Recent Advances in the Treatment of Patients with Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
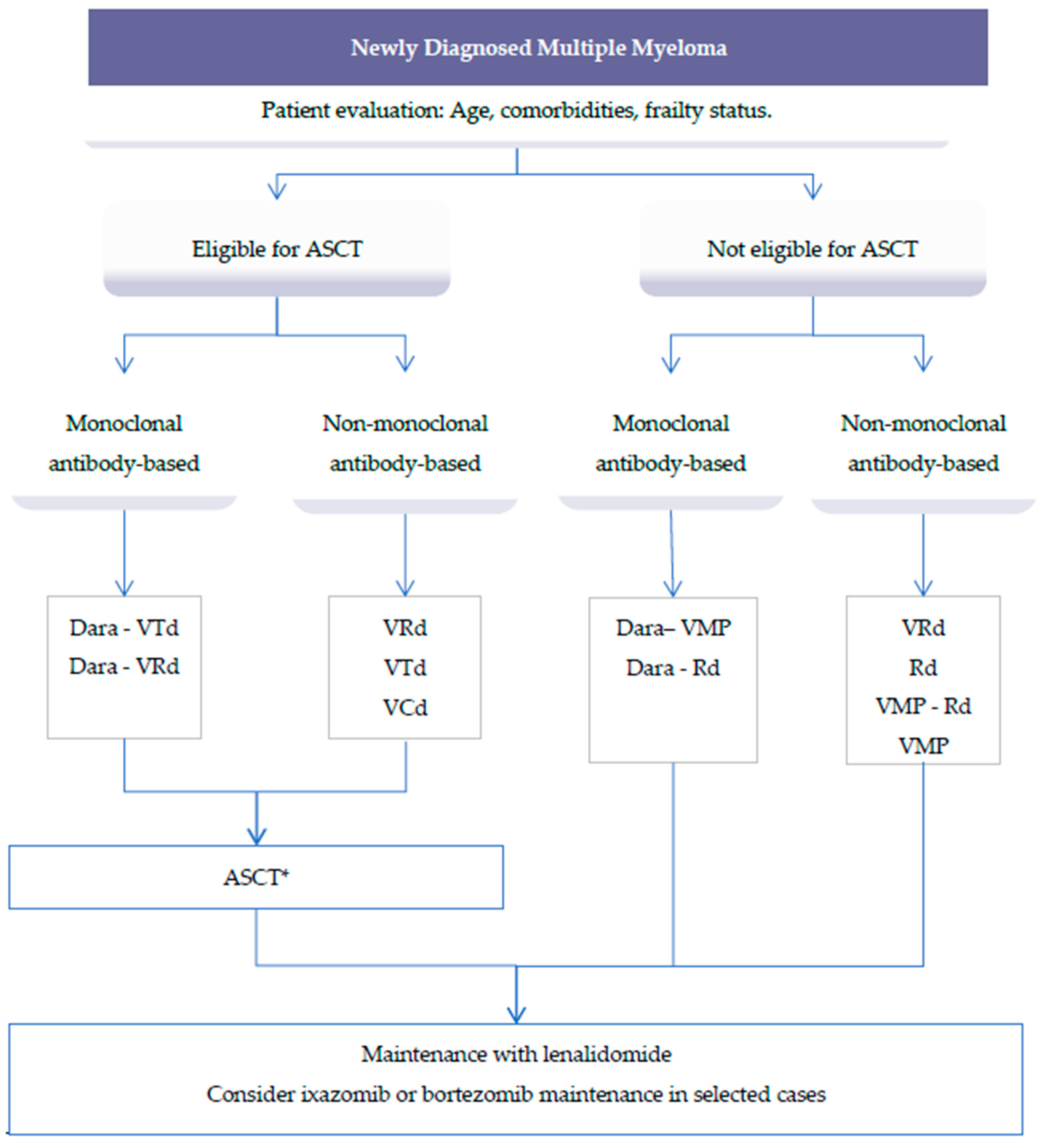
2. Treatment of Newly Diagnosed Multiple Myeloma
2.1. Front-Line Transplant Setting
2.2. Role of Autologous Stem Cell Transplant (ASCT)
2.3. Post ASCT Treatment: Consolidation and Maintenance
2.4. Front-Line Non-Transplant Setting
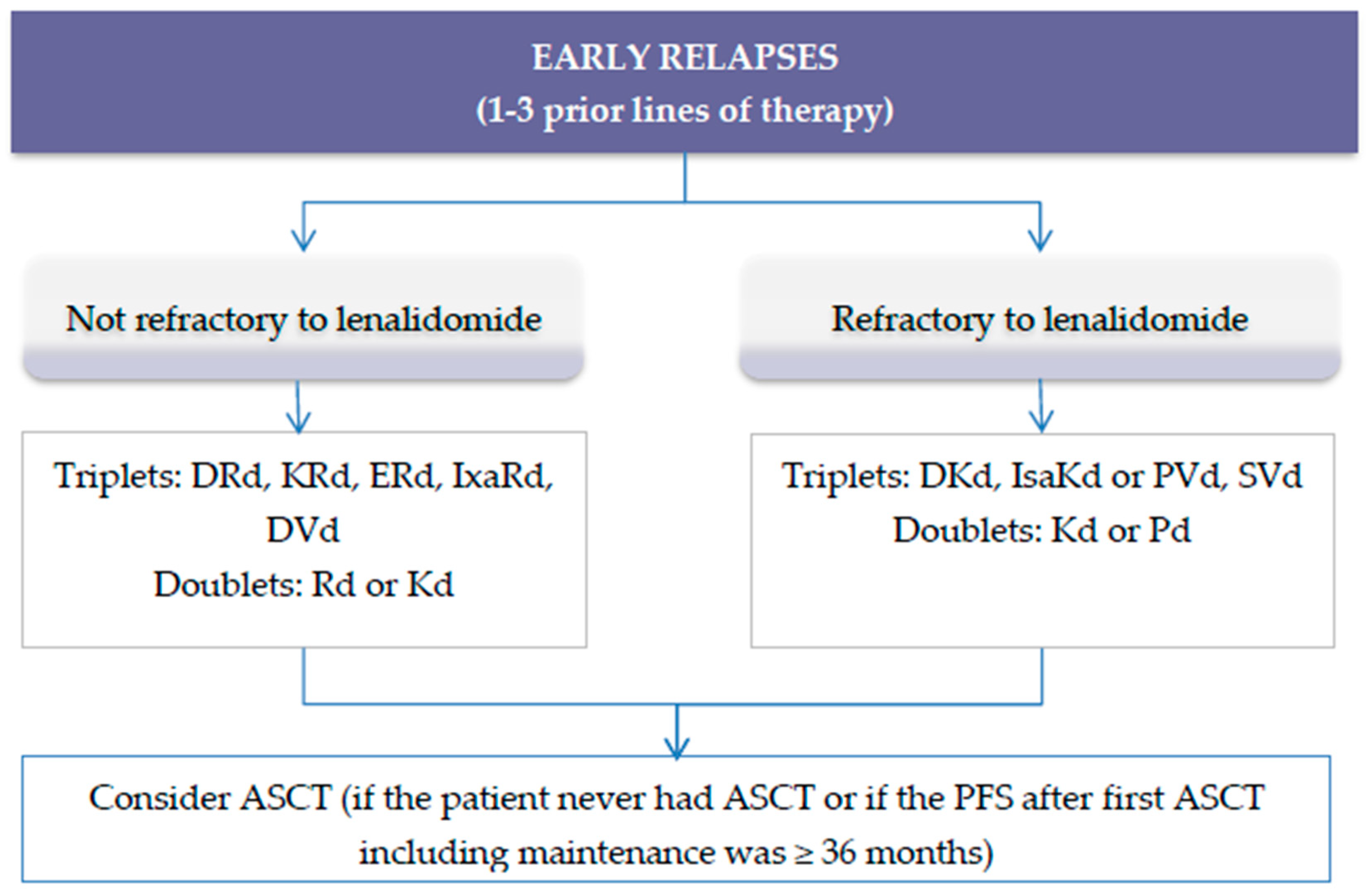
3. Treatment of Relapsed Multiple Myeloma
3.1. Treatment of Early Relapse
Second Generation Regimens for Relapsed Refractory Multiple Myeloma (RRMM)
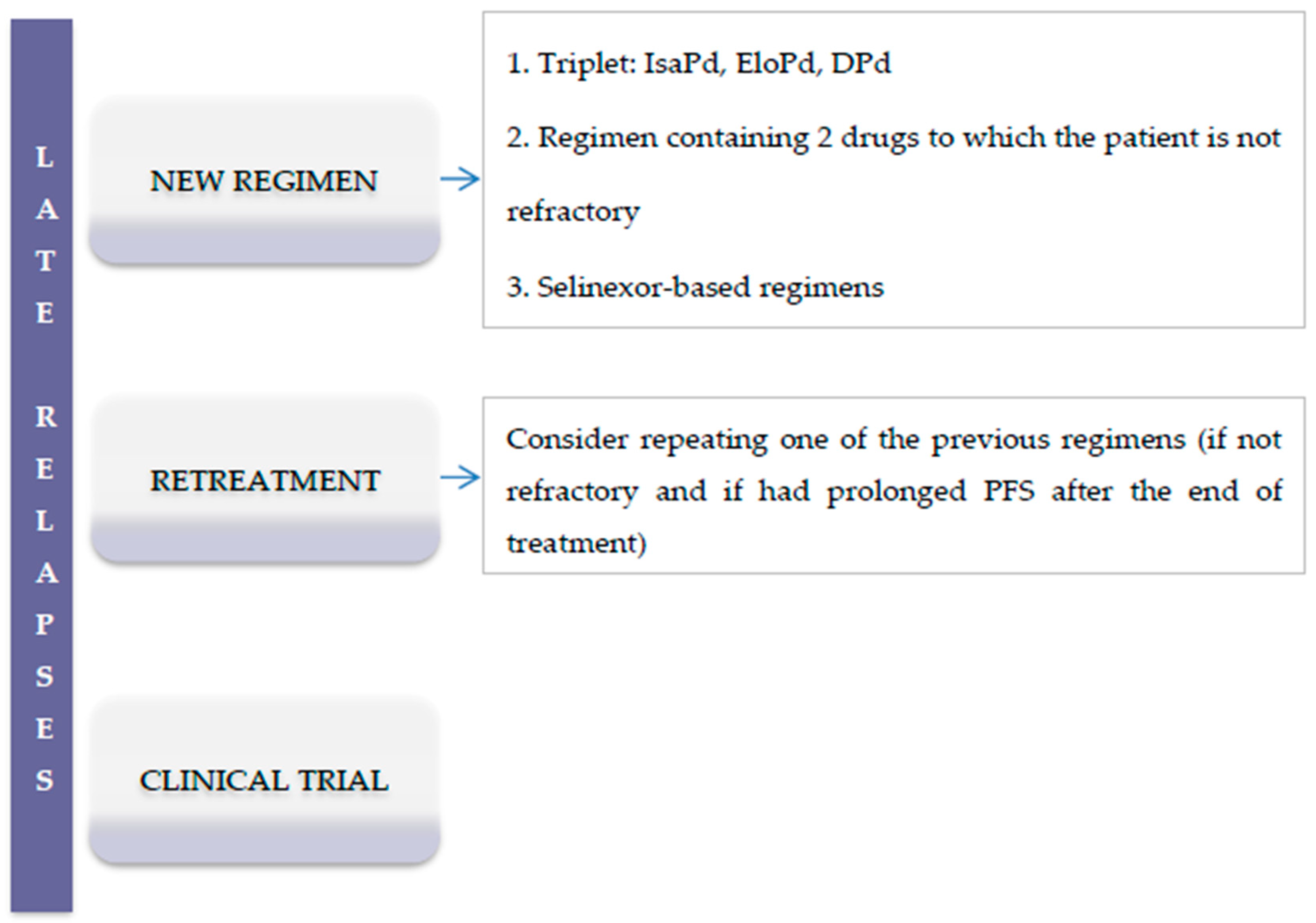
3.2. Late Relapses
4. New Generation Therapies and Future Insights
4.1. Novel Drugs
4.1.1. Selinexor
4.1.2. Melflufen
4.1.3. Iberdomide
4.1.4. Venetoclax
4.2. Immunotherapy
4.2.1. Belantamab Mafodotin
4.2.2. Bispecific Antibodies
4.2.3. CAR-T Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet Lond. Engl. 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer Oxf. Engl. 1990 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Moreau, P.; Touzeau, C.; Vij, R.; Goldsmith, S.R.; Rosko, A.E. Newly Diagnosed Myeloma in 2020. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e144–e158. [Google Scholar] [CrossRef]
- Takamatsu, H.; Takezako, N.; Zheng, J.; Moorhead, M.; Carlton, V.E.H.; Kong, K.A.; Murata, R.; Ito, S.; Miyamoto, T.; Yokoyama, K.; et al. Prognostic value of sequencing-based minimal residual disease detection in patients with multiple myeloma who underwent autologous stem-cell transplantation. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2503–2510. [Google Scholar] [CrossRef]
- Moreau, P.; Miguel, J.S.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv52–iv61. [Google Scholar] [CrossRef]
- Cavo, M.; Tacchetti, P.; Zamagni, E. Front-line treatment of multiple myeloma. HemaSphere 2019, 3, 127–130. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef]
- D’Agostino, M.; Bertamini, L.; Oliva, S.; Boccadoro, M.; Gay, F. Pursuing a Curative Approach in Multiple Myeloma: A Review of New Therapeutic Strategies. Cancers 2019, 11, 2015. [Google Scholar] [CrossRef]
- Moreau, P.; Avet-Loiseau, H.; Facon, T.; Attal, M.; Tiab, M.; Hulin, C.; Doyen, C.; Garderet, L.; Randriamalala, E.; Araujo, C.; et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood 2011, 118, 5752–5758. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Macro, M.; Caillot, D.; Chaleteix, C.; Roussel, M.; Garderet, L.; Royer, B.; Brechignac, S.; Tiab, M.; et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: Results of the prospective IFM2013-04 trial. Blood 2016, 127, 2569–2574. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Dachs, L.R.; Hebraud, B.; Oriol, A.; Colin, A.-L.; Rios, R.; Hulin, C.; Blanchard, M.J.; Caillot, D.; Sureda, A.; Hernández, M.T.; et al. Integrated Analysis of Randomized Controlled Trials Evaluating Bortezomib + Lenalidomide + Dexamethasone or Bortezomib + Thalidomide + Dexamethasone Induction in Transplant-Eligible Newly Diagnosed Multiple Myeloma. Blood 2018, 132, 3245. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Gay, F.; Cerrato, C.; Petrucci, M.T.; Zambello, R.; Gamberi, B.; Ballanti, S.; Omedè, P.; Palmieri, S.; Troia, R.; Spada, S.; et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: Results from the FORTE trial. J. Clin. Oncol. 2019, 37, 8002. [Google Scholar] [CrossRef]
