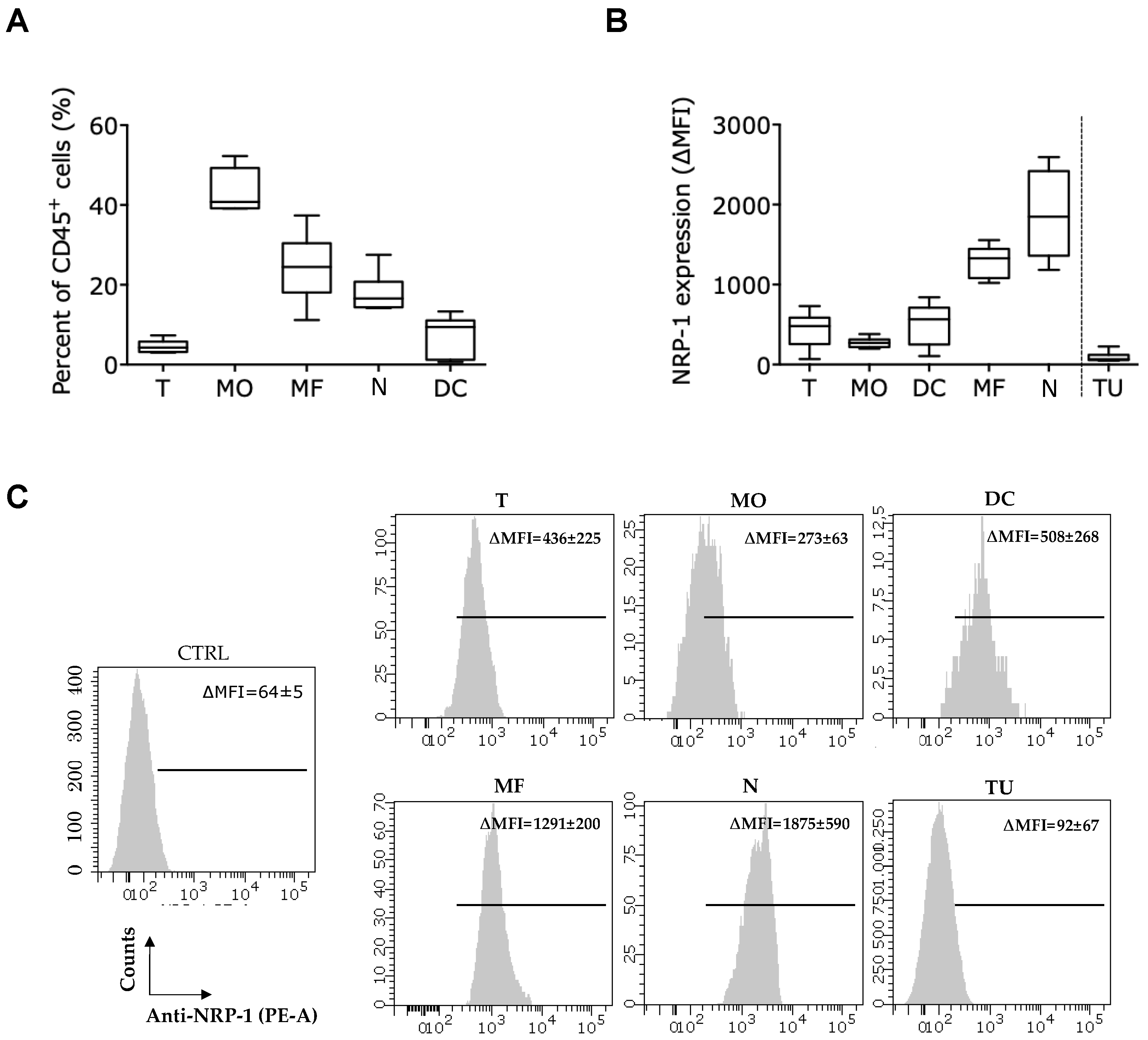
2.4. Presence of the Nb1 Anti-NRP-1 Nb in the TME of CRC Tumors Favors More Pro-Inflammatory Macrophages
2.5. Presence of the Nb1 Anti-NRP-1 Nb in the TME of CRC Tumors Favors Anti-Tumor T-Cell Responses
3. Discussion
4. Materials and Methods
4.1. Nb Generation
4.2. Bio-Layer Interferometry on the Octet®
4.3. Cell Culture
4.4. PEM Isolation
4.5. Sema3A Cell-Binding Assay
4.6. Mouse NRP1/Sema3A Competition AlphaScreen
4.7. HUVEC Collapse Assay
4.8. Mouse Bone Marrow-Derived Macrophages (BMDMs)
4.9. FluoroblokTM BMDM Migration Assay
4.10. Lentiviral Vector Production and Characterization
4.11. Generation and Quality Control of Nb-Expressing MC38 Tumor Cells
4.12. Mice
4.13. Tumor Challenge
4.14. Preparation of a Single-Cell Suspension from Tumors
4.15. Polymerase Chain Reaction (PCR)
4.16. Flow Cytometry and Fluorescence-Assisted Cell Sorting
4.17. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy. Front. Immunol. 2017, 8, 1288. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Cagnoni, G.; Battistini, C.; Bonelli, S.; Isella, C.; Van Ginderachter, J.A.; Bernards, R.; Di Nicolantonio, F.; Giordano, S.; Tamagnone, L. Neuropilin-1 upregulation elicits adaptive resistance to oncogene-targeted therapies. J. Clin. Investig. 2018, 128, 3976–3990. [Google Scholar] [CrossRef] [PubMed]
- Sarris, M.; Andersen, K.G.; Randow, F.; Mayr, L.; Betz, A.G. Neuropilin-1 Expression on Regulatory T Cells Enhances Their Interactions with Dendritic Cells during Antigen Recognition. Immunity 2008, 28, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Bourbié-Vaudaine, S.; Blanchard, N.; Hivroz, C.; Roméo, P.-H. Dendritic Cells Can Turn CD4 + T Lymphocytes into Vascular Endothelial Growth Factor-Carrying Cells by Intercellular Neuropilin-1 Transfer. J. Immunol. 2006, 177, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.; Laoui, D.; Wenes, M.; Rizzolio, S.; Bassani, N.; Mambretti, M.; Deschoemaeker, S.; Van Ginderachter, J.A.; Tamagnone, L.; Mazzone, M. Impeding Macrophage Entry into Hypoxic Tumor Areas by Sema3A/Nrp1 Signaling Blockade Inhibits Angiogenesis and Restores Antitumor Immunity. Cancer Cell 2013, 24, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Voilin, E.; Gros, G.; Corgnac, S.; de Montpréville, V.; Validire, P.; Bismuth, G.; Mami-Chouaib, F. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat. Commun. 2019, 10, 3345. [Google Scholar] [CrossRef]
- Wallerius, M.; Wallmann, T.; Bartish, M.; Ostling, J.; Mezheyeuski, A.; Tobin, N.P.; Nygren, E.; Pangigadde, P.; Pellegrini, P.; Squadrito, M.L.; et al. Guidance Molecule SEMA3A Restricts Tumor Growth by Differentially Regulating the Proliferation of Tumor-Associated Macrophages. Cancer Res. 2016, 76, 3166–3178. [Google Scholar] [CrossRef]
