Maintenance of Energy Homeostasis during Calorically Restricted Ketogenic Diet and Fasting-MR-Spectroscopic Insights from the ERGO2 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Prior Treatment MRSI Substudy
2.2. Adherence to Radiation Study Protocol
2.3. Patients’ Adherence to Recommended Dietary Intervention
2.4. Detectability of Ketone Bodies in Urine and Blood Samples
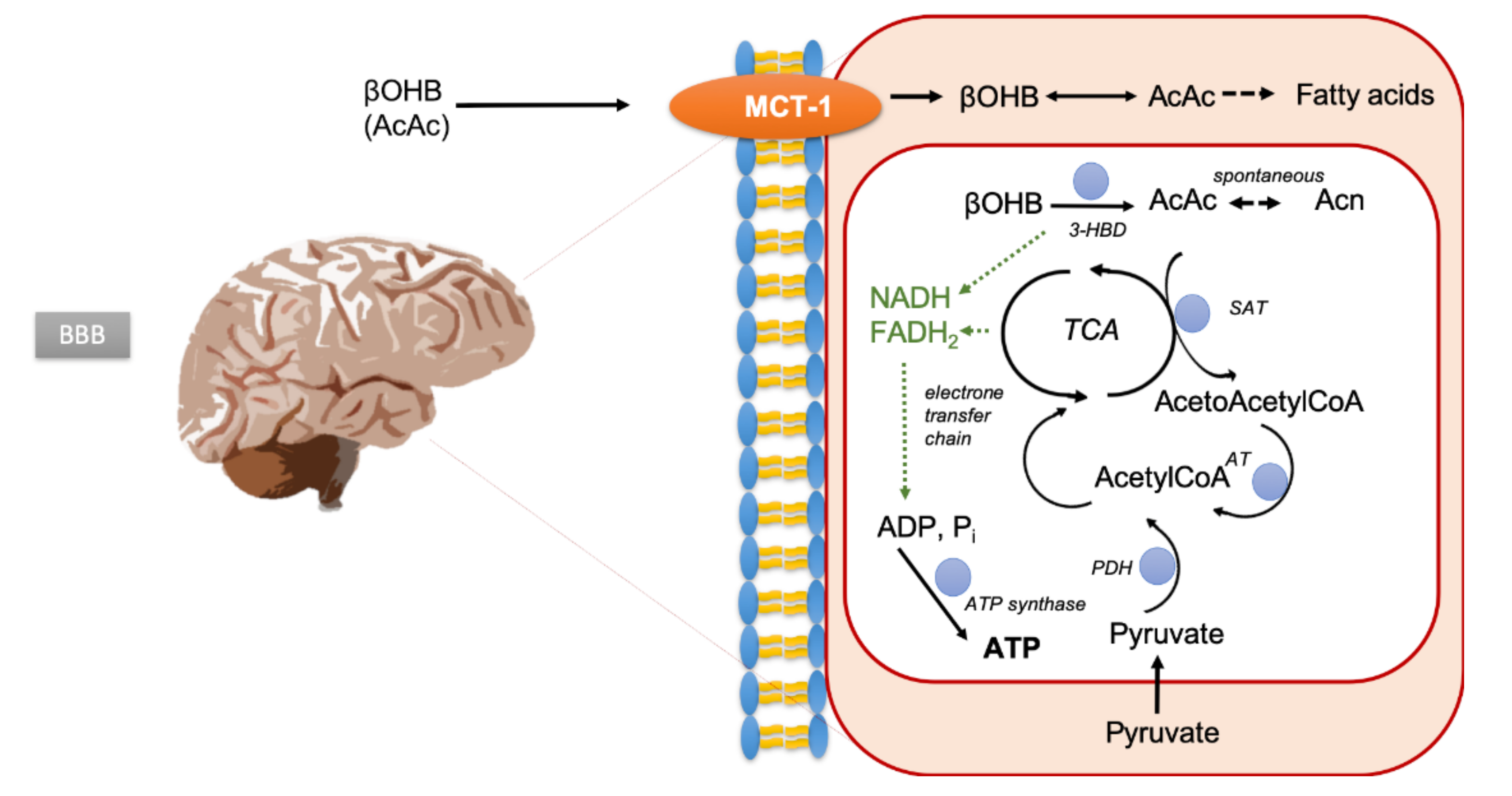
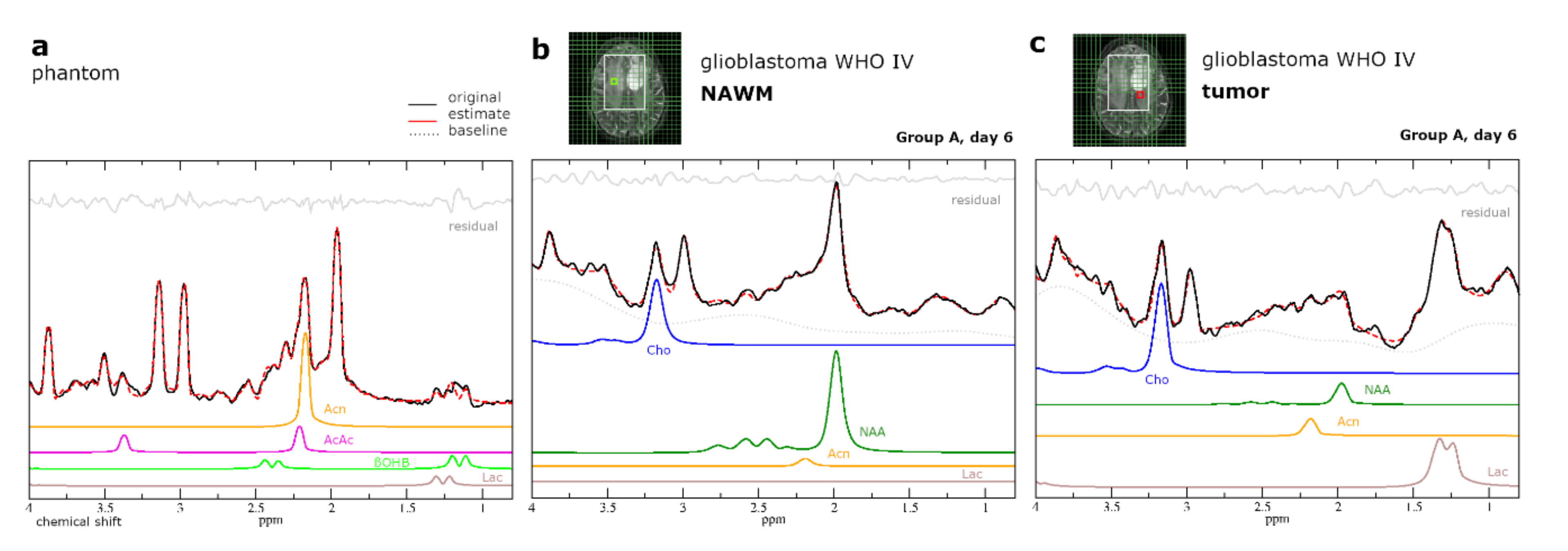
2.5. Intracerebral Detectability and Quantitation of Ketone Bodies Using MRSI
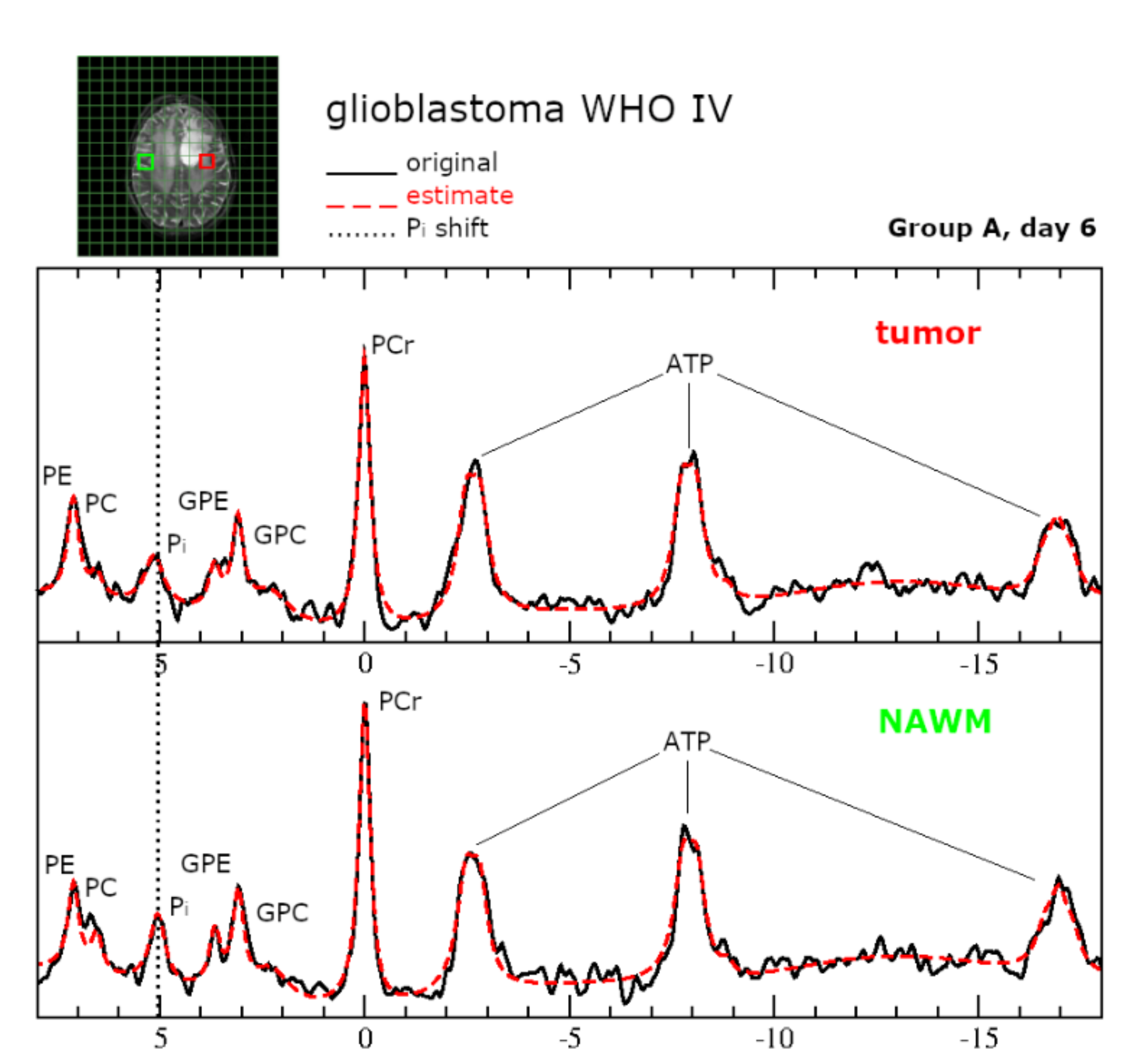
2.6.Intracellular pH and Energy Metabolism
3. Discussion
3.1. Discussion
3.2. Limitations
4. Materials and Methods
4.1. Patients and Study Design
4.2. Magnetic Resonance Imaging
4.3. Data Analysis
4.4. 31P MRSI Model
4.5. Simulation of Basis Set for 1H MRSI
4.6. Phantom Replacement
4.7. Laboratory Testing
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncology 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Hottinger, A.F.; Pacheco, P.; Stupp, R. Tumor treating fields: A novel treatment modality and its use in brain tumors. NEUONC 2016, 18, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Kommission Leitlinien der Deutschen Gesellschaft für Neurologie. Leitlinien für Diagnostik und Therapie in der Neurologie; Thieme: Stuttgart, Germany, 2013. (In German) [Google Scholar]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncology 2013, 15, 4–27. [Google Scholar] [CrossRef]
- Zemlin, A.; Märtens, B.; Wiese, B.; Merten, R.; Steinmann, D. Timing of re-irradiation in recurrent high-grade gliomas: A single institution study. J. Neuro Oncol. 2018, 138, 571–579. [Google Scholar] [CrossRef]
- Combs, S.E.; Thilmann, C.; Edler, L.; Debus, J.; Schulz-Ertner, D. Efficacy of Fractionated Stereotactic Reirradiation in Recurrent Gliomas: Long-Term Results in 172 Patients Treated in a Single Institution. JCO 2005, 23, 8863–8869. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.; Poff, A.M.; D’Agostino, D.P.; Mukherjee, P. Metabolic therapy: A new paradigm for managing malignant brain cancer. Cancer Lett. 2015, 356, 289–300. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Chakravarti, A.; Zhai, G.; Suzuki, Y.; Sarkesh, S.; Black, P.M.; Muzikansky, A.; Loeffler, J.S. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J. Clin. Oncol. 2004, 22, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Tian, R.; Liu, W.; Fei, Z.; Long, Q.; Wang, X.; Zhang, X. The expression and significance of HIF-1alpha and GLUT-3 in glioma. Brain Res. 2009, 1304, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Boitier, E.; Miccoli, L.; Rousset, S.; Dutrillaux, B.; Poupon, M.F. Gliomas are driven by glycolysis: Putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res. 1997, 17, 1903–1911. [Google Scholar] [PubMed]
- Maurer, G.D.; Brucker, D.P.; Bähr, O.; Harter, P.N.; Hattingen, E.; Walenta, S.; Mueller-Klieser, W.; Steinbach, J.P.; Rieger, J. Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Kiebish, M.A.; Han, X.; Cheng, H.; Seyfried, T.N. In Vitro Growth Environment Produces Lipidomic and Electron Transport Chain Abnormalities in Mitochondria from Non-Tumorigenic Astrocytes and Brain Tumours. ASN Neuro 2009, 1, AN20090011. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P. Targeting energy metabolism in brain cancer: Review and hypothesis. Nutr. Metab. 2005, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Trujillo, A.; Ma, X.; Beierle, E.A. Ketone bodies inhibit the viability of human neuroblastoma cells. J. Pediatric Surg. 2009, 44, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Seymour, K.J.; Bluml, S.; Sutherling, J.; Sutherling, W.; Ross, B.D. Identification of cerebral acetone by 1H-MRS in patients with epilepsy controlled by ketogenic diet. MAGMA 1999, 8, 33–42. [Google Scholar] [CrossRef]
- Berrington, A.; Schreck, K.C.; Barron, B.J.; Blair, L.; Lin, D.D.M.; Hartman, A.L.; Kossoff, E.; Easter, L.; Whitlow, C.T.; Jung, Y.; et al. Cerebral Ketones Detected by 3T MR Spectroscopy in Patients with High-Grade Glioma on an Atkins-Based Diet. AJNR 2019, 40, 1908–1915. [Google Scholar] [CrossRef]
