Simple Summary
The Wnt/β-catenin signaling pathway is aberrantly activated in most colorectal cancers and less frequently in a variety of other solid neoplasias. Many epidemiological and experimental studies and some clinical trials suggest an anticancer action of vitamin D, mainly against colorectal cancer. The aim of this review was to analyze the literature supporting the interference of Wnt/β-catenin signaling by the active vitamin D metabolite 1α,25-dihydroxyvitamin D3. We discuss the molecular mechanisms of this antagonism in colorectal cancer and other cancer types. Additionally, we summarize the available data indicating a reciprocal inhibition of vitamin D action by the activated Wnt/β-catenin pathway. Thus, a complex mutual antagonism between Wnt/β-catenin signaling and the vitamin D system seems to be at the root of many solid cancers.
Abstract
Abnormal activation of the Wnt/β-catenin pathway is common in many types of solid cancers. Likewise, a large proportion of cancer patients have vitamin D deficiency. In line with these observations, Wnt/β-catenin signaling and 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active vitamin D metabolite, usually have opposite effects on cancer cell proliferation and phenotype. In recent years, an increasing number of studies performed in a variety of cancer types have revealed a complex crosstalk between Wnt/β-catenin signaling and 1,25(OH)2D3. Here we review the mechanisms by which 1,25(OH)2D3 inhibits Wnt/β-catenin signaling and, conversely, how the activated Wnt/β-catenin pathway may abrogate vitamin D action. The available data suggest that interaction between Wnt/β-catenin signaling and the vitamin D system is at the crossroads in solid cancers and may have therapeutic applications.
1. Introduction
1.1. Wnt
Wnt proteins are extracellular signaling molecules that control many key processes during embryonic development and regulate the homeostasis of adult tissues, mainly by modulating the survival, self-renewal, and proliferation of stem cells. They are secreted by a variety of cell types and typically have a short range of action, mediating communication between neighboring cells. Wnt proteins bind to cell surface receptors, of which several classes have been described. Specific Wnt-receptor combinations and cellular contexts determine which of the existing Wnt signaling pathways is engaged [1].
The Wnt/β-catenin pathway is triggered by Wnt binding to cell membrane receptors of the Frizzled and low-density lipoprotein receptor-related protein (LRP) families. In the absence of a Wnt ligand, β-catenin protein is mainly located at cell-cell contacts and free cytoplasmic β-catenin is kept low because of a proteolytic destruction machinery. A complex containing the tumor suppressor proteins APC (adenomatous polyposis coli) and axin, and the kinases casein kinase 1 (CK1) and glycogen synthase kinase-3β (GSK-3β) targets β-catenin for N-terminal phosphorylation and subsequent ubiquitination and proteasome-mediated degradation. Wnt binding to Frizzled and LRP5/6 leads to inhibition of the β-catenin destruction complex and, therefore, to the accumulation of β-catenin in the cytoplasm. A proportion of β-catenin enters the nucleus and binds to transcription factors of the LEF/TCF family acting as a co-activator and regulating the expression of a large variety of genes. Wnt target genes are cell and tissue type-dependent and affect many cellular functions and processes, such as cell proliferation, stemness, migration, and invasion. Some of these targets include the c-MYC and CCND1/cyclin D1 oncogenes, the Wnt inhibitors DKK1 and NKD1/2, and the Wnt effector LEF1. In addition, the Wnt inhibitor AXIN2 is the most ubiquitously regulated β-catenin target gene [1,2,3].
Wnt/β-catenin signaling is highly dependent on the number of Frizzled receptor molecules present on the cell surface. Vertebrates have evolved a complex regulatory mechanism to control the amount of Frizzled on the plasma membrane that involves three types of proteins: leucine-rich repeat-containing G-protein coupled receptors (LGR4-6), their extracellular ligands R-spondins (RSPO1-4), and the E3 transmembrane ubiquitin ligases ZNRF3 and RNF43 [4,5]. In the absence of RSPO, Frizzled receptors are targeted for degradation by ZNRF3/RNF43-mediated ubiquitination, which results in low Frizzled membrane concentration and, therefore, in attenuated Wnt signaling. In contrast, RSPO binding to LGR4-6 sequesters ZNRF3/RNF43 in a ternary complex and prevents ubiquitin tagging of Frizzled. Thus, RSPOs are responsible for the accumulation of Frizzled receptors on the cell surface and the potentiation of Wnt/β-catenin signaling in target cells [6,7,8].
Dysregulation of Wnt/β-catenin signaling is involved in human diseases including cancer. In many types of cancer, e.g., colorectal, breast, and liver carcinoma, melanoma and leukemia, β-catenin constitutively accumulates within the nucleus of tumor cells [9,10,11,12,13]. In fact, aberrant activation of the Wnt/β-catenin pathway is the most common event in human colorectal cancer (CRC) [14,15] in which massive sequencing has estimated that over 94% of primary colon tumors carry mutations in one or more genes involved in this pathway [16]. Truncation mutations and allelic losses in the tumor suppressor gene APC are present in around 80% of sporadic CRC cases, whereas a small proportion carries mutations in AXIN2 or CTNNB1/β-catenin genes. Moreover, chromosomal rearrangements in R-spondin family members RSPO2 and RSPO3 have been found in 10% of human CRC leading to enhanced Wnt signaling [17,18]. In addition, RNF43 is mutated in a proportion of mismatch repair-deficient colon tumors. Alterations in genes encoding components of the Wnt/β-catenin pathway are frequently mutually exclusive, which confirms that aberrant activation of this pathway is a hallmark of CRC. Mutations in CTNNB1/β-catenin or AXIN2 have been reported in other human tumors, e.g., hepatocellular carcinomas [19,20,21], whereas overexpression of Wnt factors/receptors or silencing of extracellular Wnt inhibitors are the preferred mechanisms of Wnt/β-catenin sustained activation in other cancers, e.g., breast and lung cancers [22,23,24,25,26,27].
1.2. Vitamin D
Vitamin D3 (cholecalciferol) is a natural seco-steroid whose main source is non-enzymatic production in human skin from UV-B exposed 7-dehydrocholesterol, an abundant cholesterol precursor [28,29]. Vitamin D3 from skin production and from dietary uptake is hydroxylated in the liver to 25-hydroxyvitamin D3 (25(OH)D3, calcidiol), a stable compound that is used as a biomarker for the vitamin D status of a person [29,30,31]. Subsequent hydroxylation of 25(OH)D3 at carbon 1, which occurs mainly in the kidneys but also in several types of epithelial and immune cells, renders 1,25-dihydroxyvitamin D3 (1,25(OH)2D3, calcitriol). This is the most active vitamin D3 metabolite and a high affinity ligand for the vitamin D receptor (VDR) [29,31,32].
VDR is a member of the nuclear receptor superfamily of transcription factors, which includes receptors for other hormones such as estrogen, progesterone or glucocorticoids, as well as a number of orphan receptors. Nuclear receptors present a highly conserved ligand-binding domain, which in the case of VDR fixes 1,25(OH)2D3 or its synthetic analogues with high specificity [33]. Binding of 1,25(OH)2D3 to VDR promotes the formation of complexes with RXR, the receptor for 9-cis-retinoic acid, and the binding of these VDR/RXR heterodimers to DNA. This leads to epigenetic changes that affect the transcription rate of hundreds of target genes involved in many cellular processes, including proliferation, differentiation and survival [29,31]. Moreover, a proportion of VDR molecules locate in the cytoplasm of some cell types where, on ligand binding, they trigger rapid, non-genomic, modulatory effects on signaling pathways by acting on kinases, phosphatases, and ion channels [34,35].
Current evidence indicates that 1,25(OH)2D3 and its derivatives modulate signaling pathways that affect cell survival, growth, and differentiation [29,36,37], key processes that are dysregulated in human cancers. One of these signaling routes is the Wnt/β-catenin pathway. This review will focus on the crosstalk between Wnt and vitamin D in solid tumors.
2. Antagonism of Wnt/β-Catenin Signaling by 1,25(OH)2D3 in Solid Cancers
2.1. Colorectal Cancer
Four decades ago, an epidemiological study hinted at the protective effects of vitamin D3 against CRC by indicating that high UVB exposure or life at lower latitudes, both of which result in higher vitamin D3 synthesis, lead to lower incidence of CRC [38]. Since then, a large number of epidemiological studies, experimental work performed in cultured cells and animal models, and also some, but not all, vitamin D3 supplementation human clinical trials have strongly suggested that 1,25(OH)2D3 has anticancer effects, particularly in CRC [31,37,39,40,41,42,43,44].
Our group was a pioneer in demonstrating that 1,25(OH)2D3 antagonizes the Wnt/β-catenin signaling pathway in colon carcinoma cells [45], a mechanism that could at least partly account for the protective effects of vitamin D3 observed in epidemiological and animal studies. Previously, other groups had reported a crosstalk between Wnt signaling and other nuclear receptors, such as those for retinoid acid and androgen [46,47].