- Gay, F.; Cerrato, C.; Scalabrini, R.; Belotti, A.; Galli, M.; Zamagni, E.; Offidani, M.; Omedè, P.; Monaco, F.; Tosi, P.; et al. Carfilzomib lenalidomide dexamethasone (krd) with or without transplantation in newly diagnosed myeloma (forte trial): Efficacy according to risk status: S872. HemaSphere 2019, 3, 390–391. [Google Scholar] [CrossRef]
- Gay, F.; Scalabrini, D.R.; Belotti, A.; Offidani, M.; Petrucci, M.; Esma, F.; Palmas, A.D.; Caravita, T.; Grasso, M.; Aquino, S.; et al. Carfilzomib-lenalidomide-dexamethasone (KRd) vs carfilzomib-cyclophosphamide-dexamethasone (KCd) induction: Planned interim analysis of the randomized FORTE trial in newly diagnosed multiple myeloma (NDMM). J. Clin. Oncol. 2017, 35, 8003. [Google Scholar] [CrossRef]
- Kumar, S.K.; Jacobus, S.J.; Cohen, A.D.; Weiss, M.; Callander, N.; Singh, A.K.; Parker, T.L.; Menter, A.; Yang, X.; Parsons, B.; et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Harousseau, J.-L.; Stoppa, A.-M.; Sotto, J.-J.; Fuzibet, J.-G.; Rossi, J.-F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Child, J.A.; Morgan, G.J.; Davies, F.E.; Owen, R.G.; Bell, S.E.; Hawkins, K.; Brown, J.; Drayson, M.T.; Selby, P.J. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003, 348, 1875–1883. [Google Scholar] [CrossRef]
- Fermand, J.-P.; Katsahian, S.; Divine, M.; Leblond, V.; Dreyfus, F.; Macro, M.; Arnulf, B.; Royer, B.; Mariette, X.; Pertuiset, E.; et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: Long-term results of a randomized control trial from the group myelome-autogreffe. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 9227–9233. [Google Scholar] [CrossRef]
- Barlogie, B.; Kyle, R.A.; Anderson, K.C.; Greipp, P.R.; Lazarus, H.M.; Hurd, D.D.; McCoy, J.; Jr, D.F.M.; Dakhil, S.R.; Lanier, K.S.; et al. Standard Chemotherapy Compared With High-Dose Chemoradiotherapy for Multiple Myeloma: Final Results of Phase III US Intergroup Trial S9321. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Bladé, J.; Rosiñol, L.; Sureda, A.; Ribera, J.M.; Díaz-Mediavilla, J.; García-Laraña, J.; Mateos, M.V.; Palomera, L.; Fernández-Calvo, J.; Martí, J.M.; et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: Long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood 2005, 106, 3755–3759. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.M.; Patriarca, F.; Zamagni, E.; Montefusco, V.; Dozza, L.; Galli, M.; Bringhen, S.; Testoni, N.; Grasso, M.; et al. Double Autologous Stem Cell Transplantation Significantly Prolongs Progression-Free Survival and Overall Survival in Comparison with Single Autotransplantation in Newly Diagnosed Multiple Myeloma: An Analysis of Phase 3 EMN02/HO95 Study. Blood 2017, 130, 401. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Pasquini, M.C.; Blackwell, B.; Knust, K.; Bashey, A.; Devine, S.M.; Efebera, Y.A.; Ganguly, S.; Gasparetto, C.; Geller, N.; et al. Comparison of Autologous Hematopoietic Cell Transplant (autoHCT), Bortezomib, Lenalidomide (Len) and Dexamethasone (RVD) Consolidation with Len Maintenance (ACM), Tandem Autohct with Len Maintenance (TAM) and Autohct with Len Maintenance (AM) for up-Front Treatment of Patients with Multiple Myeloma (MM): Primary Results from the Randomized Phase III Trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702—StaMINA Trial). Blood 2016, 128, LBA-1. [Google Scholar] [CrossRef]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef]