- Artzi, M.; Liberman, G.; Vaisman, N.; Bokstein, F.; Vitinshtein, F.; Aizenstein, O.; Ben Bashat, D. Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: 1H-MRS study. J. Neuro Oncol. 2017, 132, 267–275. [Google Scholar] [CrossRef]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Thio, L.L.; Erbayat-Altay, E.; Rensing, N.; Yamada, K.A. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr. Res. 2006, 60, 413–417. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Rao, J.U.; Madden-Hennessey, K.; Ip, K.L.; Hyder, F.; Drewes, L.R.; Geschwind, J.-F.; de Graaf, R.A.; Rothman, D.L. A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro-Oncology 2016, 18, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P. Antiangiogenic and Proapoptotic Effects of Dietary Restriction on Experimental Mouse and Human Brain Tumors. Clin. Cancer Res. 2004, 10, 5622–5629. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Sanderson, T.M.; El-Abbadi, M.M.; McGowan, R.; Mukherjee, P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br. J. Cancer 2003, 89, 1375–1382. [Google Scholar] [CrossRef]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef]
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220. [Google Scholar] [CrossRef]
- Di Biase, S.; Shim, H.S.; Kim, K.H.; Vinciguerra, M.; Rappa, F.; Wei, M.; Brandhorst, S.; Cappello, F.; Mirzaei, H.; Lee, C.; et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 2017, 15, e2001951. [Google Scholar] [CrossRef]
- Lee, C.; Safdie, F.M.; Raffaghello, L.; Wei, M.; Madia, F.; Parrella, E.; Hwang, D.; Cohen, P.; Bianchi, G.; Longo, V.D. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010, 70, 1564–1572. [Google Scholar] [CrossRef]
- Brandhorst, S.; Wei, M.; Hwang, S.; Morgan, T.E.; Longo, V.D. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp. Gerontol. 2013, 48, 1120–1128. [Google Scholar] [CrossRef]
- Moreira, J.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Pérès, S.; Paulevé, L.; Nogueira, M.; Steyaert, J.-M.; Schwartz, L. The Redox Status of Cancer Cells Supports Mechanisms behind the Warburg Effect. Metabolites 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E.A. Tumor Acidity as Evolutionary Spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Radda, G.K. Handbook of Magnetic Resonance Spectroscopy in Vivo: MRS Theory, Practice and Applications; Bottomley, P.A., Griffiths, J.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-99766-6. [Google Scholar]