Results from our laboratory showed that 1,25(OH)2D3 interferes with Wnt/β-catenin signaling in human colon carcinoma cells by at least three mechanisms (Figure 1). Firstly, ligand-activated nuclear VDR binds and sequesters β-catenin, which prevents its binding to LEF/TCF transcription factors and thus blocks β-catenin/TCF-mediated transcription of Wnt target genes [45]. VDR/β-catenin physical interaction was later confirmed in this and other cell systems and involves the C-terminal region of β-catenin and the activator function-2 domain of VDR [48]. Interestingly, wild-type APC potentiates VDR/β-catenin binding [49]. Lithocholic acid, a low affinity VDR ligand, also prompts this interaction, although less efficiently than 1,25(OH)2D3 [49].
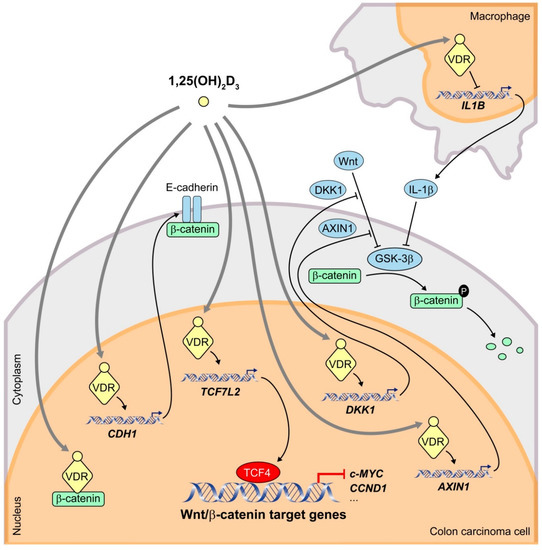
Figure 1.
Schematic representation of the mechanisms by which 1,25(OH)2D3 interferes the Wnt/β-catenin signaling pathway in human CRC cells. 1,25(OH)2D3 binds to its high affinity receptor VDR inducing the formation of β-catenin/VDR complexes and thus preventing that of transcriptionally active β-catenin/TCF4 complexes. In addition, 1,25(OH)2D3 increases the transcription of the CDH1 gene encoding E-cadherin, which sequesters newly synthesized β-catenin protein at the subcortical adherens junctions. Furthermore, 1,25(OH)2D3 upregulates the expression of the negative regulators of the Wnt/β-catenin pathway TCF7L2 (encoding TCF4), DKK1 and AXIN1. 1,25(OH)2D3 also antagonizes the pathway by reducing the secretion by nearby macrophages of IL-1β, which inhibits GSK-3β activity in CRC cells leading to an increase in β-catenin levels.
Secondly, 1,25(OH)2D3 induces the expression of E-cadherin protein, which sequesters newly synthesized β-catenin at subcortical cell-cell adherens junctions, thus avoiding its translocation to the nucleus and β-catenin/TCF-mediated transcription [45]. Our data suggest that the small GTPase RhoA, the protease inhibitor cystatin D, the regulator of tyrosine kinase receptor signaling Sprouty-2, and the histone demethylase JMJD3 are involved in this mechanism [34,50,51,52]. Induction of E-cadherin by 1,25(OH)2D3 and concomitant inhibition of the Wnt/β-catenin pathway have also been reported in other cell types [53]. However, 1,25(OH)2D3 can antagonize Wnt/β-catenin signaling in colon carcinoma cells that do not express E-cadherin, which implies that this mechanism is not strictly required [45].
Thirdly, 1,25(OH)2D3 promotes the expression of Dickkopf 1 (DKK1), a member of a family of extracellular inhibitors of the Wnt/β-catenin pathway [54]. DKK1 can inhibit Wnt/β-catenin signaling by two mechanisms. On the one hand, DKK1 direct binding to LRP5/6 blocks Wnt-LRP interaction [55]. On the other, DKK1 can engage a ternary complex with LRP5/6 and Kremen receptors, which prompts rapid endocytosis and removal of LRP5/6 from the plasma membrane [56]. In addition to a Wnt inhibitor, DKK1 is a β-catenin/TCF target gene [57,58,59]. Although most CRC carry mutations that activate the Wnt/β-catenin pathway downstream of DKK1, evidence suggests that this extracellular inhibitor has antitumor effects that are independent of β-catenin/TCF transcriptional activity [60,61,62]. Supporting the relevance of DKK1 in CRC, we and others have demonstrated that DKK1 expression is frequently downregulated in this neoplasia [59], in part due to gene promoter hypermethylation [62,63,64]. Moreover, the expression levels of VDR and DKK1 in human CRC biopsies directly correlate [54], and dietary vitamin D intake is inversely associated with DKK1 promoter methylation in a large cohort of CRC patients [65].
DKK4 is another member of the Dickkopf family of Wnt extracellular inhibitors, although it is a weaker Wnt antagonist than DKK1. We reported that 1,25(OH)2D3 downregulates the expression of DKK4 in both human colon and breast cancer cells. Accordingly, a significant inverse correlation between DKK4 and VDR expression exists in human CRC biopsies [66]. Unexpectedly, overexpression of DKK4 in human CRC cells enhances their migratory, invasive, and angiogenic potential [66]. These effects are probably unrelated to Wnt/β-catenin inhibition and imply additional mechanisms of action of DKK4. In this regard, we and others found that DKK4 transcripts are overexpressed in human CRC samples and in biopsies from patients with inflammatory bowel disease [66,67,68]. These data suggest that downregulation of DKK4 by 1,25(OH)2D3 may be another mechanism for the antitumor action of 1,25(OH)2D3 in CRC.
The c-MYC oncogene is a well-known β-catenin/TCF target gene that is frequently deregulated in human cancers and activates genetic programs that orchestrate biological processes to promote cell growth and proliferation [69]. Therefore, targeting the function of MYC oncoproteins holds the promise of achieving new, effective anticancer therapies that can be applied to a broad range of tumors [70]. In particular, mutational and integrative analyses have stressed the essential role of c-MYC in CRC [16]. A study reported by Meyer and colleagues using chromatin immunoprecipitation assays followed by high-throughput DNA sequencing (ChIP-Seq) in the CRC cell line LS180 concluded that β-catenin/TCF4 and VDR/RXR heterodimers colocalize at 74 sites near a limited set of genes that included c-MYC and c-FOS oncogenes [71]. These data strongly suggest a direct action of both complexes at these gene loci. In fact, 1,25(OH)2D3 effects on c-MYC gene expression may count as another mechanism of crosstalk between 1,25(OH)2D3 and Wnt/β-catenin signaling pathways. Firstly, ligand-activated VDR represses c-MYC expression by direct interaction with two vitamin D response elements (VDRE) in the promoter region [71,72]. Secondly, the antagonism exerted by 1,25(OH)2D3 on Wnt/β-catenin signaling impairs the transcription of c-MYC mediated by β-catenin/TCF complexes through their binding to several Wnt responsive elements (WRE) at the c-MYC promoter [45,73].
Some authors have proposed additional mechanisms of 1,25(OH)2D3 crosstalk with Wnt/β-catenin signaling in CRC cells (Figure 1). Beildeck and colleagues showed that 1,25(OH)2D3 increases TCF4 expression in several human CRC cell lines. The effect is indirect but completely dependent on VDR [74]. Tang and colleagues have reported that TCF4 functions as a transcriptional repressor that restricts CRC cell growth [75]. Therefore, 1,25(OH)2D3/VDR-mediated upregulation of TCF4 possibly has a protective effect on CRC. Furthermore, 1,25(OH)2D3/VDR induces expression of the negative regulator of the Wnt/β-catenin pathway AXIN1 in CRC cells through a VDRE localized in the regulatory region of the gene [76]. In addition, Gröschel and colleagues found that 1,25(OH)2D3 reduces nuclear β-catenin levels in LT97 colon microadenoma cells and thus downregulates the expression of Wnt target genes such as BCL2, CCND1/cyclin D1, SNAI1, CD44, and LGR5 [77]. Moreover, in healthy colon of mice fed a high vitamin D diet, β-catenin protein expression is decreased and the same effect is observed for TCF4 [77], which contrasts with the results of Beildeck and colleagues [74].
Kaler and colleagues described a paracrine mechanism that involves not only a crosstalk between 1,25(OH)2D3 and Wnt/β-catenin signaling pathways but also between carcinoma cells and the tumor microenvironment (Figure 1). They demonstrated that colon carcinoma cells induce the release of interleukin-1β (IL-1β) from macrophagic THP-1 cells in a process that requires constitutive activation of STAT1 [78]. Secreted IL-1β then acts on colon carcinoma cells where it triggers the inactivation of GSK-3β and thus the stabilization of β-catenin and subsequent expression of Wnt target genes. 1,25(OH)2D3 interrupts this crosstalk by blocking the constitutive activation of STAT1 and thus the production of IL-1β in macrophages in a VDR-dependent manner, which hampers the ability of macrophages to activate Wnt/β-catenin signaling in CRC cells [78]. The possibility that this mechanism works in vivo with tumor-associated macrophages is highly interesting.