- Podojil, J.R.; Chiang, M.-Y.; Ifergan, I.; Copeland, R.; Liu, L.N.; Maloveste, S.; Langermann, S.; Liebenson, D.; Balabanov, R.; Chi, H.; et al. B7-H4 Modulates Regulatory CD4 + T Cell Induction and Function via Ligation of a Semaphorin 3a/Plexin A4/Neuropilin-1 Complex. J. Immunol. 2018, 201, 897–907. [Google Scholar] [CrossRef]
- Rivera, L.B.; Bergers, G. Location, Location, Location: Macrophage Positioning within Tumors Determines Pro- or Antitumor Activity. Cancer Cell 2013, 24, 687–689. [Google Scholar] [CrossRef]
- Bruder, D.; Probst-Kepper, M.; Westendorf, A.M.; Geffers, R.; Beissert, S.; Loser, K.; von Boehmer, H.; Buer, J.; Hansen, W. Frontline: Neuropilin-1: A surface marker of regulatory T cells. Eur. J. Immunol. 2004, 34, 623–630. [Google Scholar] [CrossRef]
- Hansen, W.; Hutzler, M.; Abel, S.; Alter, C.; Stockmann, C.; Kliche, S.; Albert, J.; Sparwasser, T.; Sakaguchi, S.; Westendorf, A.M.; et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 2012, 209, 2001–2016. [Google Scholar] [CrossRef] [PubMed]
- Overacre-Delgoffe, A.E.; Chikina, M.; Dadey, R.E.; Yano, H.; Brunazzi, E.A.; Shayan, G.; Horne, W.; Moskovitz, J.M.; Kolls, J.K.; Sander, C.; et al. Interferon-γ Drives T reg Fragility to Promote Anti-tumor Immunity. Cell 2017, 169, 1130–1141.e11. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, X.; Shen, G.; Wang, W.; Li, J.; Zhao, J.; Wei, Y.-Q.; Edwards, C.K. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci. Rep. 2016, 6, 24249. [Google Scholar] [CrossRef] [PubMed]
- Weekes, C.D.; Beeram, M.; Tolcher, A.W.; Papadopoulos, K.P.; Gore, L.; Hegde, P.; Xin, Y.; Yu, R.; Shih, L.M.; Xiang, H.; et al. A phase I study of the human monoclonal anti-NRP1 antibody MNRP1685A in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; LoRusso, P.M.; Messersmith, W.A.; Papadopoulos, K.P.; Gore, L.; Beeram, M.; Ramakrishnan, V.; Kim, A.H.; Beyer, J.C.; Mason Shih, L.; et al. A Phase Ib study evaluating MNRP1685A, a fully human anti-NRP1 monoclonal antibody, in combination with bevacizumab and paclitaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 951–960. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Movahedi, K.; Schoonooghe, S.; Laoui, D.; Houbracken, I.; Waelput, W.; Breckpot, K.; Bouwens, L.; Lahoutte, T.; De Baetselier, P.; Raes, G.; et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012, 72, 4165–4177. [Google Scholar] [CrossRef]
- Schoonooghe, S.; Laoui, D.; Van Ginderachter, J.A.; Devoogdt, N.; Lahoutte, T.; De Baetselier, P.; Raes, G. Novel applications of nanobodies for in vivo bio-imaging of inflamed tissues in inflammatory diseases and cancer. Immunobiology 2012, 217, 1266–1272. [Google Scholar] [CrossRef]