- Meidenbauer, J.J.; Mukherjee, P.; Seyfried, T.N. The glucose ketone index calculator: A simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr. Metab. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Wagner, M.; von Mettenheim, N.; Harter, P.N.; Wenger, K.J.; Franz, K.; Bojunga, J.; Vetter, M.; Gerlach, R.; Glatzel, M.; et al. ERGO2: A prospective randomized trial of calorie restricted ketogenic diet and fasting in addition to re-irradiation for malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 987–995. [Google Scholar] [CrossRef]
- Pan, J.W.; Rothman, T.L.; Behar, K.L.; Stein, D.T.; Hetherington, H.P. Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J. Cereb. Blood Flow Metab. 2000, 20, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Cecil, K.M.; Mulkey, S.B.; Ou, X.; Glasier, C.M. Brain ketones detected by proton magnetic resonance spectroscopy in an infant with Ohtahara syndrome treated with ketogenic diet. Pediatr. Radiol. 2015, 45, 133–137. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family-Role and regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef]
- Leino, R.L.; Gerhart, D.Z.; Duelli, R.; Enerson, B.E.; Drewes, L.R. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 2001, 38, 519–527. [Google Scholar] [CrossRef]
- Pifferi, F.; Tremblay, S.; Croteau, E.; Fortier, M.; Tremblay-Mercier, J.; Lecomte, R.; Cunnane, S.C. Mild experimental ketosis increases brain uptake of 11 C-acetoacetate and 18 F-fluorodeoxyglucose: A dual-tracer PET imaging study in rats. Nutr. Neurosci. 2011, 14, 51–58. [Google Scholar] [CrossRef]
- Musa-Veloso, K. Non-invasive detection of ketosis and its application in refractory epilepsy. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sulway, M.J.; Malins, J.M. Acetone in diabetic ketoacidosis. Lancet 1970, 2, 736–740. [Google Scholar] [CrossRef]
- Likhodii, S.S.; Burnham, W.M. Ketogenic diet: Does acetone stop seizures? Med. Sci. Monit. 2002, 8, HY19-24. [Google Scholar] [PubMed]
- Boros, L.G.; Collins, T.Q.; Somlyai, G. What to eat or what not to eat-that is still the question. Neuro-Oncology 2017, 19, 595–596. [Google Scholar] [CrossRef]
