Evidences of a Direct Relationship between Cellular Fuel Supply and Ciliogenesis Regulated by Hypoxic VDAC1-ΔC
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Pharmacological Inhibitors and Chemicals
2.1.2. RNA Interference
2.1.3. Mutations and Stable Transfections
2.1.4. Colony-Forming Assay
2.1.5. Respirometry and Extracellular Acidification
2.1.6. Phenotype MicroArray on Omnilog™ Analyser
2.1.7. ATP Determination
2.1.8. Lactate Measurement
2.1.9. FACS Analysis
2.1.10. Microtubule Assay
2.1.11. Electron Microscopy
2.2. Immunoblotting
Co-Immunoprecipitation Assay
2.3. Immunocytochemistry
2.3.1. Microarray Experiments
2.3.2. Statistics
3. Results
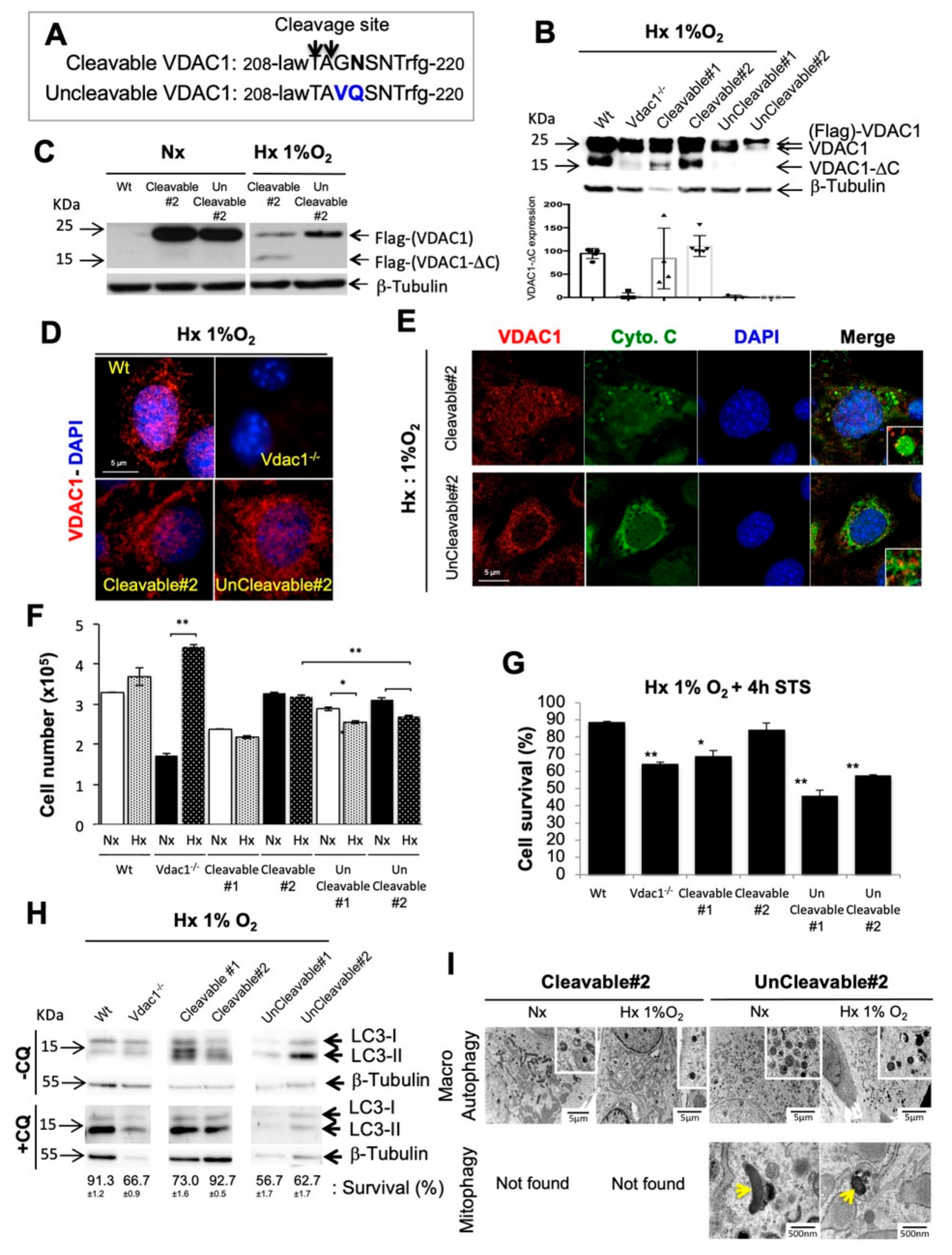
3.1. Genetic Proof of Concept in Cellular Model
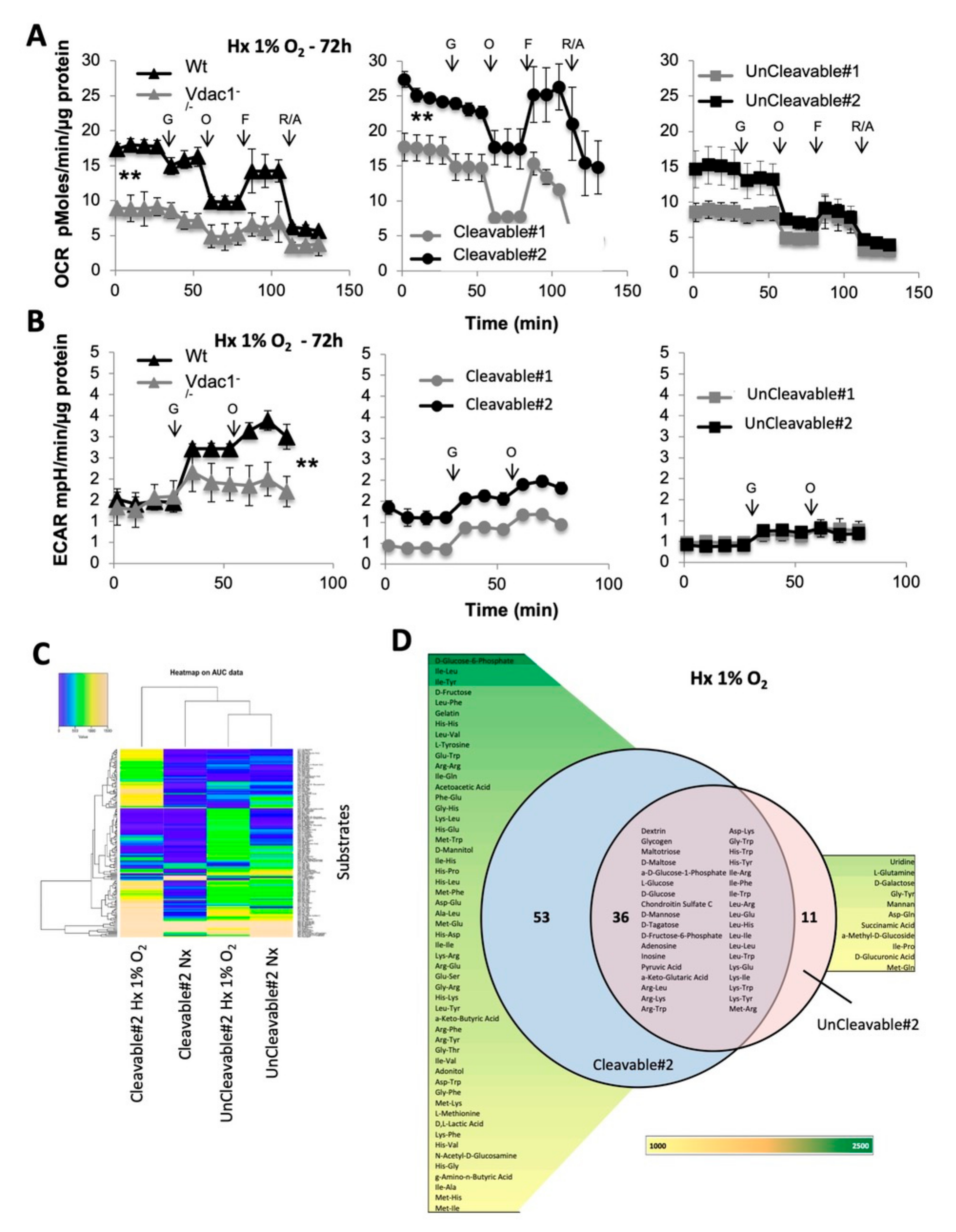
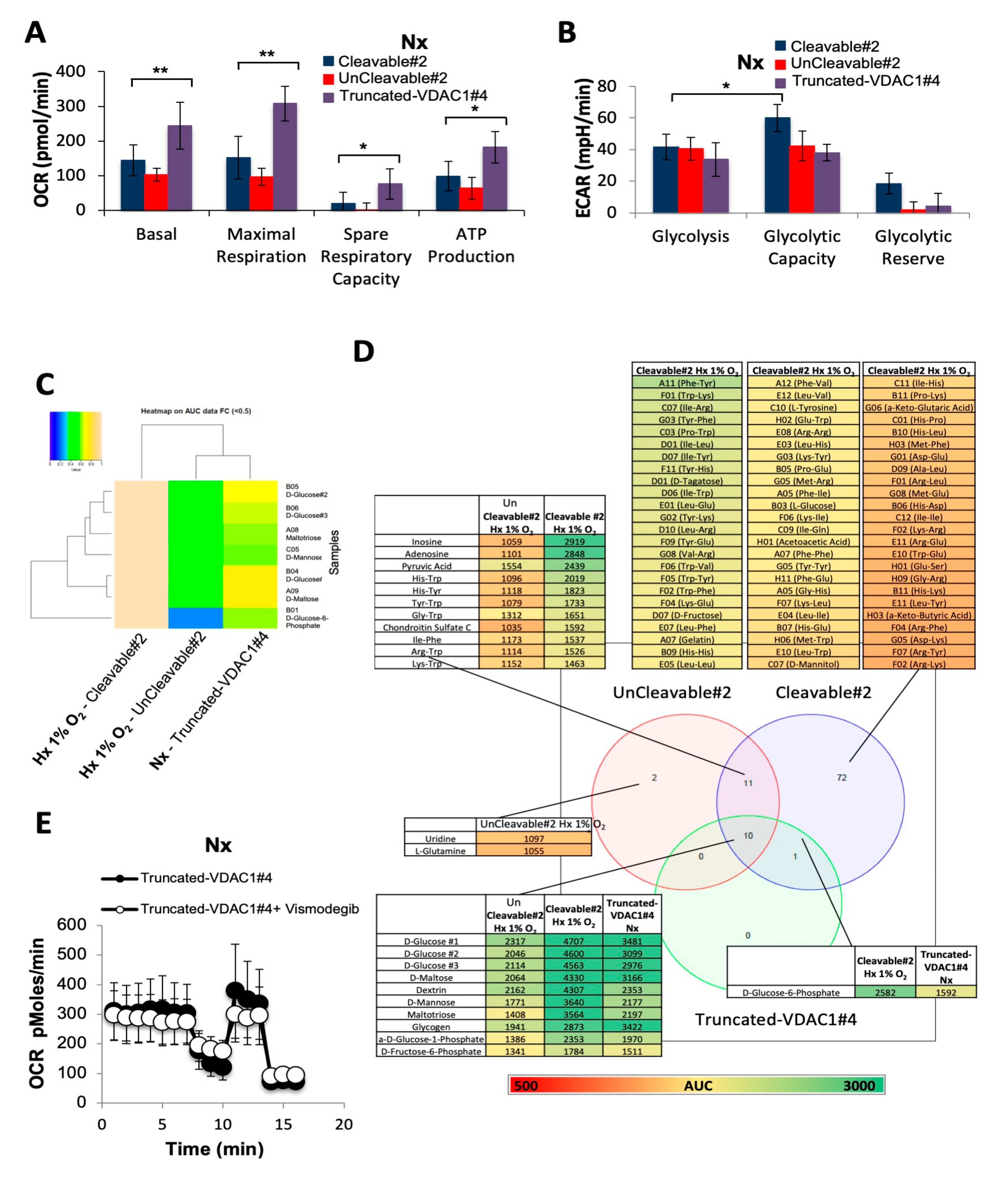
3.2. Metabolic Adaptability is Controlled by VDAC1-ΔC
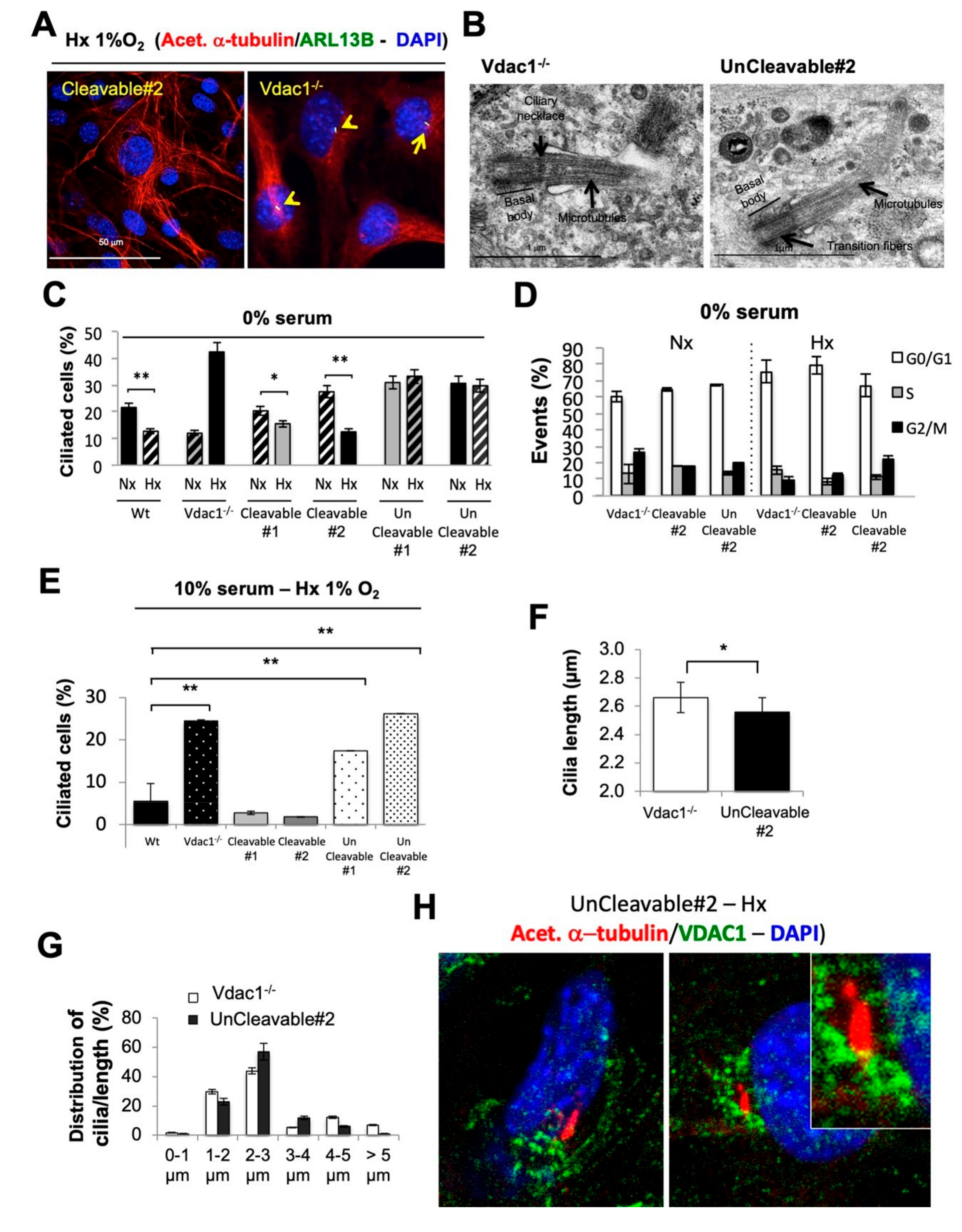
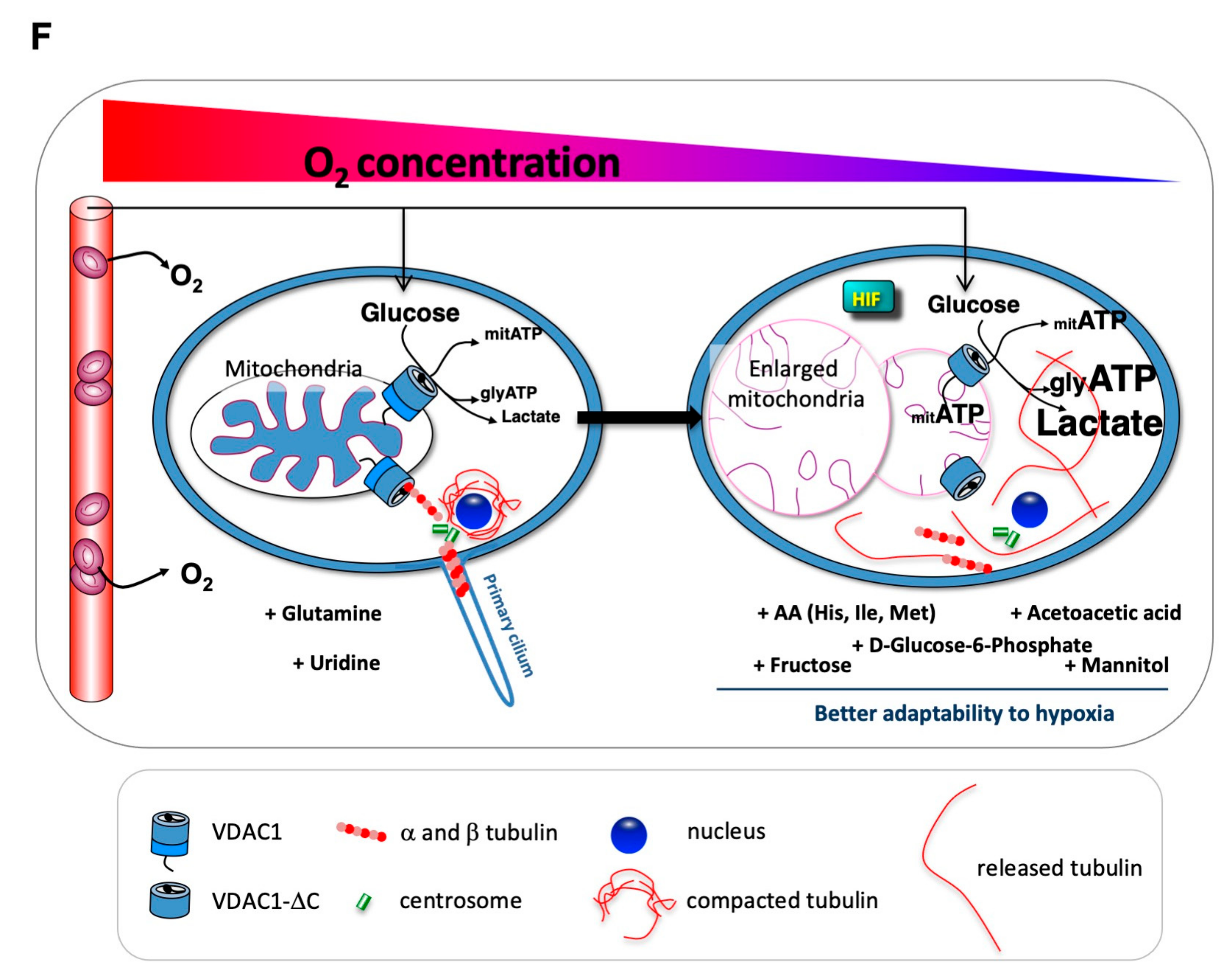
3.3. Mitochondrial VDAC1-ΔC Represses Ciliogenesis
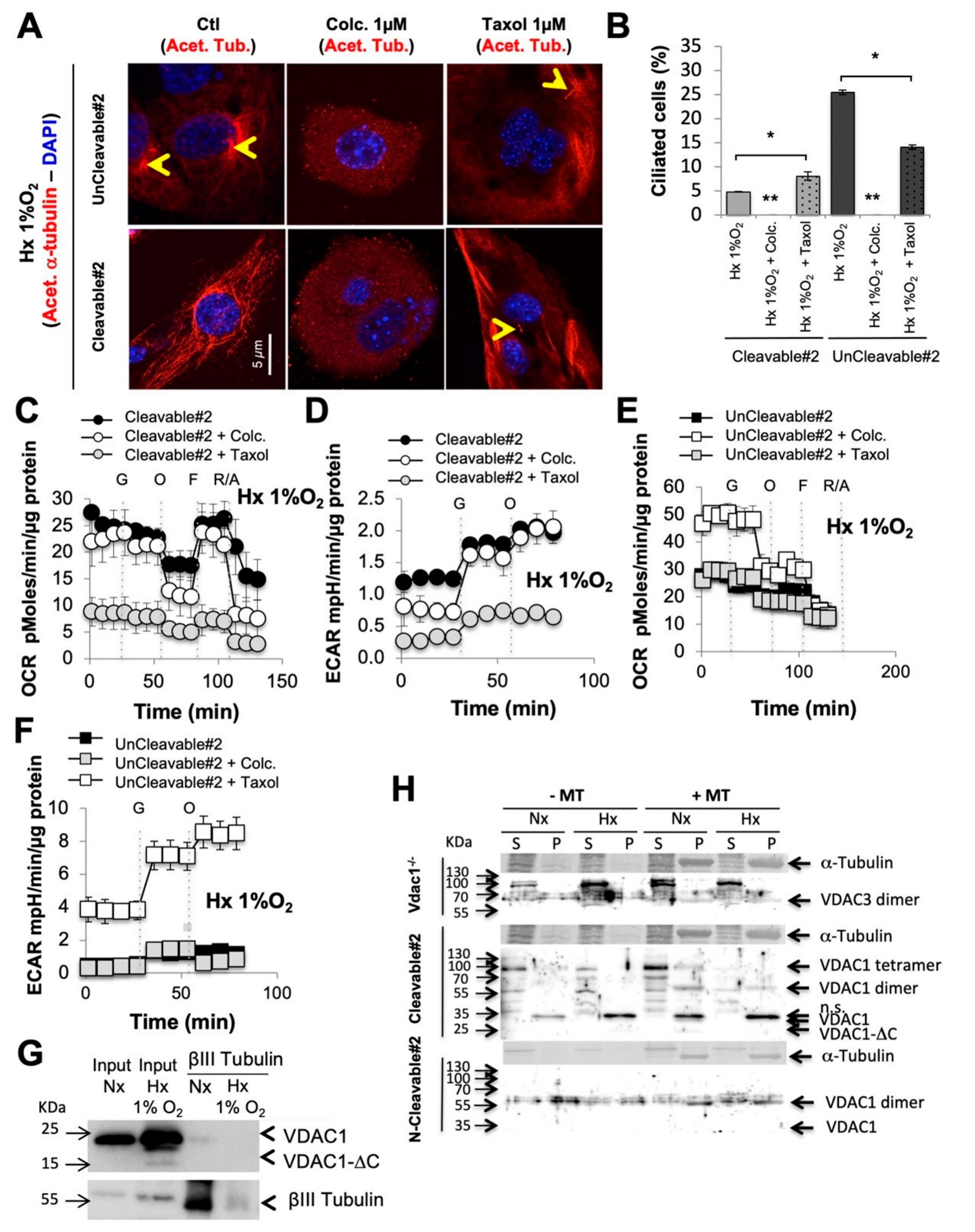
3.4. When VDAC1 Meets Tubulin to Control Primary Cilium and Metabolism
3.5. Direct Impact of VDAC1-ΔC on Cell Survival