Our group has also studied the interplay between 1,25(OH)2D3 and Wnt3A (an activator of the Wnt/β-catenin pathway) in human colon fibroblasts. Both agents strongly modulate the gene expression profile and phenotype of these cells. However, in contrast to the antagonism exerted by 1,25(OH)2D3 on the Wnt/β-catenin pathway in colon carcinoma cells, they have a partially overlapping effect. Both compounds inhibit fibroblast proliferation and migration, but while 1,25(OH)2D3 reduces, Wnt3A increases fibroblast capacity to remodel the extracellular matrix [79]. In addition, in contrast to the effects observed in established colon carcinoma cell lines, 1,25(OH)2D3 does not affect the expression of key genes of the Wnt/β-catenin pathway (AXIN2, CCND1, DKK1 and c-MYC) in human colon tumor or normal organoids derived from CRC patients, where only the DKK4 Wnt/β-catenin target gene is repressed by 1,25(OH)2D3 [80]. This shows that antagonism of the Wnt/β-catenin pathway is not a universal action of 1,25(OH)2D3 in tumor contexts. Moreover, 1,25(OH)2D3 cooperates with Wnt factors in the differentiation of bone (osteoblasts), skin (keratinocytes) and brain (neuronal precursors) cells under physiologic conditions [81,82]. Together, available data indicate a mostly repressive action of 1,25(OH)2D3 on overactivation of the Wnt/β-catenin pathway with different effects in particular scenarios.
The interplay between 1,25(OH)2D3 and Wnt/β-catenin signaling in CRC has also been studied in vivo in animal models and patients. Our group showed that the 1,25(OH)2D3 analogue EB1089 reduces the growth of xenografts generated by human CRC cells in immunosuppressed mice. In line with data obtained in cell cultures, this inhibition is associated with an increase of E-cadherin and DKK1 levels, and a decrease of β-catenin nuclear content and of the expression of the β-catenin/TCF target gene ENC1 in the xenografts [54,83,84]. Likewise, the antitumor action of 1,25(OH)2D3 on chemically induced mouse intestinal tumors is concomitant with increased expression of E-cadherin and the inhibition of β-catenin/TCF target genes such as c-Myc and Ccnd1/cyclin D1 in the intestinal crypts of these animals [85,86]. Concordantly, Xu and colleagues reported that 1,25(OH)2D3 and two of its analogues reduce the tumor load in the Apcmin/+ mouse model of intestinal tumorigenesis associated with an increase of E-cadherin protein and a decrease of nuclear β-catenin levels and of the expression of the Wnt target genes c-Myc and Tcf1 [87].
A Western-style diet that is high in fat and low in calcium and vitamin D is a risk factor for gastrointestinal carcinogenesis. This diet increases the frequency of intestinal tumors in normal mice and speeds up tumor formation in mouse models for intestinal cancer [88]. Several groups have shown that a Western-style diet alters components of the Wnt/β-catenin pathway in intestinal epithelial cells of normal mice [88,89]. These effects can be reversed by calcium and vitamin D supplementation, which prevents the increase of β-catenin/TCF transcriptional activity and reduces the expression of β-catenin, Ephb2 and Frizzled-2, -5, and -10 [89,90].
Vdr knockout mice have also been used to study the role of the vitamin D pathway on CRC. Larriba and colleagues [91] and Zheng and colleagues [92] generated Apcmin/+ Vdr−/− mice and discovered that the absence of Vdr results in a higher tumor load and an increased number of premalignant lesions. Interestingly, nuclear staining of β-catenin and expression of Wnt target genes Ccnd1/cyclin D1 and Lef1 are higher in Apcmin/+ Vdr−/− than in Apcmin/+ Vdr+/+ mice. This suggests that Vdr inactivation facilitates intestinal tumorigenesis fostered by Wnt/β-catenin activation [91,92].
Remarkably, in a randomized, double-blinded, placebo-controlled clinical trial, Bostick’s group reported that vitamin D supplements increase the expression of APC, E-cadherin and other differentiation markers, and decrease that of β-catenin in the upper part of the crypt of normal rectal mucosa from sporadic colorectal adenoma patients [93,94,95,96]. In addition, a recent study with 67 CRC patients has revealed that a high circulating 25(OH)D3 level associates with low promoter methylation of secreted frizzled-related protein 2 (SFRP2) gene that encodes a soluble inhibitor of the Wnt/β-catenin pathway [97]. These data support an inhibitory effect of vitamin D on Wnt signaling in the human colon in vivo.
2.2. Other Solid Tumors
Although Wnt signaling was first described as inducing breast tumors in mice [98] and Wnt/β-catenin signaling is activated in a proportion of multiple subtypes of human breast cancers [10,99], the typical mutations in components of the pathway found in CRC (APC, CTNNB1, AXIN2) are rare in breast carcinomas [27]. The elevated level of nuclear β-catenin and Wnt signaling in these tumors may be due to high expression of Wnt factors in the tumor environment, loss of APC, Wnt inhibitors (DKK1, SFRPs), and/or E-cadherin expression by epigenetic modification/gene silencing, or alterations in the expression of other genes that encode constituents of the pathway (RSPO2, FZD6) [27].
Notably, nuclear β-catenin accumulation in a subset of triple-negative and basal-like breast cancer subtypes has been associated with a poor patient outcome [10,99]. Our group has shown that 1,25(OH)2D3 downregulates the expression of myoepithelial/basal markers, such as P-cadherin, smooth muscle α-actin, and α6 and β4 integrins in a panel of breast carcinoma cells, and that Vdr−/− mice express higher levels of P-cadherin and smooth muscle α-actin in the mammary gland than wt littermates [100]. These results suggest that 1,25(OH)2D3/VDR antagonizes the Wnt/β-catenin pathway in breast cancer cells, which might protect against the triple-negative and basal-like phenotype. In line with this, 1,25(OH)2D3 induces DKK1 expression and reduces β-catenin transcriptional activity in R7 murine breast cancer cells, and Vdr deletion and 1,25(OH)2D3 treatment increases and inhibits, respectively, the tumor expression of several Wnt/β-catenin target genes in breast cancer mouse models [101,102]. The capacity of 1,25(OH)2D3 to inhibit spheroid formation by breast cancer stem cells is overcome by β-catenin overexpression, which suggests that inhibition of the Wnt/β-catenin pathway is essential for this action of 1,25(OH)2D3 [101]. Furthermore, Zheng and colleagues have reported that VDR overexpression in a stem cell-enriched subpopulation of MCF-7 breast cancer cells inhibits Wnt/β-catenin signaling and increases cell sensitivity to tamoxifen [103]. Surprisingly, however, in another study the stable knockdown of VDR expression leads to attenuation of the Wnt/β-catenin pathway in MDA-MB-231 breast cancer cells: cytoplasmic and nuclear levels of β-catenin are reduced with the subsequent downregulation of its target genes AXIN2, CCND1/cyclin D1, IL6, and IL8 [104].
Long non-coding RNA colon cancer-associated transcript 2 (CCAT2) is upregulated in ovarian cancer cells and promotes epithelial-mesenchymal transition (EMT) at least partially through the Wnt/β-catenin pathway. CCAT2 knockdown represses the expression of β-catenin and the activity of TCF/LEF factors and inhibits EMT by upregulating E-cadherin and downregulating N-cadherin, Snail1, and Twist1 [105]. Of note, 1,25(OH)2D3 inhibits CCAT2 expression in ovarian cancer cells concomitantly with a reduction in cell proliferation, migration, and invasion. This is linked to decreased binding of TCF4 to the c-MYC promoter and, thus, to repression of c-MYC protein expression [106]. Thus, inhibition of CCAT2 represents a novel mechanism of Wnt/β-catenin antagonism by 1,25(OH)2D3. In addition, Srivastava and colleagues have shown that 1,25(OH)2D3/VDR can deplete ovarian cancer stem cells via inhibition of the Wnt/β-catenin pathway [107].
A recent study has investigated whether 1,25(OH)2D3 can affect Wnt/β-catenin signaling in human uterine leiomyoma primary cells using a Wnt pathway PCR array. Up to 75% of the β-catenin/TCF target genes analyzed are repressed by 1,25(OH)2D3. Similarly, 1,25(OH)2D3 inhibits the expression of 73.3% and 77.2% of the Wnt-related genes involved in tissue polarity and cell migration, and in cell cycle, cell growth and proliferation, respectively [108]. These results suggest that not only Wnt/β-catenin but probably also Wnt non-canonical pathways are inhibited by 1,25(OH)2D3 in this cellular context.