- Yang, E.Y.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [Google Scholar] [CrossRef]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.J.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 13, 500–515. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Woo, S.R.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D.; Bonnevier, J.; et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013, 501, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Nakhlé, J.; Sundstedt, A.; Plas, P.; Bauchet, A.-L.; Pierron, V.; Bruetschy, L.; Deronic, A.; Törngren, M.; Liberg, D.; et al. Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment. J. Immunother. Cancer 2015, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; Van den Bossche, J.; Mack, M.; Pipeleers, D.; In’t Veld, P.; De Baetselier, P.; et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef] [PubMed]
- Laoui, D.; Van Overmeire, E.; Di Conza, G.; Aldeni, C.; Keirsse, J.; Morias, Y.; Movahedi, K.; Houbracken, I.; Schouppe, E.; Elkrim, Y.; et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014, 74, 24–30. [Google Scholar] [CrossRef]
- Watson, D.C.; Sargianou, M.; Panos, G. Interleukin-12 (IL-12)/IL-10 ratio as a marker of disease severity in crimean-congo hemorrhagic fever. Clin. Vaccine Immunol. 2012, 19, 823–824. [Google Scholar] [CrossRef]
- Saksida, A.; Duh, D.; Wraber, B.; Dedushaj, I.; Ahmeti, S.; Avšič-Županc, T. Interacting roles of immune mechanisms and viral load in the pathogenesis of Crimean-Congo hemorrhagic fever. Clin. Vaccine Immunol. 2010, 17, 1086–1093. [Google Scholar] [CrossRef]
- Gagnon, M.L.; Bielenberg, D.R.; Gechtman, Z.; Miao, H.-Q.; Takashima, S.; Soker, S.; Klagsbrun, M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc. Natl. Acad. Sci. USA 2000, 97, 2573–2578. [Google Scholar] [CrossRef]
- Kuo, C.J.; Farnebo, F.; Yu, E.Y.; Christofferson, R.; Swearingen, R.A.; Carter, R.; von Recum, H.A.; Yuan, J.; Kamihara, J.; Flynn, E.; et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc. Natl. Acad. Sci. USA 2001, 98, 4605–4610. [Google Scholar] [CrossRef]
- Schuch, G.; Machluf, M.; Bartsch, G.; Nomi, M.; Richard, H.; Atala, A.; Soker, S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood 2002, 100, 4622–4628. [Google Scholar] [CrossRef]
- Hong, T.-M.; Chen, Y.-L.; Wu, Y.-Y.; Yuan, A.; Chao, Y.-C.; Chung, Y.-C.; Wu, M.-H.; Yang, S.-C.; Pan, S.-H.; Shih, J.-Y.; et al. Targeting Neuropilin 1 as an Antitumor Strategy in Lung Cancer. Clin. Cancer Res. 2007, 13, 4759–4768. [Google Scholar] [CrossRef]
- Bergé, M.; Bonnin, P.; Sulpice, E.; Vilar, J.; Allanic, D.; Silvestre, J.S.; Lévy, B.I.; Tucker, G.C.; Tobelem, G.; Merkulova-Rainon, T. Small interfering RNAs induce target-independent inhibition of tumor growth and vasculature remodeling in a mouse model of hepatocellular carcinoma. Am. J. Pathol. 2010, 177, 3192–3210. [Google Scholar] [CrossRef] [PubMed]