3.6. Direct Impact of VDAC1-ΔC on Cell Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frezza, C. Metabolism and cancer: The future is now. Br. J. Cancer 2019, 122, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Mizrachi, D.; Mizrachi, D. VDAC1: From structure to cancer therapy. Front. Oncol. 2012, 2, 164. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Kmita, H.; Lemasters, J.J. Editorial: Uncovering the function of the mitochondrial protein vdac in health and disease: From structure-function to novel therapeutic strategies. Front. Oncol. 2017, 7, 320. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Dmitriev, A.A.; Krasnov, G.S.; Lakunina, V.A. Targeting VDAC-bound hexokinase II: A promising approach for concomitant anti-cancer therapy. Expert Opin. Ther. Targets 2013, 17, 1221–1233. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Weng, C.; Chen, R.; Zheng, Y.; Chen, Q.; Tang, H. Identification of the protein–protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2003, 305, 989–996. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Ben-Hail, D.; Ilie, M.; Gounon, P.; Rouleau, M.; Hofman, V.; Doyen, J.; Mari, B.; Shoshan-Barmatz, V.; Hofman, P.; et al. Expression of a truncated active form of vdac1 in lung cancer associates with hypoxic cell survival and correlates with progression to chemotherapy resistance. Cancer Res. 2012, 72, 2140–2150. