Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer?
Abstract
Simple Summary
Abstract
1. Introduction
2. Main Features of ILC2s
3. Enhancing and Regulatory Function of ILC2s
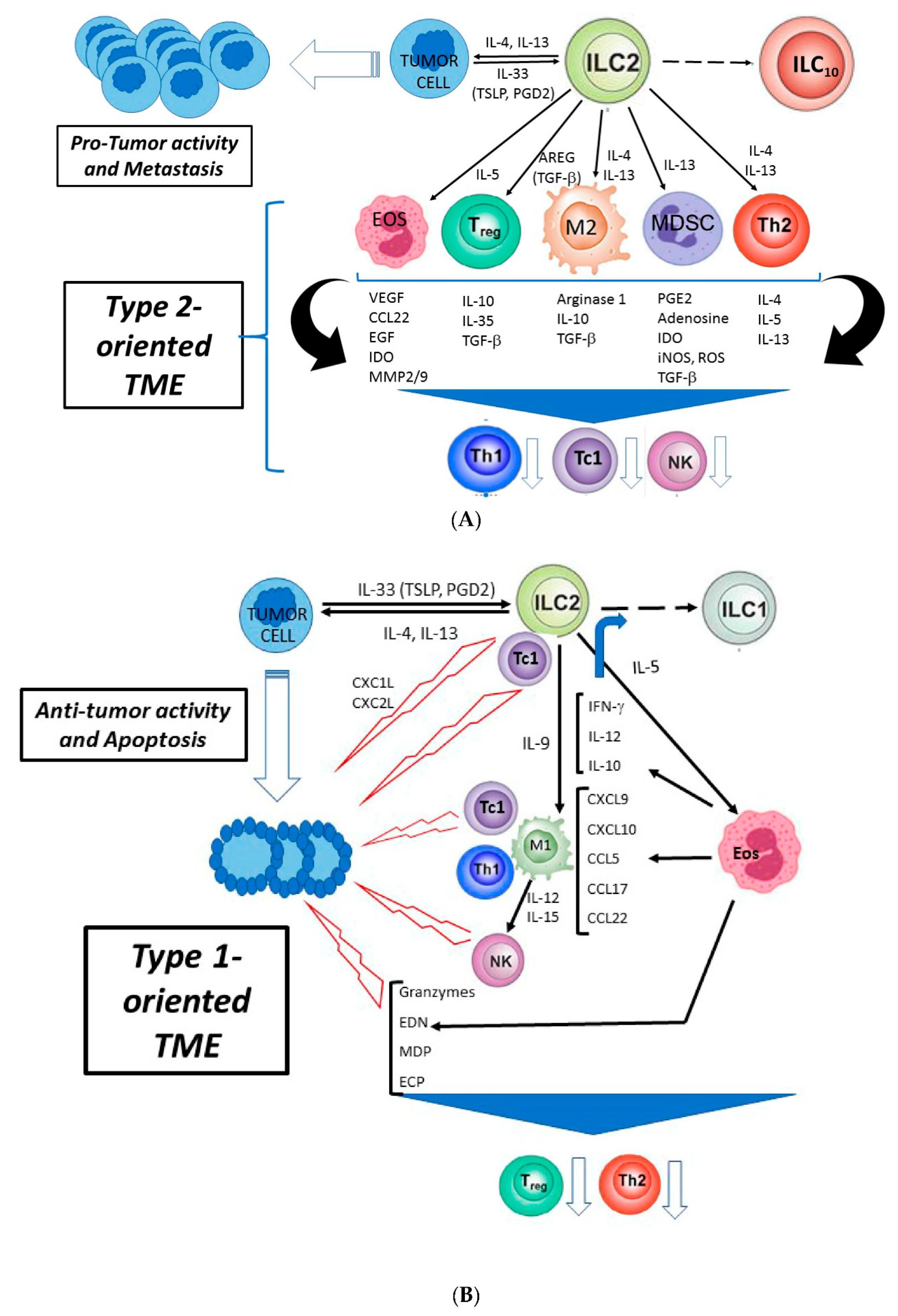
4. Pro- and Antitumor Activity of ILC2s
4.1. Protumor Activity
4.2. Antitumor Activity
5. ILC2s as Therapeutic Targets in Tumors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated C-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nat. Cell Biol. 2010, 464, 1367–1370. [Google Scholar] [CrossRef]
- Price, A.E.; Liang, H.-E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 11489–11494. [Google Scholar] [CrossRef]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP Elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015, 21, 698–708. [Google Scholar] [CrossRef]
- Yazdani, R.; Sharifi, M.; Shirvan, A.S.; Azizi, G.; Ganjalikhani-Hakemi, M. Characteristics of innate lymphoid cells (ILCs) and their role in immunological disorders (an update). Cell. Immunol. 2015, 298, 66–76. [Google Scholar] [CrossRef]
- Klose, C.S.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef]
- Constantinides, M.G.; McDonald, B.D.; Verhoef, P.A.; Bendelac, A. A committed precursor to innate lymphoid cells. Nat. Cell Biol. 2014, 508, 397–401. [Google Scholar] [CrossRef]
- Scoville, D.S.; Mundy-Bosse, B.L.; Zhang, M.H.; Chen, L.; Zhang, X.; Keller, K.A.; Hughes, T.; Chen, L.; Cheng, S.; Bergin, S.M.; et al. A progenitor cell expressing transcription factor rorgammat generates all human innate lymphoid cell subsets. Immunity 2016, 44, 1140–1150. [Google Scholar] [CrossRef]
- Withers, D.R. Lymphoid tissue inducer cells. Curr. Biol. 2011, 21, R381–R382. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Hashimoto-Hill, S.; Kim, M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol. 2016, 37, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Voisin, B.; Kim, D.Y.; Kennedy, E.A.; Jo, J.-H.; Shih, H.-Y.; Truong, A.; Doebel, T.; Sakamoto, K.; Cui, C.-Y.; et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 2019, 176, 982–997.e16. [Google Scholar] [CrossRef]
- Wong, H.S.; Walker, J.A.; Jolin, H.E.; Drynan, L.F.; Hams, E.; Camelo, A.; Barlow, J.L.; Neill, D.R.; Panova, V.; Koch, U.; et al. Transcription factor Roralpha is critical for nuocyte development. Nat. Immunol. 2012, 13, 229–236. [Google Scholar] [CrossRef]
- Spooner, C.J.; Lesch, J.; Yan, N.; A Khan, A.; Abbas, A.; Ramirez-Carrozzi, V.; Zhou, M.; Soriano, R.; Eastham-Anderson, J.; Diehl, L.; et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat. Immunol. 2013, 14, 1229–1236. [Google Scholar] [CrossRef]
- Mjösberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; Velde, A.A.T.; Fokkens, W.; Van Drunen, C.M.; Spits, H. The Transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef]
- Yang, Q.; Monticelli, L.A.; Saenz, S.A.; Chi, A.W.-S.; Sonnenberg, G.F.; Tang, J.; De Obaldia, M.E.; Bailis, W.; Bryson, J.L.; Toscano, K.; et al. T Cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity 2013, 38, 694–704. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Clare, S.; Wang, J.; Lee, S.-C.; Brandt, C.; Burke, S.; Lu, L.; He, D.; Jenkins, N.A.; et al. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J. Exp. Med. 2015, 212, 865–874. [Google Scholar] [CrossRef]
- Walker, J.A.; Oliphant, C.J.; Englezakis, A.; Yu, Y.; Clare, S.; Rodewald, H.-R.; Belz, G.; Liu, P.; Fallon, P.G.; McKenzie, A.N. Bcl11b is essential for group 2 innate lymphoid cell development. J. Exp. Med. 2015, 212, 875–882. [Google Scholar] [CrossRef]
- Halim, T.Y.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Halim, T.Y.F. Group 2 innate lymphoid cells in disease. Int. Immunol. 2015, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hochdörfer, T.; Winkler, C.; Pardali, K.; Mjösberg, J. Expression of c-Kit discriminates between two functionally distinct subsets of human type 2 innate lymphoid cells. Eur. J. Immunol. 2019, 49, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Xue, L.; Jolin, H.; Hardman, C.; Cousins, D.J.; McKenzie, A.N.J.; Ogg, G.S. Group 2 innate lymphoid cells express functional NKp30 receptor inducing type 2 cytokine production. J. Immunol. 2016, 196, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Heesters, B.A.; Kradolfer, C.M.; Krabbendam, L.; Martinez-Gonzalez, I.; De Bruijn, M.J.; Golebski, K.; Hendriks, R.W.; Stadhouders, R.; Spits, H.; et al. Correction: KLRG1 and NKp46 discriminate subpopulations of human CD117+CRTH2− ILCs biased toward ILC2 or ILC3. J. Exp. Med. 2019, 216, 2221–2222. [Google Scholar] [CrossRef]