- Raskopf, E.; Vogt, A.; Standop, J.; Sauerbruch, T.; Schmitz, V. Inhibition of Neuropilin-1 by RNA-Interference and its Angiostatic Potential in the Treatment of Hepatocellular Carcinoma. Z. Gastroenterol. 2010, 48, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, L.; Xiao, Z.; Lu, S.; Yang, R.; Han, Z.C. Neuropilin-1 in acute myeloid leukemia: Expression and role in proliferation and migration of leukemia cells. Leuk. Lymphoma 2008, 49, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Suzuki, E.; Nishie, M.; Kii, I.; Kataoka, T.R.; Hirata, M.; Inoue, M.; Pu, F.; Iwaisako, K.; Tsuda, M.; et al. Downregulation of neuropilin-1 on macrophages modulates antibody-mediated tumoricidal activity. Cancer Immunol. Immunother. 2017, 66, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, R.E.; Lipscomb, E.A.; Lin, X.; Wendt, M.A.; Chadborn, N.H.; Eickholt, B.J.; Mercurio, A.M. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003, 63, 5230–5233. [Google Scholar]
- Liang, W.-C.; Dennis, M.S.; Stawicki, S.; Chanthery, Y.; Pan, Q.; Chen, Y.; Eigenbrot, C.; Yin, J.; Koch, A.W.; Wu, X.; et al. Function Blocking Antibodies to Neuropilin-1 Generated from a Designed Human Synthetic Antibody Phage Library. J. Mol. Biol. 2007, 366, 815–829. [Google Scholar] [CrossRef]
- Chen, L.; Miao, W.; Tang, X.; Zhang, H.; Wang, S.; Luo, F.; Yan, J. Inhibitory effect of neuropilin-1 monoclonal antibody (NRP-1 MAb) on glioma tumor in mice. J. Biomed. Nanotechnol. 2013, 9, 551–558. [Google Scholar] [CrossRef]
- Zeng, F.; Luo, F.; Lv, S.; Zhang, H.; Cao, C.; Chen, X.; Wang, S.; Li, Z.; Wang, X.; Dou, X.; et al. A monoclonal antibody targeting neuropilin-1 inhibits adhesion of MCF7 breast cancer cells to fibronectin by suppressing the FAK/p130cas signaling pathway. Anticancer. Drugs 2014, 25, 663–672. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, J.; Wang, S.; Li, Y.; Mi, Y.; Gao, S.; Xu, Y.; Chen, Y.; Yan, J. Anti-neuropilin-1 monoclonal antibody suppresses the migration and invasion of human gastric cancer cells via Akt dephosphorylation. Exp. Ther. Med. 2018, 16, 537–546. [Google Scholar] [CrossRef]
- Darbonne, W.C.; Du, X.; Dhawan, P.; Hartley, D.; Tarrant, J.; Taylor, H.; Cain, G.; Shih, L.M.; Brachmann, R.K.; Phung, Q.; et al. Mechanism for platelet reduction in anti-neuropilin-1 (MNRP1685A)-treated phase I patients. J. Clin. Oncol. 2011, 29, e13598. [Google Scholar] [CrossRef]
- Broos, K.; Lecocq, Q.; Xavier, C.; Bridoux, J.; Nguyen, T.T.; Corthals, J.; Schoonooghe, S.; Lion, E.; Raes, G.; Keyaerts, M.; et al. Evaluating a Single Domain Antibody Targeting Human PD-L1 as a Nuclear Imaging and Therapeutic Agent. Cancers 2019, 16, 872. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Lecocq, Q.; Keersmaecker, B.; Raes, G.; Corthals, J.; Lion, E.; Thielemans, E.; Devoogdt, N.; Keyaerts, M. Breckpot Single Domain Antibody-Mediated Blockade of Programmed Death-Ligand 1 on Dendritic Cells Enhances CD8 T-cell Activation and Cytokine Production. Vaccines 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; De Vlaeminck, Y.; Hanssens, H.; D’Huyvetter, M.; Raes, G.; Goyvaerts, C.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. Theranostics in immuno-oncology using nanobody derivatives. Theranostics 2019, 9, 7772–7791. [Google Scholar] [CrossRef] [PubMed]