1,25(OH)2D3 and its analogue TX527 increase β-catenin protein levels in the nucleus and at the plasma membrane in a Kaposi’s sarcoma cellular model and potentiate β-catenin/VDR interaction. The net outcome is downregulation of the β-catenin/TCF target genes c-MYC, MMP9 and CCND1/cyclin D1. Moreover, VE-cadherin protein and DKK1 RNA levels are increased [109]. As in Kaposi’s sarcoma cells, 1,25(OH)2D3 augments the level of total β-catenin (both cytoplasmic and nuclear pools), while it reduces that of phosphorylated β-catenin in renal cell carcinoma cells [110]. More importantly, 1,25(OH)2D3 enhances VDR/β-catenin interaction while attenuating β-catenin/TCF binding. Accordingly, 1,25(OH)2D3 downregulates the expression of CCND1/cyclin D1 and AXIN2 genes. In addition, 1,25(OH)2D3 upregulates E-cadherin expression and blocks TGFβ1-induced nuclear translocation of ZEB1, Snail1 and Twist1, which contributes to the suppression of EMT and the inhibition of cell migration and invasion [110]. Thus, the effects of 1,25(OH)2D3 on Kaposi’s sarcoma and renal cell carcinoma cells are largely in agreement with those observed on CRC cells. Distinctly, in pancreatic carcinoma cells, the 1,25(OH)2D3 analogue calcipotriol inhibits Wnt/β-catenin signaling by a different mechanism: the promotion of lysosomal degradation of the Wnt membrane receptor LRP6 [111].
Concomitant with an increase in Wnt/β-catenin signaling, global and epidermal-specific Vdr deletion predispose mice to either chemical [112] or long-term UVB-induced [113,114] skin tumor formation. 1,25(OH)2D3 enhances β-catenin binding to E-cadherin at the plasma membrane, which promotes epidermal cell differentiation. Moreover, VDR competes with LEF/TCF to recruit β-catenin to gene promoters [115,116] and both 1,25(OH)2D3 and VDR suppress β-catenin-stimulated LEF1/TCF-driven reporter activity [116,117]. The 1,25(OH)2D3 analogue EB1089 prevents the development of β-catenin-induced trichofolliculomas, while β-catenin activation in the absence of Vdr results in basal cell carcinomas [115]. Recently, Muralidhar and colleagues analyzed 703 primary melanoma transcriptomes and found that high tumor VDR expression is associated with upregulation of pathways mediating antitumor immunity and downregulation of proliferative pathways, notably Wnt/β-catenin [118]. Functional validation in vitro showed that 1,25(OH)2D3 inhibits the expression of Wnt/β-catenin pathway genes. These results suggest that 1,25(OH)2D3/VDR inhibits the pro-proliferative and immunosuppressive Wnt/β-catenin pathway in melanoma and that this is associated with less metastatic disease and stronger host immune responses [118].
Salehi-Tabar and colleagues have reported that VDR knockdown induces, while 1,25(OH)2D3 inhibits, β-catenin binding to and activation of c-MYC promoter in head and neck squamous cell carcinoma [119]. In this neoplasia, two vitamin D hydroxyderivatives, 20(OH)D3 and 1,20(OH)2D3, interfere with β-catenin nuclear translocation [120]. In a recent study, Rubin and colleagues analyzed the antitumor effects of 1,25(OH)2D3 and mitotane, the only chemotherapeutic agent available for adrenocortical carcinoma treatment. These authors reported a reduction in adrenocortical carcinoma cell growth and migration in response to either of the two agents, which is stronger when they are combined [121]. 1,25(OH)2D3 triggers a decrease in β-catenin RNA and nuclear protein levels, and both 1,25(OH)2D3 and mitotane induce RNA expression of the Wnt inhibitor DKK1, with a more marked effect with the combined treatment, although neither of them can reduce expression of the Wnt target gene c-MYC [121].
Vitamin D deficiency has been shown to promote hepatocellular carcinoma growth in Smad3+/- mice via upregulation of toll-like receptor 7 expression and β-catenin activation and, accordingly, vitamin D supplementation reduced β-catenin levels [122]. In contrast, Matsuda and colleagues reported that neither dietary supplements of vitamin D nor treatment with vitamin D analogues affect tumor formation or growth in a mouse model of hepatocarcinogenesis induced by mutant β-catenin and c-MET overexpression. Hence, they questioned the utility of vitamin D for hepatocellular carcinoma therapy in that setting [123].
In summary, available data show that 1,25(OH)2D3 and its analogues interfere with Wnt/β-catenin signaling in a variety of human solid tumors using mechanisms that mostly resemble those observed in CRC cells (Table 1).

Table 1.
Mechanisms of Wnt/β-catenin pathway interference by 1,25(OH)2D3 in solid cancers.
3. Antagonism of 1,25(OH)2D3/VDR Signaling by the Wnt/β-Catenin Pathway
The abovementioned data indicate that 1,25(OH)2D3 antagonizes Wnt/β-catenin signaling in several neoplasias. However, the interplay between both pathways is a two-way road, that is, activation of the Wnt/β-catenin pathway may also result in 1,25(OH)2D3/VDR inhibition.
VDR is the only high affinity receptor for 1,25(OH)2D3 and mediates most if not all 1,25(OH)2D3 effects. Thus, cellular VDR expression is the main determinant of 1,25(OH)2D3 action and its downregulation leads to 1,25(OH)2D3 unresponsiveness. VDR is expressed in most normal human cell types and tissues, but also in cancer cell lines and tumors of diverse origins. In line with the antitumor effects of 1,25(OH)2D3 observed in several neoplasias, high VDR expression in human cancer is usually a hallmark of good prognosis [29,31,36,37,124]. VDR expression and activity is regulated transcriptionally, posttranscriptionally by several microRNAs (miRs), and posttranslationally (via phosphorylation, ubiquitination, acetylation, and sumoylation) [125].
3.1. Repression of VDR by Snail Transcription Factors
Wnt/β-catenin signaling is known to promote EMT through upregulation of the expression and activity of key EMT transcription factors such as Snail1, Snail2, Zeb1 and Twist1 by several mechanisms [126,127]. Snail1 and Snail2 are phosphorylated by GSK-3β and tagged for β-TrCP-mediated ubiquitination and subsequent proteasomal degradation [128,129,130]. Thus, GSK-3β inhibition in response to Wnt/β-catenin signaling results in Snail1 and Snail2 protein stabilization. Inhibition of GSK-3β also increases SNAI1 transcription via NFκB activation [131]. Furthermore, the Wnt/β-catenin target gene AXIN2 contributes to Snail1 protein stabilization in breast cancer cells by regulating GSK-3β localization. When levels of AXIN2 increase in response to β-catenin/TCF signaling, GSK-3β is exported from the nuclear compartment leaving Snail1 in its non-phosphorylated transcriptionally active form [132]. In addition, induction of SNAI2 RNA levels by Wnt3 has been described in breast cancer cells [133].
Interestingly, Snail1 and Snail2 are the best-characterized transcriptional repressors of the human VDR gene. Our group demonstrated that Snail1 represses the expression of VDR by two mechanisms [83] (Figure 2). On the one hand, Snail1 inhibits VDR gene transcription by binding to three E-box sequences in its promoter. On the other, Snail1 reduces VDR RNA half-life. As a consequence, Snail1 strongly decreases the level of VDR RNA and protein and the cellular response to 1,25(OH)2D3 [83,84]. Moreover, forced expression of Snail1 in human CRC cells prevents the upregulation of E-cadherin and the subsequent cell differentiation triggered by 1,25(OH)2D3. Therefore, by repressing VDR and CDH1/E-cadherin genes, Snail1 abolishes 1,25(OH)2D3 action and favors the accumulation of β-catenin in the nucleus and the transcription of β-catenin/TCF target genes [83,84]. Later, Snail2 was found to also inhibit VDR gene expression in CRC cells through the same E-boxes in the promoter used by Snail1 (Figure 2). Actually, both transcription factors present an additive repressive effect on the VDR gene [134]. SNAI1 and/or SNAI2 upregulation is observed in 76% of human CRC and is associated with VDR downregulation [83,134,135,136]. Not surprisingly, the lowest VDR RNA levels are found in tumors with upregulation of both SNAI1 and SNAI2 genes [134]. VDR expression is also reduced in normal colonic tissue surrounding the tumor, which suggests that Snail1 expression in tumor cells promotes the secretion of factors that reduce VDR expression in neighboring normal cells [137]. In addition to CRC cells, Snail1 and Snail2 also repress VDR gene expression and antagonize the antitumor action of 1,25(OH)2D3 in human osteosarcoma and breast cancer cells [138,139]. Knackstedt and colleagues showed that downregulation of Vdr observed in the colon of a colitis mouse model is associated with an increase in the expression of Snail1 and Snail2 [140]. Altogether, these results support that activation of the Wnt/β-catenin pathway upregulates Snail1 and Snail2, which antagonizes 1,25(OH)2D3/VDR signaling by inhibiting VDR gene expression.