- De Feyter, H.M.; Behar, K.L.; de Graaf, R.A.; Rothman, D.L. “What to eat or what not to eat-that is still the question”—Reply. Neuro-Oncology 2017, 19, 596–597. [Google Scholar] [CrossRef][Green Version]
- Rieger, J.; Steinbach, J.P. To diet or not to diet—that is still the question. Neuro-Oncology 2016, 18, 1035–1036. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Counillon, L.; Bouret, Y.; Marchiq, I.; Pouysségur, J. Na + /H + antiporter (NHE1) and lactate/H + symporters (MCTs) in pH homeostasis and cancer metabolism. BBA Mol. Cell Res. 2016, 1863, 2465–2480. [Google Scholar] [CrossRef]
- García-Cañaveras, J.C.; Chen, L.; Rabinowitz, J.D. The Tumor Metabolic Microenvironment: Lessons from Lactate. Cancer Res. 2019, 79, 3155–3162. [Google Scholar] [CrossRef]
- Voronina, P.P.; Adamovich, K.V.; Adamovich, T.V.; Dubouskaya, T.G.; Hrynevich, S.V.; Waseem, T.V.; Fedorovich, S.V. High Concentration of Ketone Body β-Hydroxybutyrate Modifies Synaptic Vesicle Cycle and Depolarizes Plasma Membrane of Rat Brain Synaptosomes. J. Mol. Neurosci. 2020, 70, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wenger, K.J.; Steinbach, J.P.; Bähr, O.; Pilatus, U.; Hattingen, E. Lower Lactate Levels and Lower Intracellular pH in Patients with IDH -Mutant versus Wild-Type Gliomas. AJNR 2020, 41, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Wenger, K.J.; Hattingen, E.; Franz, K.; Steinbach, J.P.; Bähr, O.; Pilatus, U. Intracellular pH measured by 31 P-MR-spectroscopy might predict site of progression in recurrent glioblastoma under antiangiogenic therapy. J. Magn. Reson. Imaging 2017, 46, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Henke, G.; Paulsen, F.; Steinbach, J.P.; Ganswindt, U.; Isijanov, H.; Kortmann, R.-D.; Bamberg, M.; Belka, C. Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol. 2009, 185, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Klose, U. (Ed.) Proceedings of Data Acquisition and Evaluation Methods in Proton MR Spectroscopy 2011; Shaker Verlag: Herzogenrath, Germany, 2012; ISBN 978-3-8440-1116-6. [Google Scholar]
- Kreis, R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn. Reson. Med. 2016, 75, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Naressi, A.; Couturier, C.; Castang, I.; de Beer, R.; Graveron-Demilly, D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput. Biol. Med. 2001, 31, 269–286. [Google Scholar] [CrossRef]