- Ito, M.; Maruyama, T.; Saito, N.; Koganei, S.; Yamamoto, K.; Matsumoto, N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit Nk cell cytotoxicity. J. Exp. Med. 2006, 203, 289–295. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, L.; Qiu, J.; Chen, X.; Hu-Li, J.; Siebenlist, U.; Williamson, P.R.; Urban, J.F., Jr.; Paul, W.E. Il-25-Responsive, Lineage-Negative Klrg1(Hi) Cells Are Multipotential ‘Inflammatory’ Type 2 Innate Lymphoid Cells. Nat. Immunol. 2015, 16, 161–169. [Google Scholar] [CrossRef]
- Flamar, A.-L.; Klose, C.S.; Moeller, J.B.; Mahlakõiv, T.; Bessman, N.J.; Zhang, W.; Moriyama, S.; Stokic-Trtica, V.; Rankin, L.C.; Putzel, G.G.; et al. Interleukin-33 Induces the Enzyme Tryptophan Hydroxylase 1 to Promote Inflammatory Group 2 Innate Lymphoid Cell-Mediated Immunity. Immunity 2020, 52, 606–619.e6. [Google Scholar] [CrossRef]
- Brestoff, R.J.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 Innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-W.; Odegaard, J.I.; Mukundan, L.; Qiu, Y.; Molofsky, A.B.; Nussbaum, J.C.; Yun, K.; Locksley, R.M.; Chawla, A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015, 160, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Roediger, B.; Kyle, R.; Yip, K.H.; Sumaria, N.; Guy, T.V.; Kim, B.S.; Mitchell, A.J.; Tay, S.S.; Jain, R.; Forbes-Blom, E.; et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 2013, 14, 564–573. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Mahlakõiv, T.; Moeller, J.B.; Rankin, L.C.; Flamar, A.-L.; Kabata, H.; Monticelli, L.A.; Moriyama, S.; Putzel, G.G.; Rakhilin, N.; et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nat. Cell Biol. 2017, 549, 282–286. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; Von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.-E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nat. Cell Biol. 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Maazi, H.; Patel, N.; Sankaranarayanan, I.; Suzuki, Y.; Rigas, D.; Soroosh, P.; Freeman, G.J.; Sharpe, A.H.; Akbari, O. ICOS:ICOS-Ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 2015, 42, 538–551. [Google Scholar] [CrossRef]
- Maggi, L.; Montaini, G.; Mazzoni, A.; Rossettini, B.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Eliotta, F.; Rossi, O.; Gallo, O.; et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 2017, 139, 964–976.e4. [Google Scholar] [CrossRef]
- Lei, A.-H.; Xiao, Q.; Liu, G.-Y.; Shi, K.; Yang, Q.; Li, X.; Liu, Y.-F.; Wang, H.-K.; Cai, W.-P.; Guan, Y.-J.; et al. ICAM-1 controls development and function of ILC2. J. Exp. Med. 2018, 215, 2157–2174. [Google Scholar] [CrossRef]
- Nagashima, H.; Mahlakõiv, T.; Shih, H.-Y.; Davis, F.P.; Meylan, F.; Huang, Y.; Harrison, O.J.; Yao, C.; Mikami, Y.; Urban, J.F.; et al. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity 2019, 51, 682–695.e6. [Google Scholar] [CrossRef]
- Wallrapp, A.; Burkett, P.R.; Riesenfeld, S.J.; Kim, S.-J.; Christian, E.; Abdulnour, R.-E.E.; Thakore, P.I.; Schnell, A.; Lambden, C.; Herbst, R.H.; et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 2019, 51, 709–723.e6. [Google Scholar] [CrossRef]
- Moriyama, S.; Brestoff, J.R.; Flamar, A.L.; Moeller, J.B.; Klose, C.S.N.; Rankin, L.C.; Yudanin, N.A.; Monticelli, L.A.; Putzel, G.G.; Rodewald, H.R.; et al. Beta2-Adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 2018, 359, 1056–1061. [Google Scholar] [CrossRef]
- Duerr, C.U.; A McCarthy, C.D.; Mindt, B.C.; Rubio, M.; Meli, A.P.; Pothlichet, J.; Eva, M.M.; Gauchat, J.-F.; Qureshi, S.T.; Mazer, B.D.; et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat. Immunol. 2015, 17, 65–75. [Google Scholar] [CrossRef]
- Gasteiger, G.; Hemmers, S.; Firth, M.A.; Le Floc’H, A.; Huse, M.; Sun, J.C.; Rudensky, A.Y. IL-2–dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J. Exp. Med. 2013, 210, 1167–1178. [Google Scholar] [CrossRef]