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Lacas-Gervais, S.; Adaixo, R.; Ilc, K.; Rouleau, M.; Notte, A.; Dieu, M.; Michiels, C.; Voeltzel, T.; Maguer-Satta, V.; et al. Local Mitochondrial-Endolysosomal Microfusion Cleaves Voltage-Dependent Anion Channel 1 To Promote Survival in Hypoxia. Mol. Cell. Biol. 2015, 35, 1491–1505. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Giuliano, S.; Saland, E.; Lacas-Gervais, S.; Sheiko, T.; Pelletier, J.; Bourget, I.; Bost, F.; Féral, C.C.; Boulter, E.; et al. Knockout of Vdac1 activates hypoxia-inducible factor through reactive oxygen species generation and induces tumor growth by promoting metabolic reprogramming and inflammation. Cancer Metab. 2015, 3, 8. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Bezrukov, S.M. VDAC inhibition by tubulin and its physiological implications. Biochim. Biophys. Acta BBA Biomembr. 2012, 1818, 1526–1535. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Hoogerheide, D.P.; Rovini, A.; Sirajuddin, M.; Bezrukov, S.M. Sequence diversity of tubulin isotypes in regulation of the mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2018, 293, 10949–10962. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.N. VDAC–Tubulin, an Anti-Warburg Pro-Oxidant Switch. Front. Oncol. 