- Bonanad, S.; De La Rubia, J.; Gironella, M.; Persona, E.P.; González, B.; Lago, C.F.; Arnan, M.; Zudaire, M.; Rivas, J.H.; Soler, A.; et al. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: The GAH Scale. J. Geriatr. Oncol. 2015, 6, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Domm, A.-S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H.; et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Dimopoulos, M.A.; Meuleman, N.; Belch, A.; Mohty, M.; Chen, W.-M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; Renwick, W.; et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia 2020, 34, 224–233. [Google Scholar] [CrossRef]
- Stege, C.A.M.; Van Der Holt, B.; Dinmohamed, A.G.; Sonneveld, P.; Levin, M.-D.; Van De Donk, N.W.C.J.; Mellqvist, U.-H.; Waage, A.; Zweegman, S. Validation of the FIRST simplified frailty scale using the ECOG performance status instead of patient-reported activities. Leukemia 2020, 34, 1964–1966. [Google Scholar] [CrossRef]
- Mateos, M.V.; Martínez-López, J.; Hernández, M.-T.; Ocio, E.M.; Rosiñol, L.; Martínez, R.; Teruel, A.-I.; Gutiérrez, N.C.; Ramos, M.-L.M.; Oriol, A.; et al. Sequential vs alternating administration of VMP and Rd in elderly patients with newly diagnosed MM. Blood 2016, 127, 420–425. [Google Scholar] [CrossRef]
- Lahuerta, J.-J.; Paiva, B.; Vidriales, M.-B.; Cordón, L.; Cedena, M.-T.; Puig, N.; Martinez-Lopez, J.; Rosiñol, L.; Gutierrez, N.C.; Martín-Ramos, M.-L.; et al. Depth of Response in Multiple Myeloma: A Pooled Analysis of Three PETHEMA/GEM Clinical Trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2900–2910. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Sexton, R.; Abidi, M.H.; Epstein, J.; Rajkumar, S.V.; Dispenzieri, A.; Kahanic, S.P.; Thakuri, M.C.; Reu, F.J.; et al. Longer term follow-up of the randomized phase III trial SWOG S0777: Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lúcio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet Lond. Engl. 2020, 395, 132–141. [Google Scholar] [CrossRef]
- Facon, T.; San-Miguel, J.; Usmani, S.Z.; Dimopoulos, M.A.; Kumar, S.K.; Mateos, M.-V.; Cavo, M.; Heeg, B.; Van Beekhuizen, S.; Pisini, M.; et al. A Network Meta-Analysis (NMA) to Evaluate Comparative Effectiveness of Frontline Treatments for Patients (Pts) with Newly Diagnosed Multiple Myeloma (NDMM) Who Are Transplant-Ineligible (TIE). Blood 2019, 134, 2144. [Google Scholar] [CrossRef]
- Yong, K.L.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.; Raab, M.S.; et al. Multiple myeloma: Patient outcomes in real-world practice. Br. J. Haematol. 2016, 175, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Therneau, T.M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Rajkumar, S.V.; Fonseca, R.; Witzig, T.E.; Lust, J.A.; Larson, D.R.; et al. Clinical Course of Patients With Relapsed Multiple Myeloma. Mayo Clin. Proc. 2004, 79, 867–874. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, F.; Wu, S.; Liu, Y.; Guo, H.; Liu, Y. Triplet versus doublet combination regimens for the treatment of relapsed or refractory multiple myeloma: A meta-analysis of phase III randomized controlled trials. Crit. Rev. Oncol. Hematol. 2017, 113, 249–255. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Siegel, D.S.; Dimopoulos, M.A.; Ludwig, H.; Facon, T.; Goldschmidt, H.; Jakubowiak, A.J.; San-Miguel, J.; Obreja, M.; Blaedel, J.; Stewart, A.K. Improvement in Overall Survival With Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 728–734. [Google Scholar] [CrossRef]