- Lambrecht, N.B.; Hammad, H.; Fahy, J.V. The cytokines of asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Maric, J.; Ravindran, A.; Mazzurana, L.; Van Acker, A.; Rao, A.; Kokkinou, E.; Ekoff, M.; Thomas, D.; Fauland, A.; Nilsson, G.; et al. Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation. J. Allergy Clin. Immunol. 2019, 143, 2202–2214.e5. [Google Scholar] [CrossRef]
- Ebihara, T. Dichotomous regulation of acquired immunity by innate lymphoid cells. Cells 2020, 9, 1193. [Google Scholar] [CrossRef]
- Lim, A.I.; Menegatti, S.; Bustamante, J.; Le Bourhis, L.; Allez, M.; Rogge, L.; Casanova, J.-L.; Yssel, H.; Di Santo, J.P. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med. 2016, 213, 569–583. [Google Scholar] [CrossRef]
- Silver, J.S.; Kearley, J.; Copenhaver, A.M.; Sanden, C.; Mori, M.; Yu, L.; Pritchard, G.H.; A Berlin, A.; A Hunter, C.; Bowler, R.; et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 2016, 17, 626–635. [Google Scholar] [CrossRef]
- Maggi, L.; Capone, M.; Mazzoni, A.; Liotta, F.; Cosmi, L.; Annunziato, F. Plasticity and regulatory mechanisms of human ILC2 functions. Immunol. Lett. 2020, 227, 109–116. [Google Scholar] [CrossRef]
- Krabbendam, L.; Bernink, J.H.; Spits, H. Innate lymphoid cells: from helper to killer. Curr. Opin. Immunol. 2021, 68, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, X.; Pasha, M.A.; Siebel, C.W.; Costello, A.; Haczku, A.; MacNamara, K.; Liang, T.; Zhu, J.; Bhandoola, A.; et al. Cutting edge: Notch signaling promotes the plasticity of group-2 innate lymphoid cells. J. Immunol. 2017, 198, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Ohne, Y.; Teunissen, M.B.M.; Wang, J.; Wu, J.; Krabbendam, L.; Guntermann, C.; Volckmann, R.; Koster, J.; Van Tol, S.; et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 2019, 20, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; MacLaren, A.; Romanish, M.T.; Gold, M.J.; McNagny, K.M.; Takei, F. Retinoic-Acid-Receptor-Related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity 2012, 37, 463–474. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.-C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Doherty, T.A.; Scott, D.L.; Walford, H.H.; Khorram, N.; Lund, S.; Baum, R.; Chang, J.; Rosenthal, P.; Beppu, A.; Miller, M.; et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J. Allergy Clin. Immunol. 2014, 133, 1203–1205.e7. [Google Scholar] [CrossRef]
- Brüggen, M.-C.; Bauer, W.M.; Reininger, B.; Clim, E.; Captarencu, C.; Steiner, G.E.; Brunner, P.M.; Meier, B.; French, L.E.; Stingl, G. In situ mapping of innate lymphoid cells in human skin: evidence for remarkable differences between normal and inflamed skin. J. Investig. Dermatol. 2016, 136, 2396–2405. [Google Scholar] [CrossRef]
- Oliphant, J.C.; Hwang, Y.Y.; Walker, J.A.; Salimi, M.; Wong, S.H.; Brewer, J.M.; Englezakis, A.; Barlow, J.L.; Hams, E.; Scanlon, S.T.; et al. Mhcii-mediated dialog between group 2 innate lymphoid cells and Cd4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41, 283–295. [Google Scholar] [CrossRef]
- Cosmi, L.; Maggi, L.; Mazzoni, A.; Liotta, F.; Annunziato, F. Biologicals targeting type 2 immunity: Lessons learned from asthma, chronic urticaria and atopic dermatitis. Eur. J. Immunol. 2019, 49, 1334–1343. [Google Scholar] [CrossRef]
- Cosmi, L.; Liotta, F.; Maggi, L.; Annunziato, F. Role of type 2 innate lymphoid cells in allergic diseases. Curr. Allergy Asthma Rep. 2017, 17, 66. [Google Scholar] [CrossRef]
- Jeffery, H.C.; McDowell, P.; Lutz, P.; Wawman, R.E.; Roberts, S.; Bagnall, C.; Birtwistle, J.; Adams, D.H.; Oo, Y.H. Human intrahepatic ILC2 are IL-13positive amphiregulinpositive and their frequency correlates with model of end stage liver disease score. PLoS ONE 2017, 12, e0188649. [Google Scholar] [CrossRef]
- Elder, J.M.; Webster, S.J.; Williams, D.L.; Gaston, J.S.; Goodall, J.C. Tslp production by dendritic cells is modulated by il-1beta and components of the endoplasmic reticulum stress response. Eur. J. Immunol. 2016, 46, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Dunican, M.E.; Elicker, B.M.; Gierada, D.S.; Nagle, S.K.; Schiebler, M.L.; Newell, J.D.; Raymond, W.W.; Lachowicz-Scroggins, M.E.; di Maio, S.; Hoffman, E.A.; et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Investig. 2018, 128, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Rana, B.M.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.-R.; et al. Tissue-Restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 2018, 48, 1195–1207.e6. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Kim, H.Y.; A Albacker, L.; Baumgarth, N.; McKenzie, A.N.J.; Smith, G.J.; DeKruyff, R.H.; Umetsu, D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef]