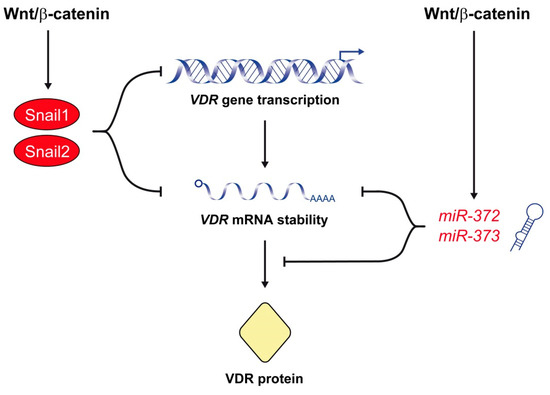
Figure 2.
The Wnt/β-catenin signaling pathway represses VDR expression. A major mechanism of this effect is the upregulation of Snail1 and Snail2, which repress VDR gene transcription and decrease VDR RNA half-life. The Wnt/β-catenin pathway also antagonizes 1,25(OH)2D3/VDR signaling by the upregulation of miR-372 and miR-373, which reduce the level of VDR RNA and protein.
3.2. Posttranscriptional Repression of VDR by miRNAs
A novel, recently described mechanism of Wnt/β-catenin-mediated antagonism of 1,25(OH)2D3/VDR signaling involves the miR-372/373 cluster (Figure 2). miR-372/373 expression is induced by β-catenin/TCF in several human cancer cell lines through three TCF/LEF binding sites located in its promoter region [141]. Accordingly, this cluster of stem cell-specific miRs is dysregulated in various cancers, particularly in CRC due to the constitutive activation of the Wnt/β-catenin pathway [142,143,144]. Wang and colleagues have shown that overexpression of miR-372/373 enhances the stemness of CRC cells and promotes their self-renewal, chemotherapy resistance and invasive potential [145]. These authors found that overexpression of miR-372/373 results in upregulation of stemness-related pathways, e.g., Nanog and Hedgehog and, conversely, downregulation of differentiation-related pathways, e.g., NFκB, MAPK/ERK, and VDR. Interestingly, they demonstrated that miR-372/373 overexpression leads to reduced expression of VDR RNA and protein in CRC cells, which contributes to the maintenance of the cancer stem cell phenotype [145]. These data suggest that the Wnt/β-catenin pathway also inhibits VDR expression through the induction of miR-372/373.
4. Conclusions
The Wnt/β-catenin pathway is frequently overactivated in cancer and promotes tumorigenesis, which makes it an attractive candidate for therapeutic intervention. The active vitamin D metabolite 1,25(OH)2D3, a major regulator of the human genome, cooperates with the Wnt/β-catenin pathway in the physiological control of tissues and organs such as bone, skin, and brain. Conversely, 1,25(OH)2D3 attenuates aberrant activation of the Wnt/β-catenin pathway that takes place in most CRC and in a variable proportion of other solid tumors. To do this, 1,25(OH)2D3 modulates a series of genes and mechanisms acting at different levels of the Wnt/β-catenin pathway that vary among cancer types. 1,25(OH)2D3 does not completely block the pathway but rather reduces its overactivation. This probably helps to maintain the physiological effects of Wnt/β-catenin in healthy organs, with few toxic side-effects. As expected from two crucial regulators of the organism and its necessary homeostasis, 1,25(OH)2D3 action is counterbalanced by Wnt factors.
The multilevel antagonistic action of 1,25(OH)2D3 on aberrantly activated Wnt/β-catenin signaling strongly supports the therapeutic utility of vitamin D compounds in cancer prevention and treatment.
Author Contributions
Conceptualization, A.M.; writing-original draft preparation, J.M.G.-S.; writing—review and editing, M.J.L. and A.M.; artwork, M.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work in the authors’ laboratory is funded by the Agencia Estatal de Investigación (PID2019-104867RB-I00/AEI/10.13039/501100011033), the Agencia Estatal de Investigación—Fondo Europeo de Desarrollo Regional (SAF2016-76377-R, MINECO/AEI/FEDER, EU), the Ministerio de Economía y Competitividad (SAF2017-90604-REDT/NuRCaMeIn), and the Instituto de Salud Carlos III—Fondo Europeo de Desarrollo Regional (CIBERONC; CB16/12/00273).
Acknowledgments
We thank Lucille Banham and Javier Pérez for their valuable assistance in the preparation of the English manuscript and the artwork, respectively.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish it.
References
- Nusse, R.; Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of beta-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Carmon, K.S.; Lin, Q.; Thomas, A.; Yi, J.; Liu, Q. LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor. PLoS ONE 2012, 7, e34739. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Chen, X.; Lin, Z.; Fang, D.; He, X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013, 27, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457. [Google Scholar] [CrossRef]
- De Lau, W.; Barker, N.; Low, T.Y.; Koo, B.-K.; Li, V.S.W.; Teunissen, H.; Kajula, P.; Haegebarth, A.; Peters, P.J.; van de Wetering, M.; et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signaling. Nature 2011, 476, 293–297. [Google Scholar] [CrossRef]
- Hao, H.X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef]
- Kobayashi, M.; Honma, T.; Matsuda, Y.; Suzuki, Y.; Narisawa, R.; Ajioka, Y.; Asakura, H. Nuclear translocation of beta-catenin in colorectal cancer. Br. J. Cancer 2000, 82, 1689–1693. [Google Scholar]
- Khramtsov, A.I.; Khramtsova, G.F.; Tretiakova, M.; Huo, D.; Olopade, O.I.; Goss, K.H. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010, 176, 2911–2920. [Google Scholar] [CrossRef]
- Damsky, W.E.; Curley, D.P.; Santhanakrishnan, M.; Rosenbaum, L.E.; Platt, J.T.; Gould Rothberg, B.E.; Taketo, M.M.; Dankort, D.; Rimm, D.L.; McMahon, M.; et al. beta-Catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 2011, 20, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Calvisi, D.F.; Ranganathan, S.; Cigliano, A.; Zhou, L.; Singh, S.; Jiang, L.; Fan, B.; Terracciano, L.; Armeanu-Ebinger, S.; et al. Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology 2014, 147, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Gekas, C.; D’Altri, T.; Aligué, R.; González, J.; Espinosa, L.; Bigas, A. beta-Catenin is required for T-cell leukemia initiation and MYC transcription downstream of Notch1. Leukemia 2016, 30, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008052. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Seshagiri, S.; Stawiski, E.W.; Durinck, S.; Modrusan, Z.; Storm, E.E.; Conboy, C.B.; Chaudhuri, S.; Guan, Y.; Janakiraman, V.; Jaiswal, B.S.; et al. Recurrent R-spondin fusions in colon cancer. Nature 2012, 488, 660–664. [Google Scholar] [CrossRef]
- Han, T.; Schatoff, E.M.; Murphy, C.; Zafra, M.P.; Wilkinson, J.E.; Elemento, O.; Dow, L.E. R-Spondin chromosome rearrangements drive Wnt-dependent tumour initiation and maintenance in the intestine. Nat. Commun. 2017, 8, 15945. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Jeannot, E.; Van Nhieu, J.T.; Scoazec, J.-Y.; Guettier, C.; Rebouissou, S.; Bacq, Y.; Leteurtre, E.; Paradis, V.; Michalak, S.; et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology 2006, 43, 515–524. [Google Scholar] [CrossRef]
- Austinat, M.; Dunsch, R.; Wittekind, C.; Tannapfel, A.; Gebhardt, R.; Gaunitz, F. Correlation between beta-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol. Cancer 2008, 7, 21. [Google Scholar] [CrossRef]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Brown, A.M. Wnt signaling and breast cancer. Cancer Biol. Ther. 2004, 3, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Klarmann, G.J.; Decker, A.; Farrar, W. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics 2008, 3, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Prior, J.; Piwnica-Worms, D.; Bu, G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 5136–5141. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Wang, Y.; Zhang, K.; Wu, J.; Yuan, Y.C.; Deng, X.; Chen, L.; Kim, C.C.; Lau, S.; et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 2011, 30, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Sanchez-Rivera, F.J.; Cetinbas, N.M.; Wu, K.; Joshi, N.S.; Helenius, K.; Park, Y.; Azimi, R.; Kerper, N.R.; Wesselhoeft, R.A.; et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017, 545, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, E.H.; van Amerongen, R. Aberrant WNT/CTNNB1 signaling as a therapeutic target in human breast cancer: Weighing the evidence. Front. Cell Dev. Biol. 2020, 8, 25. [Google Scholar] [CrossRef]