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.; Dufies, M.; Lacas-Gervais, S.; Gardie, B.; Gad-Lapiteau, S.; Parola, J.; Nottet, N.; De Padua, M.M.C.; Contenti, J.; Borchiellini, D.; et al. Identification of a new aggressive axis driven by ciliogenesis and absence of VDAC1-ΔC in clear cell Renal Cell Carcinoma patients. Theranostics 2020, 10, 2696–2713. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Slabodnick, M.; Pike, A.; Marquardt, J.; Fisk, H.A. VDAC3 regulates centriole assembly by targeting Mps1 to centrosomes. Cell Cycle 2012, 11, 3666–3678. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Cash, A.; Fisk, H.A. Non-Overlapping distributions and functions of the vdac family in ciliogenesis. Cells 2015, 4, 331–353. [Google Scholar] [CrossRef] [PubMed]
- Kozjak-Pavlovic, V.; Ross, K.; Götz, M.; Goosmann, C.; Rudel, T. A Tag at the Carboxy Terminus Prevents Membrane Integration of VDAC1 in Mammalian Mitochondria. J. Mol. Biol. 2010, 397, 219–232. [Google Scholar] [CrossRef]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouysségur, J.; Mazure, N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef]
- Bilton, R.; Mazure, N.; Trottier, E.; Hattab, M.; Déry, M.-A.; Richard, D.E.; Pouysségur, J.; Brahimi-Horn, M.C. Arrest-defective-1 protein, an acetyltransferase, does not alter stability of hypoxia-inducible Factor (HIF)-1α and Is Not Induced by Hypoxia or HIF. J. Biol. Chem. 2005, 280, 31132–31140. [Google Scholar] [CrossRef]