- Vanhamme, L.; van den Boogaart, A.; Van Huffel, S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 1997, 129, 35–43. [Google Scholar] [CrossRef]
- Raghunand, N.; Howison, C.; Sherry, A.D.; Zhang, S.; Gillies, R.J. Renal and systemic pH imaging by contrast-enhanced MRI. Magn. Reson. Med. 2003, 49, 249–257. [Google Scholar] [CrossRef]
- Hattingen, E.; Bähr, O.; Rieger, J.; Blasel, S.; Steinbach, J.; Pilatus, U. Phospholipid Metabolites in Recurrent Glioblastoma: In Vivo Markers Detect Different Tumor Phenotypes before and under Antiangiogenic Therapy. PLoS ONE 2013, 8, e56439. [Google Scholar] [CrossRef]
- Petroff, O.A.C.; Prichard, J.W.; Behar, K.L.; Rothman, D.L.; Alger, J.R.; Shulman, R.G. Cerebral metabolism in hyper- and hypocarbia: 31P and 1H nuclear magnetic resonance studies. Neurology 1985, 35, 1681. [Google Scholar] [CrossRef]
- Starcuk, Z.; Strbak, O.; Starcukova, J.; Graveron-Demilly, D. Simulation of steady state free precession acquisition mode in coupled spin systems for fast MR spectroscopic imaging. In Proceedings of the 2008 IEEE International Workshop on Imaging Systems and Techniques, Crete, Greece, 10–12 September 2008; IEEE: New York, NY, USA, 2008; pp. 302–306. [Google Scholar]
- Tofts, P.S.; Waldman, A.D. Spectroscopy:1H Metabolite Concentrations. In Quantitative MRI of the Brain; Tofts, P., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2003; pp. 299–339. ISBN 978-0-470-84721-3. [Google Scholar]
- Wenger, K.J.; Hattingen, E.; Harter, P.N.; Richter, C.; Franz, K.; Steinbach, J.P.; Bähr, O.; Pilatus, U. Fitting algorithms and baseline correction influence the results of non-invasive in vivo quantitation of 2-hydroxyglutarate with 1 H-MRS. NMR Biomed. 2019, 32, e4027. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, T.; Merboldt, K.D.; Bruhn, H.; Hänicke, W.; Frahm, J. Absolute concentrations of metabolites in the adult human brain in vivo: Quantification of localized proton MR spectra. Radiology 1993, 187, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.K.; Banerjee, A.; Patel, A.M.; Zhao, Y.D.; Dimitrov, I.E.; Browning, J.D.; Sherwood Brown, E.; Maher, E.A.; Choi, C. T2 measurement of J-coupled metabolites in the human brain at 3T: T2 OF J-COUPLED METABOLITES AT 3T. NMR Biomed. 