- Holick, M.F.; Frommer, J.E.; McNeill, S.C.; Richtand, N.M.; Henley, J.W.; Potts, J.T., Jr. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 1977, 76, 107–114. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Molnár, F.; Peräkylä, M.; Carlberg, C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. J. Biol. Chem. 2006, 281, 10516–10526. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Morán, P.; Larriba, M.J.; Pálmer, H.G.; Valero, R.A.; Barbáchano, A.; Dunach, M.; García de Herreros, A.; Villalobos, C.; Berciano, M.T.; Lafarga, M.; et al. RhoA—ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J. Cell Biol. 2008, 183, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Fernández-Barral, A.; Bustamante-Madrid, P.; Ferrer-Mayorga, G.; Barbáchano, A.; Larriba, M.J.; Muñoz, A. Vitamin D effects on cell differentiation and stemness in cancer. Cancers 2020, 12, 2413. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 65–71. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Calderwood, A.H.; Baron, J.A.; Mott, L.A.; Ahnen, D.J.; Bostick, R.M.; Figueiredo, J.C.; Passarelli, M.N.; Rees, J.R.; Robertson, D.J.; Barry, E.L. No evidence for posttreatment effects of vitamin D and calcium supplementation on risk of colorectal adenomas in a randomized trial. Cancer Prev. Res. 2019, 12, 295–304. [Google Scholar] [CrossRef]
- Markotic, A.; Langer, S.; Kelava, T.; Vucic, K.; Turcic, P.; Tokic, T.; Stefancic, L.; Radetic, E.; Farrington, S.; Timofeeva, M.; et al. Higher post-operative serum vitamin D level is associated with better survival outcome in colorectal cancer patients. Nutr. Cancer 2019, 71, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The SUNSHINE randomized clinical trial. J. Am. Med. Assoc. 2019, 321, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.; Malki, A. Vitamin D signaling in inflammation and cancer: Molecular mechanisms and therapeutic implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-induced molecular mechanisms to potentiate cancer therapy and to reverse drug-resistance in cancer cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef] [PubMed]
- Pálmer, H.G.; González-Sancho, J.M.; Espada, J.; Berciano, M.T.; Puig, I.; Baulida, J.; Quintanilla, M.; Cano, A.; García de Herreros, A.; Lafarga, M.; et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001, 154, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, V.; Pishvaian, M.; Salimuddin; Byers, S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr. Biol. 1999, 9, 1415–1418. [Google Scholar] [CrossRef]
- Truica, C.I.; Byers, S.; Gelmann, E.P. beta-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000, 60, 4709–4713. [Google Scholar]
- Shah, S.; Islam, M.N.; Dakshanamurthy, S.; Rizvi, I.; Rao, M.; Herrell, R.; Zinser, G.; Valrance, M.; Aranda, A.; Moras, D.; et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol. Cell 2006, 21, 799–809. [Google Scholar] [CrossRef]
- Egan, J.B.; Thompson, P.A.; Vitanov, M.V.; Bartik, L.; Jacobs, E.T.; Haussler, M.R.; Gerner, E.W.; Jurutka, P.W. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta-catenin activity in colon cancer cells. Mol. Carcinog. 2010, 49, 337–352. [Google Scholar] [CrossRef]
- Álvarez-Díaz, S.; Valle, N.; García, J.M.; Peña, C.; Freije, J.M.; Quesada, V.; Astudillo, A.; Bonilla, F.; López-Otín, C.; Muñoz, A. Cystatin D is a candidate tumor suppressor gene induced by vitamin D in human colon cancer cells. J. Clin. Investig. 2009, 119, 2343–2358. [Google Scholar] [CrossRef]
- Barbáchano, A.; Ordóñez-Morán, P.; García, J.M.; Sánchez, A.; Pereira, F.; Larriba, M.J.; Martínez, N.; Hernández, J.; Landolfi, S.; Bonilla, F.; et al. SPROUTY-2 and E-cadherin regulate reciprocally and dictate colon cancer cell tumourigenicity. Oncogene 2010, 29, 4800–4813. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Barbáchano, A.; Silva, J.; Bonilla, F.; Campbell, M.J.; Muñoz, A.; Larriba, M.J. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum. Mol. Genet. 2011, 20, 4655–4665. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; McCann, M.; Zhang, Z.; Posner, G.H.; Bingham, V.; El-Tanani, M.; Campbell, F.C. Vitamin D receptor modulates the neoplastic phenotype through antagonistic growth regulatory signals. Mol. Carcinog. 2009, 48, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Peña, C.; García, J.M.; Larriba, M.J.; Ordóñez-Morán, P.; Navarro, D.; Barbáchano, A.; López de Silanes, I.; Ballestar, E.; Fraga, M.F.; et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007, 28, 1877–1884. [Google Scholar] [CrossRef]
- Semenov, M.V.; Tamai, K.; Brott, B.K.; Kuhl, M.; Sokol, S.; He, X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Mao, B.; Wu, W.; Davidson, G.; Marhold, J.; Li, M.; Mechler, B.M.; Delius, H.; Hoppe, D.; Stannek, P.; Walter, C.; et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 2002, 417, 664–667. [Google Scholar] [CrossRef]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef]
- Chamorro, M.N.; Schwartz, D.R.; Vonica, A.; Brivanlou, A.H.; Cho, K.R.; Varmus, H.E. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005, 24, 73–84. [Google Scholar] [CrossRef]
- González-Sancho, J.M.; Aguilera, O.; García, J.M.; Pendás-Franco, N.; Peña, C.; Cal, S.; García de Herreros, A.; Bonilla, F.; Muñoz, A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene 2005, 24, 1098–1103. [Google Scholar] [CrossRef]
- Lee, A.Y.; He, B.; You, L.; Xu, Z.; Mazieres, J.; Reguart, N.; Mikami, I.; Batra, S.; Jablons, D.M. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem. Biophys. Res. Commun. 2004, 323, 1246–1250. [Google Scholar] [CrossRef]
- Mikheev, A.M.; Mikheeva, S.A.; Liu, B.; Cohen, P.; Zarbl, H. A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf-1 as suppressor of HeLa cell transformation. Carcinogenesis 2004, 25, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Fraga, M.F.; Ballestar, E.; Paz, M.F.; Herranz, M.; Espada, J.; García, J.M.; Muñoz, A.; Esteller, M.; González-Sancho, J.M. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 2006, 25, 4116–4121. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Suzuki, H.; Toyota, M.; Nojima, M.; Maruyama, R.; Sasaki, S.; Takagi, H.; Sogabe, Y.; Sasaki, Y.; Idogawa, M.; et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis 2007, 28, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.B.; Manno, M.; Mrkonjic, M.; Daftary, D.; Dicks, E.; Buchanan, D.D.; Younghusband, H.B.; Parfrey, P.S.; Young, J.P.; Pollett, A.; et al. Promoter methylation of Wnt antagonists DKK1 and SFRP1 is associated with opposing tumor subtypes in two large populations of colorectal cancer patients. Carcinogenesis 2011, 32, 741–747. [Google Scholar] [CrossRef]
- Rawson, J.B.; Sun, Z.; Dicks, E.; Daftary, D.; Parfrey, P.S.; Green, R.C.; Gallinger, S.; McLaughlin, J.R.; Wang, P.P.; Knight, J.A.; et al. Vitamin D intake is negatively associated with promoter methylation of the Wnt antagonist gene DKK1 in a large group of colorectal cancer patients. Nutr. Cancer 2012, 64, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Pendás-Franco, N.; García, J.M.; Peña, C.; Valle, N.; Pálmer, H.G.; Heinaniemi, M.; Carlberg, C.; Jiménez, B.; Bonilla, F.; Muñoz, A.; et al. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene 2008, 27, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Nguyen, A.V.; Albers, C.G.; Lin, F.; Holcombe, R.F. Wnt pathway-related gene expression in inflammatory bowel disease. Dig. Dis. Sci. 2008, 53, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Yamaguchi, T.; Maekawa, S.; Miyazaki, C.; Takano, S.; Uetake, T.; Inoue, T.; Otaka, M.; Otsuka, H.; Sato, T.; et al. DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer. Cancer Sci. 2009, 100, 1923–1930. [Google Scholar] [CrossRef]
- Hsieh, A.L.; Walton, Z.E.; Altman, B.J.; Stine, Z.E.; Dang, C.V. MYC and metabolism on the path to cancer. Semin. Cell Dev. Biol. 2015, 43, 11–21. [Google Scholar] [CrossRef]
- Wolf, E.; Eilers, M. Targeting MYC proteins for tumor therapy. Annu. Rev. Cancer Biol. 2020, 4, 61–75. [Google Scholar] [CrossRef]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: Impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012, 26, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Toropainen, S.; Väisänen, S.; Heikkinen, S.; Carlberg, C. The down-regulation of the human MYC gene by the nuclear hormone 1alpha,25-dihydroxyvitamin D3 is associated with cycling of corepressors and histone deacetylases. J. Mol. Biol. 2010, 400, 284–294. [Google Scholar] [PubMed]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Beildeck, M.E.; Islam, M.; Shah, S.; Welsh, J.; Byers, S.W. Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS ONE 2009, 4, e7872. [Google Scholar]