- Sun, Y.; Vashisht, A.A.; Tchieu, J.; Wohlschlegel, J.A.; Dreier, L. Voltage-dependent anion channels (vdacs) recruit parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 2012, 287, 40652–40660. [Google Scholar] [CrossRef]
- Colombini, M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann. N. Y. Acad. Sci. 1980, 341, 552–563. [Google Scholar] [CrossRef]
- De Pinto, V.; Tomasello, M.F.; Messina, A.; Guarino, F.; Benz, R.; La Mendola, D.; Magrì, A.; Milardi, D.; Pappalardo, G. Determination of the Conformation of the Human VDAC1 N-Terminal Peptide, a Protein Moiety Essential for the Functional Properties of the Pore. Chem. Bio Chem. 2007, 8, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Holmuhamedov, E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—Thinking outside the box. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.N.; Sheldon, K.L.; Dehart, D.N.; Patnaik, J.; Manevich, Y.; Townsend, D.M.; Bezrukov, S.M.; Rostovtseva, T.K.; Lemasters, J.J. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells. J. Biol. Chem. 2013, 288, 11920–11929. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Hoek, J.B. Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 2008, 40, 171–182. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De, S.; Meir, A. The mitochondrial voltage-dependent anion channel 1, ca2+ transport, apoptosis, and their regulation. Front. Oncol. 