- Rigas, D.; Lewis, G.; Aron, J.L.; Wang, B.; Banie, H.; Sankaranarayanan, I.; Galle-Treger, L.; Maazi, H.; Lo, R.; Freeman, G.J.; et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator–inducible T-cell costimulator ligand interaction. J. Allergy Clin. Immunol. 2017, 139, 1468–1477.e2. [Google Scholar] [CrossRef]
- Rauber, S.; Luber, M.; Weber, S.; Maul, L.; Soare, A.; Wohlfahrt, T.; Lin, N.-Y.; Dietel, K.; Bozec, A.; Herrmann, M.; et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat. Med. 2017, 23, 938–944. [Google Scholar] [CrossRef]
- Miyamoto, C.; Kojo, S.; Yamashita, M.; Moro, K.; Lacaud, G.; Shiroguchi, K.; Taniuchi, I.; Ebihara, T. Runx/Cbfbeta complexes protect group 2 innate lymphoid cells from exhausted-like hyporesponsiveness during allergic airway inflammation. Nat. Commun. 2019, 10, 447. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From basic science to clinical translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Howard, E.; Lewis, G.; Galle-Treger, L.; Hurrell, B.P.; Helou, D.G.; Shafiei-Jahani, P.; Painter, J.D.; Muench, G.A.; Soroosh, P.; Akbari, O. IL-10 production by ILC2s requires Blimp-1 and cMaf, modulates cellular metabolism, and ameliorates airway hyperreactivity. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bando, J.K.; Gilfillan, S.; Di Luccia, B.; Fachi, J.L.; Sécca, C.; Cella, M.; Colonna, M. ILC2s are the predominant source of intestinal ILC-derived IL-10. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, S.; Zhang, B.; Zhang, Y. Cancer immunology and cancer immunodiagnosis. J. Immunol. Res. 2014, 2014, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Wang, R.; Yao, X.; Li, X.; Wang, X.; Hu, Y.; Chang, X.; Fan, P.; Dong, T.; Ogg, G.S. Activated innate lymphoid cell populations accumulate in human tumour tissues. BMC Cancer 2018, 18, 341. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, P.I.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/St2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, E.; de Monte, L.; Balzano, G.; Camisa, B.; Laino, V.; Riba, M.; Heltai, S.; Bianchi, M.; Bordignon, C.; Falconi, M.; et al. The Il-1/Il-1 receptor axis and tumor cell released inflammasome adaptor Asc are key regulators of Tslp secretion by cancer associated fibroblasts in pancreatic cancer. J. Immunother. Cancer 2019, 7, 45. [Google Scholar] [CrossRef]
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Protti, M.P.; De Monte, L. Thymic stromal lymphopoietin and cancer: Th2-Dependent and -independent mechanisms. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Munneke, J.M.; Björklund, A.T.; Mjösberg, J.M.; Garming-Legert, K.; Bernink, J.H.; Blom, B.; Huisman, C.; Van Oers, M.H.J.; Spits, H.; Malmberg, K.-J.; et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 2014, 124, 812–821. [Google Scholar] [CrossRef]
- Bruchard, M.; Ghiringhelli, F. Deciphering the roles of innate lymphoid cells in cancer. Front. Immunol. 2019, 10, 656. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, Y.; Su, Z.; Bie, Q.; Chen, X.; Barnie, P.A.; Guo, Q.; Wang, S.; Xu, H. Enhanced circulating ILC2s and MDSCs may contribute to ensure maintenance of Th2 predominant in patients with lung cancer. Mol. Med. Rep. 2017, 15, 4374–4381. [Google Scholar] [CrossRef]
- Carrega, P.; LoIacono, F.; Di Carlo, E.; Scaramuccia, A.; Mora, M.; Conte, R.; Benelli, R.; Spaggiari, G.M.; Cantoni, C.; Campana, S.; et al. NCR+ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 2015, 6, 8280. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Fehlings, M.; Kloverpris, H.N.; McGovern, N.; Koo, S.L.; Loh, C.Y.; Lim, S.; Kurioka, A.; Fergusson, J.R.; Tang, C.L.; et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 2017, 46, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Bie, Q.; Zhang, P.; Su, Z.; Zheng, D.; Ying, X.; Wu, Y.; Yang, H.; Chen, D.; Wang, S.; Xu, H. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Forkel, M.; Van Tol, S.; Höög, C.; Michaëlsson, J.; Almer, S.; Mjösberg, J. Distinct alterations in the composition of mucosal innate lymphoid cells in newly diagnosed and established Crohn’s disease and ulcerative colitis. J. Crohns Colitis 2019, 13, 67–78. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S.; et al. ILC2-modulated T cell–to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nat. Cell Biol. 2020, 579, 130–135. [Google Scholar] [CrossRef]
- Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015, 75, 79–88. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging Functions of Amphiregulin in Orchestrating Immunity, Inflammation, and Tissue Repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef]
- Nagaraj, S.; Youn, J.-I.; Gabrilovich, D.I. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J. Immunol. 2013, 191, 17–23. [Google Scholar] [CrossRef]
- Villarreal, D.O.; Wise, M.C.; Walters, J.N.; Reuschel, E.L.; Choi, M.J.; Obeng-Adjei, N.; Yan, J.; Morrow, M.P.; Weiner, D.B. Alarmin IL-33 Acts as an Immunoadjuvant to Enhance Antigen-Specific Tumor Immunity. Cancer Res. 2014, 74, 1789–1800. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Yang, Q.; Zhao, X.; Wen, W.; Li, G.; Lu, J.; Qin, W.; Qi, Y.; Xie, F.; et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+T and NK cells. J. Immunol. 2015, 194, 438–445. [Google Scholar] [CrossRef]
- Long, A.; Dominguez, D.; Qin, L.; Chen, S.; Fan, J.; Zhang, M.; Fang, D.; Zhang, Y.; Kuzel, T.M.; Zhang, B. Type 2 innate lymphoid cells impede IL-33–mediated tumor suppression. J. Immunol. 2018, 201, 3456–3464. [Google Scholar] [CrossRef]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.-M.; Barkauskas, D.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef]
- Vijayan, D.; Young, A.; Teng, M.W.; Smyth, M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 2017, 17, 709–724. [Google Scholar] [CrossRef]
- Bi, J.; Cui, L.; Yu, G.; Yang, X.; Chen, Y.; Wan, X. NK cells alleviate lung inflammation by negatively regulating group 2 innate lymphoid cells. J. Immunol. 2017, 198, 3336–3344. [Google Scholar] [CrossRef]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Warner, K.; Ohashi, P.S. ILC regulation of T cell responses in inflammatory diseases and cancer. Semin. Immunol. 2019, 41, 101284. [Google Scholar] [CrossRef]
- Joshi, B.H.; Leland, P.; Lababidi, S.; Varrichio, F.; Puri, R.K. Interleukin-4 receptor alpha overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer Med. 2014, 3, 1615–1628. [Google Scholar] [CrossRef]
- Fujisawa, T.; Joshi, B.; Nakajima, A.; Puri, R.K. A novel role of interleukin-13 receptor Alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009, 69, 8678–8685. [Google Scholar] [CrossRef]
- Fujisawa, T.; Joshi, B.H.; Puri, R.K. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int. J. Cancer 2011, 131, 344–356. [Google Scholar] [CrossRef]
- Halim, Y.T.; Hwang, Y.Y.; Scanlon, S.T.; Zaghouani, H.; Garbi, N.; Fallon, P.G.; McKenzie, A.N. Group 2 Innate lymphoid cells license dendritic cells to potentiate memory Th2 cell responses. Nat. Immunol. 2016, 17, 57–64. [Google Scholar] [CrossRef]
- Bouchery, T.; Kyle, R.; Camberis, M.; Shepherd, A.; Filbey, K.; Smith, A.; Harvie, M.; Painter, G.; Johnston, K.; Ferguson, P.; et al. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat. Commun. 2015, 6, 6970. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, Y.; Lee, H.; Mun, J.; Sim, S.; Lee, D.; Le Pham, D.; Kim, S.; Shin, Y.S.; Lee, S.; et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy 2019, 75, 95–103. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Sehmi, R.; Ambrose, C.S.; Griffiths, J.M. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin. Ther. Targets 2020, 24, 777–792. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Saadalla, A.M.; Osman, A.; Gurish, M.F.; Dennis, K.L.; Blatner, N.R.; Pezeshki, A.; McNagny, K.M.; Cheroutre, H.; Gounari, F.; Khazaie, K. Mast cells promote small bowel cancer in a tumor stage-specific and cytokine-dependent manner. Proc. Natl. Acad. Sci. USA 2018, 115, 1588–1592. [Google Scholar] [CrossRef]