2012, 25, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hattingen, E.; Pilatus, U.; Franz, K.; Zanella, F.E.; Lanfermann, H. Evaluation of optimal echo time for1H-spectroscopic imaging of brain tumors at 3 Tesla. J. Magn. Reson. Imaging 2007, 26, 427–431. [Google Scholar] [CrossRef]



| Characteristics | All Patients (n = 19) | Group B (n = 8) |
|---|---|---|
| Group A (n = 11) | ||
| General | ||
| Age, median | 53 (range 38–64) | 42.5 (range 25–61) |
| Age, mean | 53 (SD = 7) | 41 (SD = 14) |
| Karnofsky index, mean rounded to nearest tenth (SD) | 90 (SD = 13) | 90 (SD = 6) |
| Histology | ||
| Glioblastoma, IDH wildtype, WHO IV (n) | 5 | 3 |
| Glioblastoma IDH-mutant, WHO IV (n) | 1 | 1 |
| Glioblastoma IDH-mutant, IDH unknown, WHO IV (n) | 4 | 1 |
| Anaplastic astrocytoma, IDH wildtype, WHO III (n) | 1 | 0 |
| Oligodendroglioma, IDH-mutant and 1p/19q co-deleted, WHO II (n) | 0 | 1 |
| Pleomorphic Xanthoastrocytoma, IDH wildtype (n) | 0 | 1 |
| Undifferentiated neuroepithelial tumor, NOS, WHO IV (n) | 0 | 1 |
| MGMT promotor methylation status | ||
| Not methylated | 46% (n = 5) | 63% (n = 5) |
| Methylated | 46% (n = 5) | 25% (n = 2) |
| Not known | 9% (n = 1) | 13% (n = 1) |
| Prior treatment | ||
| Radiation therapy up to 60 Gy | 100% (n = 11) | 100% (n = 8) |
| Concomitant TMZ | 100% (n = 11) | 88% (n = 7) |
| Median of adjuvant cycles of TMZ | 6 | 3 |
| Prior treatment with Bevacizumab | 18% (n = 2) | 13% (n = 1) |
| Resection prior to study treatment | 9% (n = 1) | 50% (n = 4) |
| Time interval first and second irradiation (months) | ||
| Median | 9 | 11 |
| Mean | 14 (SD = 9) | 17 (SD = 16) |
| Patient ID | Group | Drop out during 9 Days of crKD-IF | Histology at First Diagnosis | Glucose Baseline (mg/dL) | Glucose Day 6 (mg/dL) | Blood Ketone Levels Day 6 (mmol/L) | Urine Ketosis Day 6 (mmol/L) | Acn Signal Quantitation (mmol/L) |
|---|---|---|---|---|---|---|---|---|
| 26 | A | yes | Glioblastoma | 78.0 | 59.0 | 0.1 | 0 | tumor tissue 0.12; NAWM 0.14 |