2017, 7, 60. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Krelin, Y.; Shteinfer-Kuzmine, A.; Arif, T. Voltage-Dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics. Front. Oncol. 2017, 7, 154. [Google Scholar] [CrossRef]
- Heiden, M.G.; Chandel, N.S.; Schumacker, P.T.; Thompson, C.B. Bcl-xL Prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 1999, 3, 159–167. [Google Scholar] [CrossRef]
- Wenner, C.E. Targeting mitochondria as a therapeutic target in cancer. J. Cell. Physiol. 2011, 227, 450–456. [Google Scholar] [CrossRef]
- Zalk, R.; Israelson, A.; Garty, E.S.; Azoulay-Zohar, H.; Shoshan-Barmatz, V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J. 2005, 386, 73–83. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Chen, M.-Y.; Bezrukov, S.M.; Giri, P.K.; Jing-Song, F.; Shanmugam, M.K.; Ding, J.L.; Sethi, G.; Swaminathan, K.; et al. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2012, 287, 29589–29598. [Google Scholar] [CrossRef] [PubMed]
- Puurand, M.; Tepp, K.; Timohhina, N.; Aid, J.; Shevchuk, I.; Chekulayev, V.; Kaamber, T. Tubulin betaII and betaIII isoforms as the regulators of VDAC channel permeability in health and disease. Cells 2019, 8, 239. [Google Scholar] [CrossRef]