- Saleh, R.; Toor, S.M.; Nair, V.S.; Elkord, E. Role of epigenetic modifications in inhibitory immune checkpoints in cancer development and progression. Front. Immunol. 2020, 11, 1469. [Google Scholar] [CrossRef]
- Yu, Y.; Tsang, J.C.H.; Wang, C.; Clare, S.; Wang, J.; Chen, X.; Brandt, C.; Kane, L.; Campos, L.S.; Lu, L.; et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nat. Cell Biol. 2016, 539, 102–106. [Google Scholar] [CrossRef]
- Chiossone, L.; Vivier, E. Immune checkpoints on innate lymphoid cells. J. Exp. Med. 2017, 214, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Huang, Y.; Mallett, G.; Stathopoulou, C.; Felizardo, T.C.; Sun, M.-A.; Martin, E.L.; Zhu, N.; Woodward, E.L.; Elias, M.S.; et al. PD-1 regulates KLRG1+ group 2 innate lymphoid cells. J. Exp. Med. 2017, 214, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Helou, D.G.; Shafiei-Jahani, P.; Lo, R.; Howard, E.; Hurrell, B.P.; Galle-Treger, L.; Painter, J.; Lewis, G.; Soroosh, P.; Sharpe, A.H.; et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Mchedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.J.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef]
- Li, J.; Razumilava, N.; Gores, G.J.; Walters, S.; Mizuochi, T.; Mourya, R.; Bessho, K.; Wang, Y.-H.; Glaser, S.S.; Shivakumar, P.; et al. Biliary repair and carcinogenesis are mediated by IL-33–dependent cholangiocyte proliferation. J. Clin. Investig. 2014, 124, 3241–3251. [Google Scholar] [CrossRef]
- Hong, J.; Kim, S.; Lin, P.C. Interleukin-33 and ST2 signaling in tumor microenvironment. J. Interferon Cytokine Res. 2019, 39, 61–71. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Zhu, J. Mysterious ILC2 tissue adaptation. Nat. Immunol. 2018, 19, 1042–1044. [Google Scholar] [CrossRef]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert Tnf-Alpha and granzyme a-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.-E.; Lefèvre, G.; Capron, M. IL-18 Is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef]
- Furbert-Harris, P.M.; Parish-Gause, D.; A Hunter, K.; Vaughn, T.R.; Howland, C.; Okomo-Awich, J.; Forrest, K.; Laniyan, I.; AbdelNaby, A.; Oredipe, O. Activated eosinophils upregulate the metastasis suppressor molecule E-cadherin on prostate tumor cells. Cell. Mol. Biol. 2003, 49, 1009–1016. [Google Scholar]
- Kataoka, S.; Konishi, Y.; Nishio, Y.; Fujikawa-Adachi, K.; Tominaga, A. Antitumor activity of eosinophils activated by Il-5 and Eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004, 23, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ikutani, M.; Yanagibashi, T.; Ogasawara, M.; Tsuneyama, K.; Yamamoto, S.; Hattori, Y.; Kouro, T.; Itakura, A.; Nagai, Y.; Takaki, S.; et al. Identification of innate IL-5–producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2011, 188, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Simson, L.; Ellyard, J.I.; A Dent, L.; Matthaei, K.I.; Rothenberg, M.E.; Foster, P.S.; Smyth, M.J.; Parish, C.R. Regulation of carcinogenesis by IL-5 and CCL11: A Potential role for eosinophils in tumor immune surveillance. J. Immunol. 2007, 178, 4222–4229. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Moon, U.J.; Kim, H.J.; Choi, H.-J.; Sin, J.-I.; Park, N.H.; Cho, H.R.; Kwon, B. Intratumorally establishing type 2 innate lymphoid cells blocks tumor growth. J. Immunol. 2016, 196, 2410–2423. [Google Scholar] [CrossRef]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef]
- Saranchova, I.; Han, J.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.G.; Welch, I.; Wyatt, A.W.; Fazli, L.; et al. Discovery of a metastatic immune escape mechanism initiated by the loss of expression of the tumour biomarker interleukin-33. Sci. Rep. 2016, 6, 30555. [Google Scholar] [CrossRef]
- Saranchova, I.; Han, J.; Zaman, R.; Arora, H.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.G.; Welch, I.; et al. Type 2 innate lymphocytes actuate immunity against tumours and limit cancer metastasis. Sci. Rep. 2018, 8, 2924. [Google Scholar] [CrossRef]
- Lu, B.; Yang, M.; Wang, Q. Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy. J. Mol. Med. 2016, 94, 535–543. [Google Scholar] [CrossRef]