- Lonial, S.; A Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; Betts, K.A.; Chen, C.; Zichlin, M.L.; Brun, A.; Signorovitch, J.; Makenbaeva, D.; Mekan, S.; Sy, O.; et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer 2018, 124, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.S.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Moreau, P.; Niesvizky, R.; Ludwig, H.; Oriol, A.; Chng, W.J.; Goldschmidt, H.; Yang, Z.; Kimball, A.S.; Dimopoulos, M. Carfilzomib-Dexamethasone Versus Bortezomib-Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Overall Survival, Safety, and Subgroups. Clin. Lymphoma Myeloma Leuk. 2019, 19, 522–530.e1. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Sonneveld, P.; Hungria, V.; Nooka, A.K.; Estell, J.A.; Barreto, W.; Corradini, P.; Min, C.-K.; Medvedova, E.; Weisel, K.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin. Lymphoma Myeloma Leuk. 2020, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.U.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.-D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef]
- Durer, C.; Durer, S.; Lee, S.; Chakraborty, R.; Malik, M.N.; Rafae, A.; Zar, M.A.; Kamal, A.; Rosko, N.; Samaras, C.; et al. Treatment of relapsed multiple myeloma: Evidence-based recommendations. Blood Rev. 2020, 39, 100616. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Ashcroft, J.; Szabo, Z.; Garderet, L. Navigating the treatment landscape in multiple myeloma: Which combinations to use and when? Ann. Hematol. 2019, 98, 1–18. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Ludwig, H.; Bazarbachi, A.; Beksac, M.; Bladé, J.; Boccadoro, M.; Cavo, M.; Delforge, M.; Dimopoulos, M.A.; Facon, T.; et al. Insights on Multiple Myeloma Treatment Strategies. HemaSphere 2019, 3, e163. [Google Scholar] [CrossRef]
- Kumar, S.K.; Callander, N.S.; Hillengass, J.; Liedtke, M.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Chandler, J.C.; Cornell, R.F.; Costello, C.; et al. NCCN Guidelines Insights: Multiple Myeloma, Version 1.2020. J. Natl. Compr. Cancer Netw. 2019, 17, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Quach, H.; Mateos, M.-V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet Lond. Engl. 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Mikhael, J. Isatuximab plus carfilzomib and dexamethasone vs carfilzomib and dexamethasone in relapsed/refractory multiple myeloma (ikema): Interim analysis of a phase 3, randomized, open-label study. EHA25 Virtual Congr. Abstract LB2603.
- Dimopoulos, M.A.; Delimpasi, S.; Simonova, M.; Spicka, I.; Pour, L.; Kryachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; et al. Weekly selinexor, bortezomib, and dexamethasone (SVd) versus twice weekly bortezomib and dexamethasone (Vd) in patients with multiple myeloma (MM) after one to three prior therapies: Initial results of the phase III BOSTON study. J. Clin. Oncol. 2020, 38, 8501. [Google Scholar] [CrossRef]
- Miguel, J.S.; Hungria, V.T.M.; Yoon, S.-S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Na Nakorn, T.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- Richardson, P.G.; Hungria, V.T.M.; Yoon, S.-S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Guenther, A.; Na Nakorn, T.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: Outcomes by prior treatment. Blood 2016, 127, 713–721. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef]
- Cook, G.; Ashcroft, A.J.; Cairns, D.; Williams, C.D.; Brown, J.M.; Cavenagh, J.D.; Snowden, J.; Parrish, C.; Yong, K.; Cavet, J.; et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): A randomised, open-label, phase 3 trial. Lancet Haematol. 2016, 3, e340–e351. [Google Scholar] [CrossRef]