| 27 | A | yes | Glioblastoma | 84.0 | 98.0 | N/A | N/A | NAWM 0.28 |
| 34 | A | no | Anaplastic astrocytoma | 112.0 | 78.0 | 0.4 | 1.5 | NAWM 0.21 |
| 38 | A | no | Glioblastoma | 81.0 | 54.0 | 4.5 | N/A | NAWM 0.25 |
| Sequence | Slice Thickness | TR; Excitation Flip Angle | TE | Matrix Size; in Plane Resolution | Vector Size; Bandwidth | Scan Time |
|---|---|---|---|---|---|---|
| 3D FID 31P CSI | 25 mm * | 2000 ms; 60° | 2.3 *** ms | 8 × 8 × 8 at 240 × 240 × 200 mm3 FOV interpolated to 16 × 16 × 16; 30 × 30 mm2 * | 1024; 2000 Hz | 10:44 m |
| 2D 1H PRESS sequence | 12.5 mm ** | 1500 ms; 90° | 30 ms | 16 × 16 at 240 × 240, interpolated to 32 × 32; 15 × 15 mm2 * | 1024; 2000 Hz | 4:45 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenger, K.J.; Wagner, M.; Harter, P.N.; Franz, K.; Bojunga, J.; Fokas, E.; Imhoff, D.; Rödel, C.; Rieger, J.; Hattingen, E.; et al. Maintenance of Energy Homeostasis during Calorically Restricted Ketogenic Diet and Fasting-MR-Spectroscopic Insights from the ERGO2 Trial. Cancers 2020, 12, 3549. https://doi.org/10.3390/cancers12123549
Wenger KJ, Wagner M, Harter PN, Franz K, Bojunga J, Fokas E, Imhoff D, Rödel C, Rieger J, Hattingen E, et al. Maintenance of Energy Homeostasis during Calorically Restricted Ketogenic Diet and Fasting-MR-Spectroscopic Insights from the ERGO2 Trial. Cancers. 2020; 12(12):3549. https://doi.org/10.3390/cancers12123549
Chicago/Turabian StyleWenger, Katharina J., Marlies Wagner, Patrick N. Harter, Kea Franz, Jörg Bojunga, Emmanouil Fokas, Detlef Imhoff, Claus Rödel, Johannes Rieger, Elke Hattingen, and et al. 2020. "Maintenance of Energy Homeostasis during Calorically Restricted Ketogenic Diet and Fasting-MR-Spectroscopic Insights from the ERGO2 Trial" Cancers 12, no. 12: 3549. https://doi.org/10.3390/cancers12123549
APA StyleWenger, K. J., Wagner, M., Harter, P. N., Franz, K., Bojunga, J., Fokas, E., Imhoff, D., Rödel, C., Rieger, J., Hattingen, E., Steinbach, J. P., Pilatus, U., & Voss, M. (2020). Maintenance of Energy Homeostasis during Calorically Restricted Ketogenic Diet and Fasting-MR-Spectroscopic Insights from the ERGO2 Trial. Cancers, 12(12), 3549. https://doi.org/10.3390/cancers12123549