- Tumino, N.; Martini, S.; Munari, E.; Scordamaglia, F.; Besi, F.; Mariotti, F.R.; Bogina, G.; Mingari, M.C.; Vacca, P.; Moretta, L. Presence of innate lymphoid cells in pleural effusions of primary and metastatic tumors: Functional analysis and expression of PD-1 receptor. Int. J. Cancer 2019, 145, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Afferni, C.; Buccione, C.; Andreone, S.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Mattei, F.; Schiavoni, G. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front. Immunol. 2018, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Schuijs, J.M.; Png, S.; Richard, A.C.; Tsyben, A.; Hamm, G.; Stockis, J.; Garcia, C.; Pinaud, S.; Nicholls, A.; Ros, X.R.; et al. Ilc2-Driven innate immune checkpoint mechanism antagonizes Nk cell antimetastatic function in the lung. Nat. Immunol. 2020, 21, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Mahmoud, A.; Keane, J.; Murphy, C.; White, D.; Carey, S.; O’Riordain, M.; Bennett, M.W.; Brint, E.K.; Houston, A. An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. Br. J. Cancer 2015, 114, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Prokopchuk, O.; Liu, Y.; Hennebruns, D.; Kornmann, P.D.M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br. J. Cancer 2005, 92, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Barderas, R.; Bartolome, R.A.; Fernandez-Acenero, M.J.; Torres, S.; Casal, J.I. High expression of Il-13 receptor Alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012, 72, 2780–2790. [Google Scholar] [CrossRef]
- Francipane, M.G.; Alea, M.P.; Lombardo, Y.; Todaro, M.; Medema, J.P.; Stassi, G. Crucial Role of Interleukin-4 in the Survival of Colon Cancer Stem Cells. Cancer Res. 2008, 68, 4022–4025. [Google Scholar] [CrossRef]
- Todaro, M.; Alea, M.P.; Di Stefano, A.B.; Cammareri, P.; Vermeulen, L.; Iovino, F.; Tripodo, C.; Russo, A.; Gulotta, G.; Medema, J.P.; et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007, 1, 389–402. [Google Scholar] [CrossRef]
- Volonté, A.; Di Tomaso, T.; Spinelli, M.; Todaro, M.; Sanvito, F.; Albarello, L.; Bissolati, M.; Ghirardelli, L.; Orsenigo, E.; Ferrone, S.; et al. Cancer-Initiating cells from colorectal cancer patients escape from T cell–mediated immunosurveillance in vitro through membrane-bound IL-4. J. Immunol. 2013, 192, 523–532. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, J.W.; Roh, J.-L.; Park, Y.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y.; Lee, B.-H. Recurrence and cancer-specific survival according to the expression of IL-4Rα and IL-13Rα1 in patients with oral cavity cancer. Eur. J. Cancer 2015, 51, 177–185. [Google Scholar] [CrossRef]
- Yang, C.-M.; Chen, H.-C.; Hou, Y.-Y.; Lee, M.-C.; Liou, H.-H.; Huang, S.-J.; Yen, L.-M.; Eng, D.-M.; Hsieh, Y.-D.; Ger, L.-P. A high IL-4 production diplotype is associated with an increased risk but better prognosis of oral and pharyngeal carcinomas. Arch. Oral Biol. 2014, 59, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.; Shoda, J.; Horibe, T.; Warabi, E.; Kohno, M.; Yanagawa, T.; Bukawa, H.; Nakanuma, Y.; Kawakami, K. Targeting interleukin-4 receptor alpha by hybrid peptide for novel biliary tract cancer therapy. Int. J. Hepatol. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Horibe, T.; Kohno, M.; Haramoto, M.; Ohara, K.; Puri, R.K.; Kawakami, K. Targeting interleukin-4 receptor alpha with hybrid peptide for effective cancer therapy. Mol. Cancer Ther. 2012, 11, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Y.; Wong, T.-T.; Teng, M.-C.; Liu, R.-S.; Lu, M.; Liang, H.-F.; Wei, M.-C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef]
- Hsi, L.C.; Kundu, S.; Palomo, J.; Xu, B.; Ficco, R.; Vogelbaum, M.A.; Cathcart, M.K. Silencing IL-13R 2 promotes glioblastoma cell death via endogenous signaling. Mol. Cancer Ther. 2011, 10, 1149–1160. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef]
- Ou, W.; Marino, M.P.; Suzuki, A.; Joshi, B.; Husain, S.R.; Maisner, A.; Galanis, E.; Puri, R.K.; Reiser, J. Specific targeting of human interleukin (Il)-13 receptor alpha2-positive cells with lentiviral vectors displaying Il-13. Hum. Gene Ther. Methods 2012, 23, 137–147. [Google Scholar] [CrossRef]
- Pandya, H.; Gibo, D.M.; Garg, S.; Kridel, S.; Debinski, W. An interleukin 13 receptor alpha 2-specific peptide homes to human glioblastoma multiforme xenografts. Neuro Oncol. 2012, 14, 6–18. [Google Scholar] [CrossRef]