- Barlogie, B.; Attal, M.; Crowley, J.; Van Rhee, F.; Szymonifka, J.; Moreau, P.; Durie, B.G.; Harousseau, J.-L. Long-term follow-up of autotransplantation trials for multiple myeloma: Update of protocols conducted by the intergroupe francophone du myelome, southwest oncology group, and university of arkansas for medical sciences. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1209–1214. [Google Scholar] [CrossRef]
- Olin, R.L.; Vogl, D.T.; Porter, D.L.; Luger, S.M.; Schuster, S.J.; Tsai, D.E.; Siegel, D.L.; Cook, R.J.; Mangan, P.A.; Cunningham, K.; et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009, 43, 417–422. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; A Dimopoulos, M.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet Lond. Engl. 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; Leblanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R.; et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Lonial, S.; Weiss, B.M.; Suvannasankha, A.; Fay, J.; Arnulf, B.; Ifthikharuddin, J.J.; de Boer, C.; Wang, J.; Wu, K.; et al. Daratumumab in Combination with Pomalidomide and Dexamethasone for Relapsed and/or Refractory Multiple Myeloma (RRMM) Patients with ≥2 Prior Lines of Therapy: Updated Analysis of MMY1001. Blood 2017, 130, 1824. [Google Scholar] [CrossRef]
- Sonneveld, P.; Zweegman, S.; Cavo, M.; Nasserinejad, K.; Troia, R.; Pour, L.; Croockewit, S.; Corradini, P.; Patriarca, F.; Wu, K.; et al. Carfilzomib, Pomalidomide and Dexamethasone (KPd) in Patients with Multiple Myeloma Refractory to Bortezomib and Lenalidomide. the EMN011 Trial. Blood 2018, 132, 801. [Google Scholar] [CrossRef]
- Shah, J.J.; Stadtmauer, E.A.; Abonour, R.; Cohen, A.D.; Bensinger, W.I.; Gasparetto, C.; Kaufman, J.L.; Lentzsch, S.; Vogl, D.T.; Gomes, C.L.; et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 2015, 126, 2284–2290. [Google Scholar] [CrossRef]
- Krishnan, A.; Kapoor, P.; Palmer, J.M.; Tsai, N.-C.; Kumar, S.; Lonial, S.; Htut, M.; Karanes, C.; Nathwani, N.; Rosenzweig, M.; et al. Phase I/II trial of the oral regimen ixazomib, pomalidomide, and dexamethasone in relapsed/refractory multiple myeloma. Leukemia 2018, 32, 1567–1574. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Mina, R.; Jakubowiak, A.J.; Zimmerman, T.L.; Wolf, J.J.; Lewis, C.; Gleason, C.; Sharp, C.; Martin, T.; Heffner, L.T.; et al. Combining carfilzomib and panobinostat to treat relapsed/refractory multiple myeloma: Results of a Multiple Myeloma Research Consortium Phase I Study. Blood Cancer J. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of Patients with Multiple Myeloma Refractory to CD38-Targeted Monoclonal Antibody Therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Allegra, A.G.; Leanza, R.; Musolino, C. Selective Inhibitors of Nuclear Export in the Treatment of Hematologic Malignancies. Clin. Lymphoma Myeloma Leuk. 2019, 19, 689–698. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research FDA Grants Accelerated Approval to Selinexor for Multiple Myeloma. FDA 2019. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-selinexor-multiple-myeloma (accessed on 7 October 2020).
- Richter, J.; Madduri, D.; Richard, S.; Chari, A. Selinexor in relapsed/refractory multiple myeloma. Ther. Adv. Hematol. 2020, 11, 204062072093062. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Ravillah, D.; Das, D.S.; Song, Y.; Nordström, E.; Gullbo, J.; Richardson, P.G.; Chauhan, D.; Anderson, K.C. A novel alkylating agent Melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br. J. Haematol. 