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, J.T.; Caponegro, M.D.; Chen, D.; Choi, M.K.; Li, M.; Tsirka, S.E. Deletion of Neuropilin 1 from Microglia or Bone Marrow—Derived Macrophages Slows Glioma Progression. Cancer Res. 2018, 78, 685–694. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Dalton, H.J.; Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Lopez-Berestein, G.; Bar-Eli, M.; Sood, A.K. Monocyte Subpopulations in Angiogenesis. Cancer Res. 2014, 74, 1287–1293. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- De Palma, M.; Venneri, M.A.; Galli, R.; Sergi, L.S.; Politi, L.S.; Sampaolesi, M.; Naldini, L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005, 8, 211–226. [Google Scholar] [CrossRef]
- Dollt, C.; Becker, K.; Michel, J.; Melchers, S.; Weis, C.-A.; Schledzewski, K.; Krewer, A.; Kloss, L.; Gebhardt, C.; Utikal, J.; et al. The shedded ectodomain of Lyve-1 expressed on M2-like tumor-associated macrophages inhibits melanoma cell proliferation. Oncotarget 2017, 8, 103682. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Klebba, I.; McCready, J.; Arendt, L.M.; Betancur-Boissel, M.; Wu, M.-F.; Zhang, X.; Lewis, M.T.; Kuperwasser, C. Estrogen Promotes ER-Negative Tumor Growth and Angiogenesis through Mobilization of Bone Marrow-Derived Monocytes. Cancer Res. 2012, 72, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Somasundaram, A.; Manne, S.; Gocher, A.M.; Szymczak-Workman, A.L.; Vignali, K.M.; Scott, E.N.; Normolle, D.P.; John Wherry, E.; Lipson, E.J.; et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat. Immunol. 2020, 21, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Keyaerts, M.; Lecocq, Q.; Renmans, D.; Nguyen, T.; Escors, D.; Liston, A.; Raes, G.; Breckpot, K.; Devoogdt, N. Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers. Oncotarget 2017, 8, 41932. [Google Scholar] [CrossRef]
- De Vlaeminck, Y.; Lecocq, Q.; Giron, P.; Heirman, C.; Geeraerts, X.; Bolli, E.; Movahedi, K.; Massa, S.; Schoonooghe, S.; Thielemans, K.; et al. Single-domain antibody fusion proteins can target and shuttle functional proteins into macrophage mannose receptor expressing macrophages. J. Control. Release 2019, 299, 107–120. [Google Scholar] [CrossRef]
- Breckpot, K.; Dullaers, M.; Bonehill, A.; Van Meirvenne, S.; Heirman, C.; De Greef, C.; van der Bruggen, P.; Thielemans, K. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 2003, 5, 654–667. [Google Scholar] [CrossRef]
- Breckpot, K.; Escors, D.; Arce, F.; Lopes, L.; Karwacz, K.; Van Lint, S.; Keyaerts, M.; Collins, M. HIV-1 Lentiviral Vector Immunogenicity Is Mediated by Toll-Like Receptor 3 (TLR3) and TLR7. J. Virol. 2010, 84, 5627–5636. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]


| RT-PCR | Forward | Reverse |
|---|---|---|
| Actin | 5′-CTG TCC CTG TAT GCC TCT G-3′ | 5′-ATG TCA CGC ACG ATT TCC-3′ |
| BCII10 | 5′-TCC TGC TAT GGG TAC TGC TGC T-3′ | 5′-CTC AGG TTT CAG GTT GTT CAT TT-3′ |