- Iwami, K.; Shimato, S.; Ohno, M.; Okada, H.; Nakahara, N.; Sato, Y.; Yoshida, J.; Suzuki, S.; Nishikawa, H.; Shiku, H.; et al. Peptide-Pulsed dendritic cell vaccination targeting interleukin-13 receptor alpha2 chain in recurrent malignant glioma patients with Hla-a*24/a*02 Allele. Cytotherapy 2012, 14, 733–742. [Google Scholar] [CrossRef]
- Kong, S.; Sengupta, S.; Tyler, B.; Bais, A.J.; Ma, Q.; Doucette, S.; Zhou, J.; Sahin, A.; Carter, B.S.; Brem, H.; et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin. Cancer Res. 2012, 18, 5949–5960. [Google Scholar] [CrossRef]
- Cortez, S.V.; Ulland, T.K.; Cervantes-Barragan, L.; Bando, J.K.; Robinette, M.L.; Wang, Q.; White, A.J.; Gilfillan, S.; Cella, M.; Colonna, M. Smad4 impedes the conversion of Nk cells into Ilc1-like cells by curtailing non-canonical Tgf-beta signaling. Nat. Immunol. 2017, 18, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.; Van Loosdregt, J.; Gorlani, A.; Bekker, C.P.; Gröne, A.; Sibilia, M.; Henegouwen, P.M.P.V.B.E.; Roovers, R.C.; Coffer, P.J.; Sijts, A.J.A.M. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Ealey, K.N.; Tetsu, H.; Kiniwa, T.; Motomura, Y.; Moro, K.; Koyasu, S. Tumor-Derived lactic acid contributes to the paucity of intratumoral ILC2s. Cell Rep. 2020, 30, 2743–2757.e5. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, W.; Yuan, X.; Liu, S.; Li, M.; Niu, J.; Zhang, P.; Li, D. Vitamin A deficiency execrates Lewis lung carcinoma via induction of type 2 innate lymphoid cells and alternatively activates macrophages. Food Sci. Nutr. 2019, 7, 1288–1294. [Google Scholar] [CrossRef]
- Trabanelli, S.; Chevalier, M.F.; Derré, L.; Jandus, C. The pro- and anti-tumor role of ILC2s. Semin. Immunol. 2019, 41, 101276. [Google Scholar] [CrossRef]
- Ercolano, G.; Falquet, M.; Vanoni, G.; Trabanelli, S.; Jandus, C. ILC2s: New actors in tumor immunity. Front. Immunol. 2019, 10, 2801. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, P.; Chen, Y.; Chai, Y. Ambiguous roles and potential therapeutic strategies of innate lymphoid cells in different types of tumor (Review). Oncol. Lett. 2020, 20, 1513–1525. [Google Scholar] [CrossRef]
| Tumor Types | Phenotype | Sites | Pro/Anti-Tumor | Associated Cells | Serum Cytokines | Functions | Ref |
|---|---|---|---|---|---|---|---|
| AML | ILC2s (also ILC1/ILC3) | PBMC | Protumor | Reduction | [79] | ||
| APL | Lin-CD127+NKp46- CRTH2+c-kit−/+ | PBMC | Protumor | MDSC | IL-13 | Increased | [32] |
| Breast cancer | Lin-CD127+CD56- CRTH2+CD117−/+ | Tissue | Protumor | MDSC | IL-13 | Increased Tregs and PD1+CTLA4+KLRG2+ILC2s (vs circulating ILC2s) | [32,80] |
| Lung cancer | Lin-ICOS+IL−17RB+ | PBMC | Protumor | MDSC | IL-5, IL-13, IL-33, Arg1 | Increased Reduction (vs blood) | [81] [82,83] |
| Gastric cancer | Lin-CD127+CRTH2+ CD161+ Lin-ICOS+IL−17RB+ | Tissue PBMC | Protumor | MDSC, M2 type Macrophages | IL-4, IL-5, IL-25, IL33 | Increased | [84] |
| Colorectal cancer | Lin-CD127+CRTH2+ | Tissue | Protumor | Increased among Non-NK ILCs | [83,85] | ||
| Bladder cancer | Lin-CD127+CRTH2+ CD117−/+ | Urine during BCG treatment | Protumor | MDSC | IL-13 | T/MDSC ratio predictive of relapse | [86] |
| Prostate cancer | Lin-CD127+CRTH2+ CD117−/+ | PBMC | Protumor | NKp30+ILC2 MDSC | ILC2s related to disease severity | [32] | |
| Pancreatic Ductal Cancer | Lin-CD127+ST2+ | Tissue | Antitumor | Activated CD8+T cells | IL-33 | Increased in long-term survivors | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggi, E.; Veneziani, I.; Moretta, L.; Cosmi, L.; Annunziato, F. Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer? Cancers 2020, 12, 3452. https://doi.org/10.3390/cancers12113452
Maggi E, Veneziani I, Moretta L, Cosmi L, Annunziato F. Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer? Cancers. 2020; 12(11):3452. https://doi.org/10.3390/cancers12113452
Chicago/Turabian StyleMaggi, Enrico, Irene Veneziani, Lorenzo Moretta, Lorenzo Cosmi, and Francesco Annunziato. 2020. "Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer?" Cancers 12, no. 11: 3452. https://doi.org/10.3390/cancers12113452
APA StyleMaggi, E., Veneziani, I., Moretta, L., Cosmi, L., & Annunziato, F. (2020). Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer? Cancers, 12(11), 3452. https://doi.org/10.3390/cancers12113452