2016, 174, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Oriol, A.; LaRocca, A.; Otero, P.R.; Bladé, J.; Cavo, M.; Hassoun, H.; Leleu, X.; Amor, A.A.; Maisel, C.; et al. Clinical Activity of Melflufen in Patients with Triple-Class Refractory Multiple Myeloma and Poor-Risk Features in an Updated Analysis of HORIZON (OP-106), a Phase 2 Study in Patients with Relapsed/Refractory Multiple Myeloma Refractory to Pomalidomide and/or Daratumumab. Blood 2019, 134, 1883. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Oriol, A.; LaRocca, A.; Blade, J.; Cavo, M.; Otero, P.R.; Leleu, X.; Hiemenz, J.W.; Hassoun, H.; Touzeau, C.; et al. HORIZON (OP-106): An exploratory analysis of time-to-next treatment (TTNT) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) who received melflufen plus dexamethasone (dex). J. Clin. Oncol. 2020, 38, e20570. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Kang, J.; Amatangelo, M.; Polonskaia, A.; Katz, M.; Chiu, H.; Couto, S.; Wang, M.; Ren, Y.; Ortiz, M.; et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia 2020, 34, 1197–1201. [Google Scholar] [CrossRef]
- Lonial, S.; van de Donk, N.W.; Popat, R.; Zonder, J.A.; Minnema, M.C.; Larsen, J.T.; Nguyen, T.V.; Chen, M.S.; Bensmaine, A.; Biyukov, T.; et al. S1603 First clinical (phase 1b/2a) study of iberdomide (cc-220; iber), a celmod, in combination with dexamethasone in patients with relapsed/refractory multiple mieloma. HemaSphere 2019, 3, 738. [Google Scholar] [CrossRef]
- Kumar, S.; Harrison, S.J.; Cavo, M.; De La Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Updated results from BELLINI, a phase III study of venetoclax or placebo in combination with bortezomib and dexamethasone in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38, 8509. [Google Scholar] [CrossRef]
- Algarín, E.M.; Díaz-Tejedor, A.; Mogollón, P.; Hernández-García, S.; Corchete, L.A.; San-Segundo, L.; Martín-Sánchez, M.; González-Méndez, L.; Schoumacher, M.; Banquet, S.; et al. Preclinical evaluation of the simultaneous inhibition of MCL-1 and BCL-2 with the combination of S63845 and venetoclax in multiple myeloma. Haematologica 2020, 105, e116–e120. [Google Scholar] [CrossRef]
- Costa, L.J.; Stadtmauer, F.E.A.; Morgan, G.; Monohan, G.; Kovacsovics, T.; Burwick, N.; Jakubowiak, A.; Kaufman, J.L.; Mobasher, M.M.; Freise, K.J.; et al. Phase 2 Study of Venetoclax Plus Carfilzomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2018, 132, 303. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Baz, R.C.; Harrison, S.J.; Quach, H.; Ho, S.-J.; Vangsted, A.J.; Moreau, P.; Gibbs, S.D.; Salem, A.H.; Coppola, S.; et al. Updated analysis of a phase I/II study of venetoclax in combination with daratumumab and dexamethasone, +/- bortezomib, in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38, 8511. [Google Scholar] [CrossRef]
- Sheikh, S.; Lebel, E.; Trudel, S. Belantamab mafodotin in the treatment of relapsed or refractory multiple myeloma. Future Oncol. Lond. Engl. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.A.; Callander, N.S.; Sborov, D.W.; Suvannasankha, A.; et al. Pivotal DREAMM-2 study: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) refractory to proteasome inhibitors (PIs), immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies (mAbs). J. Clin. Oncol. 2020, 38, 8536. [Google Scholar] [CrossRef]
- Markham, A. Belantamab Mafodotin: First Approval. Drugs 2020, 80, 1607–1613. [Google Scholar] [CrossRef]