- Ferecatu, I.; Canal, F.; Fabbri, L.; Mazure, N.M.; Bouton, C.; Golinelli-Cohen, M.-P. Dysfunction in the mitochondrial Fe-S assembly machinery leads to formation of the chemoresistant truncated VDAC1 isoform without HIF-1α activation. PLoS ONE 2018, 13, e0194782. [Google Scholar] [CrossRef] [PubMed]
- Noskov, S.Y.; Rostovtseva, T.K.; Bezrukov, S.M. ATP transport through vdac and the vdac–tubulin complex probed by equilibrium and nonequilibrium md simulations. Biochemistry 2013, 52, 9246–9256. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Keinan, N.; Abu-Hamad, S.; Tyomkin, D.; Aram, L. Apoptosis is regulated by the VDAC1 N-terminal region and by VDAC oligomerization: Release of cytochrome c, AIF and Smac/Diablo. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Song, D.K.; Choi, J.H.; Kim, M.-S. Primary Cilia as a Signaling Platform for Control of Energy Metabolism. Diabetes Metab. J. 2018, 42, 117–127. [Google Scholar] [CrossRef]
- Lee, J.; Yi, S.; Won, M.; Song, Y.S.; Yi, H.-S.; Park, Y.J.; Park, K.C.; Kim, J.T.; Chang, J.Y.; Lee, M.J.; et al. Loss-of-function of IFT88 determines metabolic phenotypes in thyroid cancer. Oncogene 2018, 37, 4455–4474. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyenberg Cunha-de Padua, M.; Fabbri, L.; Dufies, M.; Lacas-Gervais, S.; Contenti, J.; Voyton, C.; Fazio, S.; Irondelle, M.; Mograbi, B.; Rouleau, M.; et al. Evidences of a Direct Relationship between Cellular Fuel Supply and Ciliogenesis Regulated by Hypoxic VDAC1-ΔC. Cancers 2020, 12, 3484. https://doi.org/10.3390/cancers12113484