- Tang, W.; Dodge, M.; Gundapaneni, D.; Michnoff, C.; Roth, M.; Lum, L. A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 9697–9702. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhang, Y.G.; Wu, S.; Lu, R.; Lin, Z.; Zheng, Y.; Chen, H.; Cs-Szabo, G.; Sun, J. Vitamin D receptor is a novel transcriptional regulator for Axin1. J. Steroid Biochem. Mol. Biol. 2017, 165, 430–437. [Google Scholar]
- Groschel, C.; Aggarwal, A.; Tennakoon, S.; Hobaus, J.; Prinz-Wohlgenannt, M.; Marian, B.; Heffeter, P.; Berger, W.; Kallay, E. Effect of 1,25-dihydroxyvitamin D3 on the Wnt pathway in non-malignant colonic cells. J. Steroid Biochem. Mol. Biol. 2016, 155, 224–230. [Google Scholar] [CrossRef]
- Kaler, P.; Augenlicht, L.; Klampfer, L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: A crosstalk interrupted by vitamin D3. Oncogene 2009, 28, 3892–3902. [Google Scholar] [CrossRef]
- Ferrer-Mayorga, G.; Niell, N.; Cantero, R.; González-Sancho, J.M.; Del Peso, L.; Muñoz, A.; Larriba, M.J. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci. Rep. 2019, 9, 8085. [Google Scholar]
- Fernández-Barral, A.; Costales-Carrera, A.; Buira, S.P.; Jung, P.; Ferrer-Mayorga, G.; Larriba, M.J.; Bustamante-Madrid, P.; Domínguez, O.; Real, F.X.; Guerra-Pastrian, L.; et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020, 287, 53–72. [Google Scholar] [CrossRef]
- Larriba, M.J.; González-Sancho, J.M.; Barbáchano, A.; Niell, N.; Ferrer-Mayorga, G.; Muñoz, A. Vitamin D is a multilevel repressor of Wnt/beta-catenin signaling in cancer cells. Cancers 2013, 5, 1242–1260. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Oliva, R.; Geribaldi-Doldán, N.; Domínguez-García, S.; Carrascal, L.; Verástegui, C.; Nunez-Abades, P.; Castro, C. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: A role for Wnt/beta-catenin signaling. Aging 2020, 12, 13824–13844. [Google Scholar] [CrossRef] [PubMed]
- Pálmer, H.G.; Larriba, M.J.; García, J.M.; Ordóñez-Morán, P.; Peña, C.; Peiró, S.; Puig, I.; Rodríguez, R.; de la Fuente, R.; Bernad, A.; et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat. Med. 2004, 10, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.J.; Valle, N.; Pálmer, H.G.; Ordóñez-Morán, P.; Alvarez-Díaz, S.; Becker, K.F.; Gamallo, C.; de García de Herreros, A.; González-Sancho, J.M.; Muñoz, A. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr. Relat. Cancer 2007, 14, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wali, R.K.; Khare, S.; Tretiakova, M.; Cohen, G.; Nguyen, L.; Hart, J.; Wang, J.; Wen, M.; Ramaswamy, A.; Joseph, L.; et al. Ursodeoxycholic acid and F6-D3 inhibit aberrant crypt proliferation in the rat azoxymethane model of colon cancer: Roles of cyclin D1 and E-cadherin. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1653–1662. [Google Scholar]
- Fichera, A.; Little, N.; Dougherty, U.; Mustafi, R.; Cerda, S.; Li, Y.C.; Delgado, J.; Arora, A.; Campbell, L.K.; Joseph, L.; et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J. Surg. Res. 2007, 142, 239–245. [Google Scholar] [CrossRef]
- Xu, H.; Posner, G.H.; Stevenson, M.; Campbell, F.C. ApcMIN modulation of vitamin D secosteroid growth control. Carcinogenesis 2010, 31, 1434–1441. [Google Scholar] [CrossRef]
- Yang, K.; Yang, W.; Mariadason, J.; Velcich, A.; Lipkin, M.; Augenlicht, L. Dietary components modify gene expression: Implications for carcinogenesis. J. Nutr. 2005, 135, 2710–2714. [Google Scholar] [CrossRef]
- Yang, K.; Kurihara, N.; Fan, K.; Newmark, H.; Rigas, B.; Bancroft, L.; Corner, G.; Livote, E.; Lesser, M.; Edelmann, W.; et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008, 68, 7803–7810. [Google Scholar] [CrossRef]
- Wang, D.; Peregrina, K.; Dhima, E.; Lin, E.Y.; Mariadason, J.M.; Augenlicht, L.H. Paneth cell marker expression in intestinal villi and colon crypts characterizes dietary induced risk for mouse sporadic intestinal cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 10272–10277. [Google Scholar] [CrossRef]
- Larriba, M.J.; Ordóñez-Morán, P.; Chicote, I.; Martín-Fernández, G.; Puig, I.; Muñoz, A.; Pálmer, H.G. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS ONE 2011, 6, e23524. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wong, K.E.; Zhang, Z.; Dougherty, U.; Mustafi, R.; Kong, J.; Deb, D.K.; Zheng, H.; Bissonnette, M.; Li, Y.C. Inactivation of the vitamin D receptor in APCmin/+ mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int. J. Cancer 2012, 130, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Bostick, R.M.; Flanders, W.D.; Long, Q.; Sidelnikov, E.; Shaukat, A.; Daniel, C.R.; Rutherford, R.E.; Woodard, J.J. Effects of vitamin D and calcium on proliferation and differentiation in normal colon mucosa: A randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, T.U.; Shaukat, A.; Flanders, W.D.; Rutherford, R.E.; Bostick, R.M. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on the APC/beta-catenin pathway in the normal mucosa of colorectal adenoma patients. Cancer Prev. Res. 2012, 5, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Bostick, R.M. Effects of supplemental vitamin D and calcium on normal colon tissue and circulating biomarkers of risk for colorectal neoplasms. J. Steroid Biochem. Mol. Biol. 2015, 148, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Barry, E.L.; Baron, J.A.; Rutherford, R.E.; Seabrook, M.E.; Bostick, R.M. Effects of supplemental calcium and vitamin D on the APC/beta-catenin pathway in the normal colorectal mucosa of colorectal adenoma patients. Mol. Carcinog. 2017, 56, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Cabrera-Mulero, A.; Hernández-Alonso, P.; Clemente-Postigo, M.; Casanueva, F.F.; Tinahones, F.J.; Morcillo, S.; Crujeiras, A.B.; Macias-Gonzalez, M. Association between variation of circulating 25-OH vitamin D and methylation of secreted frizzled-related protein 2 in colorectal cancer. Clin. Epigenetics 2020, 12, 83. [Google Scholar] [CrossRef]
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef]
- Geyer, F.C.; Lacroix-Triki, M.; Savage, K.; Arnedos, M.; Lambros, M.B.; MacKay, A.; Natrajan, R.; Reis-Filho, J.S. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 2011, 24, 209–231. [Google Scholar] [CrossRef]
- Pendás-Franco, N.; González-Sancho, J.M.; Suarez, Y.; Aguilera, O.; Steinmeyer, A.; Gamallo, C.; Berciano, M.T.; Lafarga, M.; Muñoz, A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation 2007, 75, 193–207. [Google Scholar] [CrossRef]
- Jeong, Y.; Swami, S.; Krishnan, A.V.; Williams, J.D.; Martin, S.; Horst, R.L.; Albertelli, M.A.; Feldman, B.J.; Feldman, D.; Diehn, M. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Mol. Cancer Ther. 2015, 14, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Zinser, G.M.; Waltz, S.E. Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of beta-catenin activity. Oncotarget 2015, 6, 16304–16320. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Duan, B.; Zhang, Q.; Ouyang, L.; Peng, W.; Qian, F.; Wang, Y.; Huang, S. Vitamin D-induced vitamin D receptor expression induces tamoxifen sensitivity in MCF-7 stem cells via suppression of Wnt/beta-catenin signaling. Biosci. Rep. 2018, 38, BSR20180595. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Trivedi, T.; Lin, R.C.; Fong-Yee, C.; Nolte, R.; Manibo, J.; Chen, Y.; Hossain, M.; Horas, K.; Dunstan, C.; et al. Loss of the vitamin D receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of Wnt/beta-catenin signaling. Bone Res. 2017, 5, 17023. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, M.; Zhuang, R.; Jiang, J.; Gao, J.; Wang, H.; Chen, H.; Zhang, Z.; Kuang, Y.; Li, P. Long non-coding RNA CCAT2 promotes epithelial-mesenchymal transition involving Wnt/beta-catenin pathway in epithelial ovarian carcinoma cells. Oncol. Lett. 2018, 15, 3369–3375. [Google Scholar] [PubMed]
- Wang, L.; Zhou, S.; Guo, B. Vitamin D suppresses ovarian cancer growth and invasion by targeting long non-coding RNA CCAT2. Int. J. Mol. Sci. 2020, 21, 2334. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Rizvi, A.; Cui, T.; Han, C.; Banerjee, A.; Naseem, I.; Zheng, Y.; Wani, A.A.; Wang, Q.E. Depleting ovarian cancer stem cells with calcitriol. Oncotarget 2018, 9, 14481–14491. [Google Scholar] [CrossRef]