- Costa, L.J.; Wong, S.W.; Bermúdez, A.; De La Rubia, J.; Mateos, M.-V.; Ocio, E.M.; Rodríguez-Otero, P.; San-Miguel, J.; Li, S.; Sarmiento, R.; et al. First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. Blood 2019, 134, 143. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti–B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, J.L.D.; Shah, N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.D.; Lin, Y.; Oriol, A.; et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. J. Clin. Oncol. 2020, 38, 8503. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]
- Madduri, D.; Usmani, S.Z.; Jagannath, S.; Singh, I.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Banerjee, A.; Goldberg, J.D.; Schecter, J.M.; et al. Results from CARTITUDE-1: A Phase 1b/2 Study of JNJ-4528, a CAR-T Cell Therapy Directed Against B-Cell Maturation Antigen (BCMA), in Patients with Relapsed and/or Refractory Multiple Myeloma (R/R MM). Blood 2019, 134, 577. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Singh, I.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Olyslager, Y.; Banerjee, A.; Goldberg, J.D.; et al. Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen (BCMA)-directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38, 8505. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zhao, W.-H.; Liu, J.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. Long-Term Follow-up of a Phase 1, First-in-Human Open-Label Study of LCAR-B38M, a Structurally Differentiated Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting B-Cell Maturation Antigen (BCMA), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 579. [Google Scholar] [CrossRef]
- Mikkilineni, L.; Kochenderfer, J.N. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lobato, L.G.; Ganzetti, M.; De Larrea, C.F.; Hudecek, M.; Einsele, H.; Danhof, S. CAR T-Cells in Multiple Myeloma: State of the Art and Future Directions. Front. Oncol. 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Chanan-Khan, A.; Leung, N.; Ludwig, H.; Jagannath, S.; Niesvizky, R.; Giralt, S.; Fermand, J.-P.; Bladé, J.; et al. Renal Impairment in Patients With Multiple Myeloma: A Consensus Statement on Behalf of the International Myeloma Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Evison, F.; Sangha, J.; Yadav, P.; Aung, Y.S.; Sharif, A.; Pinney, J.A.; Drayson, M.T.; Cook, M.; Cockwell, P. A population-based study of the impact of dialysis on mortality in multiple myeloma. Br. J. Haematol. 2018, 180, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Delimpasi, S.; Katodritou, E.; Vassou, A.; Kyrtsonis, M.C.; Repousis, P.; Kartasis, Z.; Parcharidou, A.; Michael, M.; Michalis, E.; et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 195–200. [Google Scholar] [CrossRef]
- Uttervall, K.; Duru, A.D.; Lund, J.; Liwing, J.; Gahrton, G.; Holmberg, E.; Aschan, J.; Alici, E.; Nahi, H. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS ONE 2014, 9, e101819. [Google Scholar] [CrossRef]
| Treatment | No. of Cycles | Post-Induction Response (%) | Post-Consolidation Response (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Regimen | No. of Patients | Induction/Consolidation | ≥VGPR | ≥CR | s-CR | ≥VGPR | ≥CR | s-CR | Trial Name |
| VTd 28 d | 542 | 4/2 | 56 | 9 | 6.5 | 78 | 26 | 20 | CASSIOPEA [16] |
| D-VTd 28 d | 543 | 4/2 | 65 | 14 | 7 | 83 | 39 | 29 | |
| VRd 21 d | 102 | 4/2 | 57 | 13 | 7 | 73 | 42 | 32 | GRIFFIN [17] |
| D-VRd 21 d | 99 | 4/2 | 72 | 19 | 12 | 91 | 51.5 | 42 | |
| VRd 21 d | 350 | 3/2 | 47 | - | - | 78 | - | - | IFM 2009 [14] |
| VRD 28 d | 458 | 6/2 | 67 | 33 | - | 75.5 | 50 | - | GEM 2012 [15] |
| KRd 28 d | 158 | 4/4 | 67 | 34 | 8 | 87 | 62 | 41 | FORTE [18] |
| Treatment | No. of Patients | ORR (%) | ≥CR | ≥VGPR | PR | SD | Trial Name |