Meyenberg Cunha-de Padua M, Fabbri L, Dufies M, Lacas-Gervais S, Contenti J, Voyton C, Fazio S, Irondelle M, Mograbi B, Rouleau M, et al. Evidences of a Direct Relationship between Cellular Fuel Supply and Ciliogenesis Regulated by Hypoxic VDAC1-ΔC. Cancers. 2020; 12(11):3484. https://doi.org/10.3390/cancers12113484
Chicago/Turabian StyleMeyenberg Cunha-de Padua, Monique, Lucilla Fabbri, Maeva Dufies, Sandra Lacas-Gervais, Julie Contenti, Charles Voyton, Sofia Fazio, Marie Irondelle, Baharia Mograbi, Matthieu Rouleau, and et al. 2020. "Evidences of a Direct Relationship between Cellular Fuel Supply and Ciliogenesis Regulated by Hypoxic VDAC1-ΔC" Cancers 12, no. 11: 3484. https://doi.org/10.3390/cancers12113484
APA StyleMeyenberg Cunha-de Padua, M., Fabbri, L., Dufies, M., Lacas-Gervais, S., Contenti, J., Voyton, C., Fazio, S., Irondelle, M., Mograbi, B., Rouleau, M., Sadaghianloo, N., Rovini, A., Brenner, C., Craigen, W. J., Bourgeais, J., Herault, O., Bost, F., & Mazure, N. M. (2020). Evidences of a Direct Relationship between Cellular Fuel Supply and Ciliogenesis Regulated by Hypoxic VDAC1-ΔC. Cancers, 12(11), 3484. https://doi.org/10.3390/cancers12113484