| Nb1 | 5′-TCC TGC TAT GGG TAC TGC TGC T-3′ | 5′-GTA ATC TTT GCG ACC AAC TCG-3′ |
| Itgam (CD11b) | 5′-TCT TGG GTT TCC TAG TGT GTT AG-3′ | 5′-AGA GGA CAG CAC AGC ATT TAG-3′ |
| Adgre1 (F4/80) | 5′-CGT CAG GTA CGG GAT GAA TAT AAG-3′ | 5′-ATC TTG GAA GTG GAT GGC ATA G-3’ |
| H2 (MHC-II) | 5’-CAG CAA GGA CTG GTC TTT CTA T-3′ | 5′-AAC TCT GCA GGC GTA TGT ATC-3′ |
| Tnf (Tnfα) | 5′-CCT TCA CAG AGC AAT GAC TC-3′ | 5′-GTC TAC TCC CAG GTT CTC TTC-3′ |
| Ifng (Ifnγ) | 5′-CGG CAC AGT CAT TGA AAG CCT A-3′ | 5′- GTT GCT GAT GGC CTG ATT GTC-3′ |
| Il1b | 5′-GTG TGG ATC CAA AGC AAT AC-3′ | 5′-GTC TGC TCA TTC ATG ACA AG-3′ |
| Il12b | 5′- GAA AGA CCC TGA CCA TCA CT-3′ | 5′-CCT TCT CTG CAG ACA GAG AC-3′ |
| Mrc1 (CD206) | 5′-GCA AAT GGA GCC GTC TGT GC-3′ | 5′-CTC GTG GAT CTC CGT GAC AC-3′ |
| Arg1 | 5′-GTC CCT AAT GAC AGC TCC TTT C-3′ | 5′-CCA CAC TGA CTC TTC CAT TCT T-3′ |
| Ptgs2 (Cox2) | 5′-CAG ACA ACA TAA ACT GCG CCTT 3′ | 5′-GAT ACA CCT CTC CAC CAA TGA CC 3′ |
| Il10 | 5′-ACT CAA TAC ACA CTG CAG GTG-3′ | 5′-GGA CTT TAA GGG TTA CTT GG-3′ |
| Hif1a | 5′-ACC TGG CAA TGT CTC CTT TAC-3′ | 5′-CCA GTG ACT CTG GAC TTG ATT C-3′ |
| Stab1 | 5′-ACG GGA AAC TGC TTG ATG TC-3′ | 5′-ACT CAG CGT CAT GTT GTC CA-3′ |
| Chil3 (Ym1) | 5′-GCT AAG GAC AGG CCA ATA GAA-3′ | 5′-GCA TTC CAG CAA AGG CAT AG-3′ |
| Lyve1 | 5′-CTG GCT GTT TGC TAC GTG AA-3′ | 5′-CAT GAA ACT TGC CTC GTG TG-3′ |
| vegfa | 5′-CAC TTC CAG AAA CAC GAC AAA C-3′ | 5′-TGG AAC CGG CAT CTT TAT CTC-3′ |
| vegfr2 | 5′-CTC TGT CAA GTG GCG GTA AA-3′ | 5′-TCA GGA AGC CAC AAA GCT AAA -3′ |
| Hprt1 | 5′-CGA GAT GTC ATG AAG GAG ATG G-3′ | 5′-AGC AGG TCA GCA AAG AAC TTA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vlaeminck, Y.; Bonelli, S.; Awad, R.M.; Dewilde, M.; Rizzolio, S.; Lecocq, Q.; Bolli, E.; Santos, A.R.; Laoui, D.; Schoonooghe, S.; et al. Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development. Cancers 2020, 12, 3582. https://doi.org/10.3390/cancers12123582
De Vlaeminck Y, Bonelli S, Awad RM, Dewilde M, Rizzolio S, Lecocq Q, Bolli E, Santos AR, Laoui D, Schoonooghe S, et al. Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development. Cancers. 2020; 12(12):3582. https://doi.org/10.3390/cancers12123582
Chicago/Turabian StyleDe Vlaeminck, Yannick, Stefano Bonelli, Robin Maximilian Awad, Maarten Dewilde, Sabrina Rizzolio, Quentin Lecocq, Evangelia Bolli, Ana Rita Santos, Damya Laoui, Steve Schoonooghe, and et al. 2020. "Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development" Cancers 12, no. 12: 3582. https://doi.org/10.3390/cancers12123582
APA StyleDe Vlaeminck, Y., Bonelli, S., Awad, R. M., Dewilde, M., Rizzolio, S., Lecocq, Q., Bolli, E., Santos, A. R., Laoui, D., Schoonooghe, S., Tamagnone, L., Goyvaerts, C., Mazzone, M., Breckpot, K., & Van Ginderachter, J. A. (2020). Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development. Cancers, 12(12), 3582. https://doi.org/10.3390/cancers12123582