|---|---|---|---|---|---|---|---|
| Dara-Rd | 368 | 92.9 | 47.6 | 79.3 | 13.6 | 3 | MAIA [40] |
| Rd | 369 | 81.3 | 24.9 | 53.1 | 28.2 | 15.2 | |
| Dara-VMP | 350 | 91 | 42.6 | 71.1 | 19.7 | 5.7 | ALCYONE [41] |
| VMP | 356 | 74 | 24.4 | 41.1 | 24.2 | 21.3 | |
| VRd | 264 | 90.2 | 24.2 | 74.9 | 15.3 | 7 | SWOG S0777 [39] |
| Rd | 261 | 78.8 | 12.1 | 53.2 | 25.6 | 16.4 |
| Treatment | No. of Patients | Overall Response % | PFS—HR | PFS—Months | OS (Months) | Grade ≥ 3 AE | Median (Range) Prior Lines of Therapy | Trial Name |
|---|---|---|---|---|---|---|---|---|
| DRd vs. Rd | 569 | 93 vs. 76 | 0.44 | 44.5 vs. 17.5 | NR | 90% vs. 81% | 1 (1−11) | POLLUX [47] |
| KRd vs. Rd | 792 | 87 vs. 67 | 0.69 | 26.3 vs. 17.6 | 48.3 vs. 40.4 | 87% vs. 83% | 2 (1−4) | ASPIRE [49] |
| EloRd vs. Rd | 646 | 79 vs. 66 | 0.71 | 19.4 vs. 14.9 | 48 vs. 40 | 77% vs. 68% | 2 (1−4) | ELOQUENT 2 [50,51] |
| IxaRd vs. Rd | 722 | 78 vs. 72 | 0.74 | 20.6 vs. 14.7 | NR | 74% vs. 69% | 1 (1−3) | TOURMALINE MM1 [52] |
| DVd vs. Vd | 498 | 85 vs. 63 | 0.31 | 16.7 vs. 7.1 | NR | NA | 2 (1−9) | CASTOR [56] |
| Kd vs. Vd | 929 | 77 vs. 63 | 0.53 | 18.7 vs. 9.4 | 47.8 vs. 38.8 | 82% vs. 71% | 2 (1−2) | ENDEAVOR [55] |
| Treatment | No. of Patients | Overall Response % | PFS—HR | PFS—Months | OS—Months | Grade ≥ 3 AE | Median (Range) of Prior Lines of Therapy | Trial Name |
|---|---|---|---|---|---|---|---|---|
| Isa-Kd vs. Kd | 302 | 87 vs. 83 | 0.53 | NR vs. 19.1 | NR | 77% vs. 67% | 2 (1−4) | IKEMA [63] |
| DKd vs. Kd | 466 | 93 vs. 86 | 0.63 | NR vs. 15.8 | NR | 82% vs. 74% | 2 (1−2) | CANDOR [62] |
| PVd vs. Vd | 559 | 82 vs. 50 | 0.61 | 11.2 vs. 7.1 | NR | NA | 2 (1−3) | OPTIMISMM [67] |
| SelVd vs. Vd | 402 | 76 vs. 62 | 0.7 | 13.9 vs. 9.5 | NR vs. 25 | NA | 1 (1−3) | BOSTON [64] |
| PanVd vs. Vd | 768 | 61 vs. 55 | 0.63 | 12 vs. 8 | 33.6 vs. 30.4 | 96% vs. 82% | 1 (1−3) | PANORAMA [65] |
| Treatment | No. of Patients | Overall Response % | PFS—HR | PFS—Months | OS—Months | Grade ≥ 3 AE % | Median (Range) of Prior Lines of Therapy | Trial Name |
|---|---|---|---|---|---|---|---|---|
| IsaPd vs. Pd | 307 | 60 vs. 35 | 0.59 | 11.5 vs. 6.5 | NR | NA | 3 (2−4) | ICARIA [69] |
| EloPd vs. Pd | 117 | 53 vs. 26 | 0.54 | 10.3 vs. 4.7 | NR | 57 vs. 60 | 3 (2−8) | ELOQUENT 3 [70] |
| DPd | 103 | 66 | NA | 9.9 | 25.1 | 99 | 4 (1−13) | EQUULEUS [71] |
| Treatment | No. of Patients | Overall Response % | PFS—Months | OS—Months | Grade ≥ 3 AE | Median (Range) of Prior Lines of Therapy |
|---|---|---|---|---|---|---|
| KPd [76] | 32 | 50 | 7.2 | 20.6 | 63% | 6 (2−12) |
| IxaPd [77] | 31 | 48 | 8.6 | NR | 74% | 2 (1−5) |
| PanKd [78] | 30 | 57 | 8 | 23 | 63% | 4 (1−8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legarda, M.A.; Cejalvo, M.J.; de la Rubia, J. Recent Advances in the Treatment of Patients with Multiple Myeloma. Cancers 2020, 12, 3576. https://doi.org/10.3390/cancers12123576
Legarda MA, Cejalvo MJ, de la Rubia J. Recent Advances in the Treatment of Patients with Multiple Myeloma. Cancers. 2020; 12(12):3576. https://doi.org/10.3390/cancers12123576
Chicago/Turabian StyleLegarda, Mario A., María J. Cejalvo, and Javier de la Rubia. 2020. "Recent Advances in the Treatment of Patients with Multiple Myeloma" Cancers 12, no. 12: 3576. https://doi.org/10.3390/cancers12123576
APA StyleLegarda, M. A., Cejalvo, M. J., & de la Rubia, J. (2020). Recent Advances in the Treatment of Patients with Multiple Myeloma. Cancers, 12(12), 3576. https://doi.org/10.3390/cancers12123576