- Corachán, A.; Ferrero, H.; Aguilar, A.; Garcia, N.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/beta-catenin pathway. Fertil. Steril. 2019, 111, 397–407. [Google Scholar] [CrossRef]
- Tapia, C.; Suares, A.; De Genaro, P.; Gonzalez-Pardo, V. In vitro studies revealed a downregulation of Wnt/beta-catenin cascade by active vitamin D and TX 527 analog in a Kaposi’s sarcoma cellular model. Toxicol. In Vitro 2020, 63, 104748. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.H.; Fu, L.; Song, J.; Xie, D.D.; Yu, D.X.; Xu, D.X.; Sun, G.P. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and beta-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020, 111, 59–71. [Google Scholar] [CrossRef]
- Arensman, M.D.; Nguyen, P.; Kershaw, K.M.; Lay, A.R.; Ostertag-Hill, C.A.; Sherman, M.H.; Downes, M.; Liddle, C.; Evans, R.M.; Dawson, D.W. Calcipotriol targets LRP6 to inhibit Wnt signaling in pancreatic cancer. Mol. Cancer Res. 2015, 13, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Zinser, G.M.; Sundberg, J.P.; Welsh, J. Vitamin D3 receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 2002, 23, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Ellison, T.I.; Smith, M.K.; Gilliam, A.C.; MacDonald, P.N. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J. Investig. Dermatol. 2008, 128, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Teichert, A.E.; Elalieh, H.; Elias, P.M.; Welsh, J.; Bikle, D.D. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Investig. Dermatol. 2011, 131, 2289–2297. [Google Scholar] [CrossRef]
- Pálmer, H.G.; Anjos-Afonso, F.; Carmeliet, G.; Takeda, H.; Watt, F.M. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE 2008, 3, e1483. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Teichert, A.E.; Fong, F.; Oda, Y.; Bikle, D.D. 1alpha,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the beta-catenin pathway. J. Steroid Biochem. Mol. Biol. 2013, 136, 229–232. [Google Scholar] [CrossRef]
- Bikle, D.D.; Oda, Y.; Tu, C.L.; Jiang, Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J. Steroid Biochem. Mol. Biol. 2015, 148, 47–51. [Google Scholar] [CrossRef]
- Muralidhar, S.; Filia, A.; Nsengimana, J.; Poźniak, J.; O’Shea, S.J.; Diaz, J.M.; Harland, M.; Randerson-Moor, J.A.; Reichrath, J.; Laye, J.P.; et al. Vitamin D-VDR signaling inhibits Wnt/beta-catenin-mediated melanoma progression and promotes antitumor immunity. Cancer Res. 2019, 79, 5986–5998. [Google Scholar] [CrossRef]
- Salehi-Tabar, R.; Nguyen-Yamamoto, L.; Tavera-Mendoza, L.E.; Quail, T.; Dimitrov, V.; An, B.S.; Glass, L.; Goltzman, D.; White, J.H. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. USA 2012, 109, 18827–18832. [Google Scholar] [CrossRef]
- Oak, A.S.W.; Bocheva, G.; Kim, T.K.; Brożyna, A.A.; Janjetovic, Z.; Athar, M.; Tuckey, R.C.; Slominski, A.T. Noncalcemic vitamin D hydroxyderivatives inhibit human oral squamous cell carcinoma and down-regulate Hedgehog and WNT/beta-catenin pathways. Anticancer Res. 2020, 40, 2467–2474. [Google Scholar] [CrossRef]
- Rubin, B.; Pilon, C.; Pezzani, R.; Rebellato, A.; Fallo, F. The effects of mitotane and 1alpha,25-dihydroxyvitamin D3 on Wnt/beta-catenin signaling in human adrenocortical carcinoma cells. J. Endocrinol. Investig. 2020, 43, 357–367. [Google Scholar] [CrossRef]
- Chen, J.; Katz, L.H.; Muñoz, N.M.; Gu, S.; Shin, J.H.; Jogunoori, W.S.; Lee, M.H.; Belkin, M.D.; Kim, S.B.; White, J.C.; et al. Vitamin D deficiency promotes liver tumor growth in transforming growth factor-beta/smad3-deficient mice through Wnt and Toll-like receptor 7 pathway modulation. Sci. Rep. 2016, 6, 30217. [Google Scholar] [CrossRef]
- Matsuda, A.; Ishiguro, K.; Yan, I.K.; Patel, T. Therapeutic efficacy of vitamin D in experimental c-MET-beta-catenin-driven hepatocellular cancer. Gene Expr. 2019, 19, 151–159. [Google Scholar] [CrossRef]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Zenata, O.; Vrzal, R. Fine tuning of vitamin D receptor (VDR) activity by post-transcriptional and post-translational modifications. Oncotarget 2017, 8, 35390–35402. [Google Scholar] [CrossRef] [PubMed]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Yook, J.I.; Li, X.Y.; Ota, I.; Fearon, E.R.; Weiss, S.J. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 2005, 280, 11740–11748. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Li, X.-Y.; Hu, C.Y.; Ford, M.; Kleer, C.G.; Weiss, S.J. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc. Natl. Acad. Sci. USA 2012, 109, 16654–16659. [Google Scholar] [CrossRef]
- Bachelder, R.E.; Yoon, S.O.; Francí, C.; García de Herreros, A.; Mercurio, A.M. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: Implications for the epithelial-mesenchymal transition. J. Cell Biol. 2005, 168, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.I.; Li, X.Y.; Ota, I.; Hu, C.; Kim, H.S.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 activates Wnt/beta-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.J.; Martín-Villar, E.; García, J.M.; Pereira, F.; Peña, C.; García de Herreros, A.; Bonilla, F.; Muñoz, A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis 2009, 30, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; García, J.M.; Silva, J.; García, V.; Rodríguez, R.; Alonso, I.; Millán, I.; Salas, C.; García de Herreros, A.; Muñoz, A.; et al. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: Clinicopathological correlations. Hum. Mol. Genet. 2005, 14, 3361–3370. [Google Scholar] [CrossRef]
- Peña, C.; García, J.M.; García, V.; Silva, J.; Domínguez, G.; Rodríguez, R.; Maximiano, C.; García de Herreros, A.; Muñoz, A.; Bonilla, F. The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int. J. Cancer 2006, 119, 2098–2104. [Google Scholar] [CrossRef]
- Peña, C.; García, J.M.; Larriba, M.J.; Barderas, R.; Gómez, I.; Herrera, M.; García, V.; Silva, J.; Domínguez, G.; Rodríguez, R.; et al. SNAI1 expression in colon cancer related with CDH1 and VDR downregulation in normal adjacent tissue. Oncogene 2009, 28, 4375–4385. [Google Scholar] [CrossRef][Green Version]
- Mittal, M.K.; Myers, J.N.; Misra, S.; Bailey, C.K.; Chaudhuri, G. In vivo binding to and functional repression of the VDR gene promoter by SLUG in human breast cells. Biochem. Biophys. Res. Commun. 2008, 372, 30–34. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Zhou, Z.; Jiang, X.; Shen, A. Snail-1 regulates VDR signaling and inhibits 1,25(OH)-D3 action in osteosarcoma. Eur. J. Pharmacol. 2011, 670, 341–346. [Google Scholar] [CrossRef]
- Knackstedt, R.W.; Moseley, V.R.; Sun, S.; Wargovich, M.J. Vitamin D receptor and retinoid X receptor α status and vitamin D insufficiency in models of murine colitis. Cancer Prev. Res. 2013, 6, 585–593. [Google Scholar] [CrossRef][Green Version]
- Zhou, A.D.; Diao, L.T.; Xu, H.; Xiao, Z.D.; Li, J.H.; Zhou, H.; Qu, L.H. beta-Catenin/LEF1 transactivates the microRNA-371-373 cluster that modulates the Wnt/beta-catenin-signaling pathway. Oncogene 2012, 31, 2968–2978. [Google Scholar] [CrossRef] [PubMed]
- Loayza-Puch, F.; Yoshida, Y.; Matsuzaki, T.; Takahashi, C.; Kitayama, H.; Noda, M. Hypoxia and RAS-signaling pathways converge on, and cooperatively downregulate, the RECK tumor-suppressor protein through microRNAs. Oncogene 2010, 29, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Yamamoto, H.; Mimori, K.; Nishida, N.; Takahashi, H.; Haraguchi, N.; Tanaka, F.; Shibata, K.; Sekimoto, M.; Ishii, H.; et al. MicroRNA-372 is associated with poor prognosis in colorectal cancer. Oncology 2012, 82, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Eyking, A.; Reis, H.; Frank, M.; Gerken, G.; Schmid, K.W.; Cario, E. MiR-205 and miR-373 are associated with aggressive human mucinous colorectal cancer. PLoS ONE 2016, 11, e0156871. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Yu, P.; Li, B.; Guo, Y.H.; Liang, Z.R.; Zheng, L.L.; Yang, J.H.; Xu, H.; Liu, S.; Zheng, L.S.; et al. miR-372 and miR-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol. Oncol. 2018, 12, 1949–1964. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).