Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Colorectal Cancer Cohort
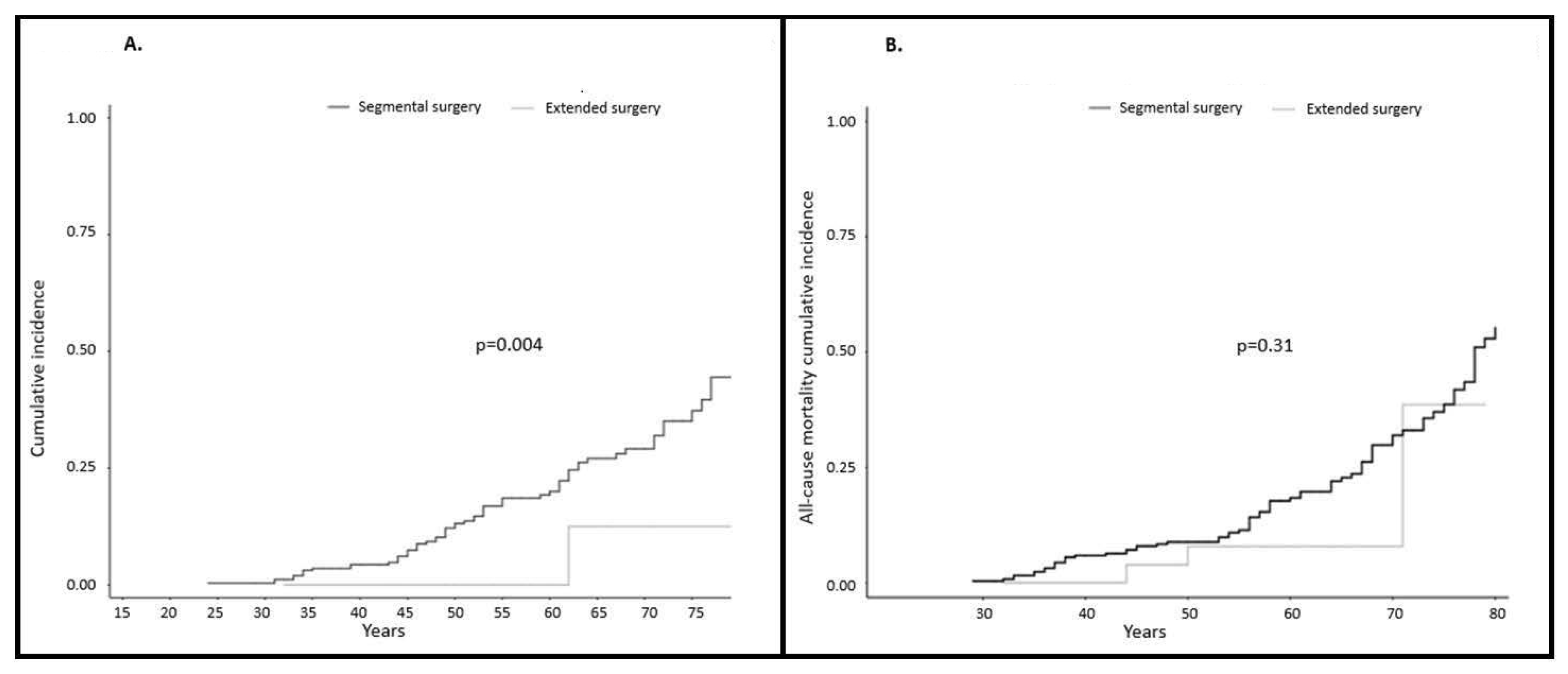
2.1.1. Incidence of Colorectal Cancer
2.1.2. Colorectal Cancer Mortality
2.2. Gynecological Cancer Cohort
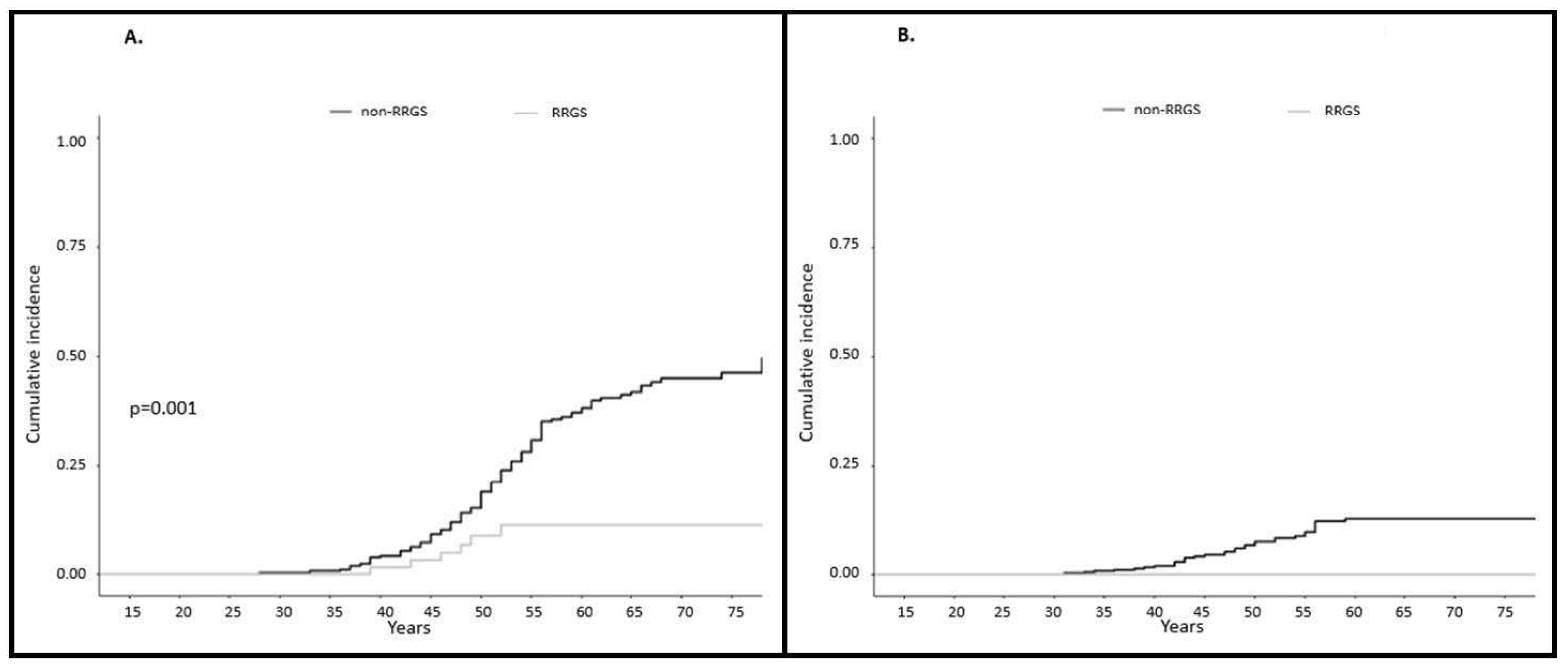
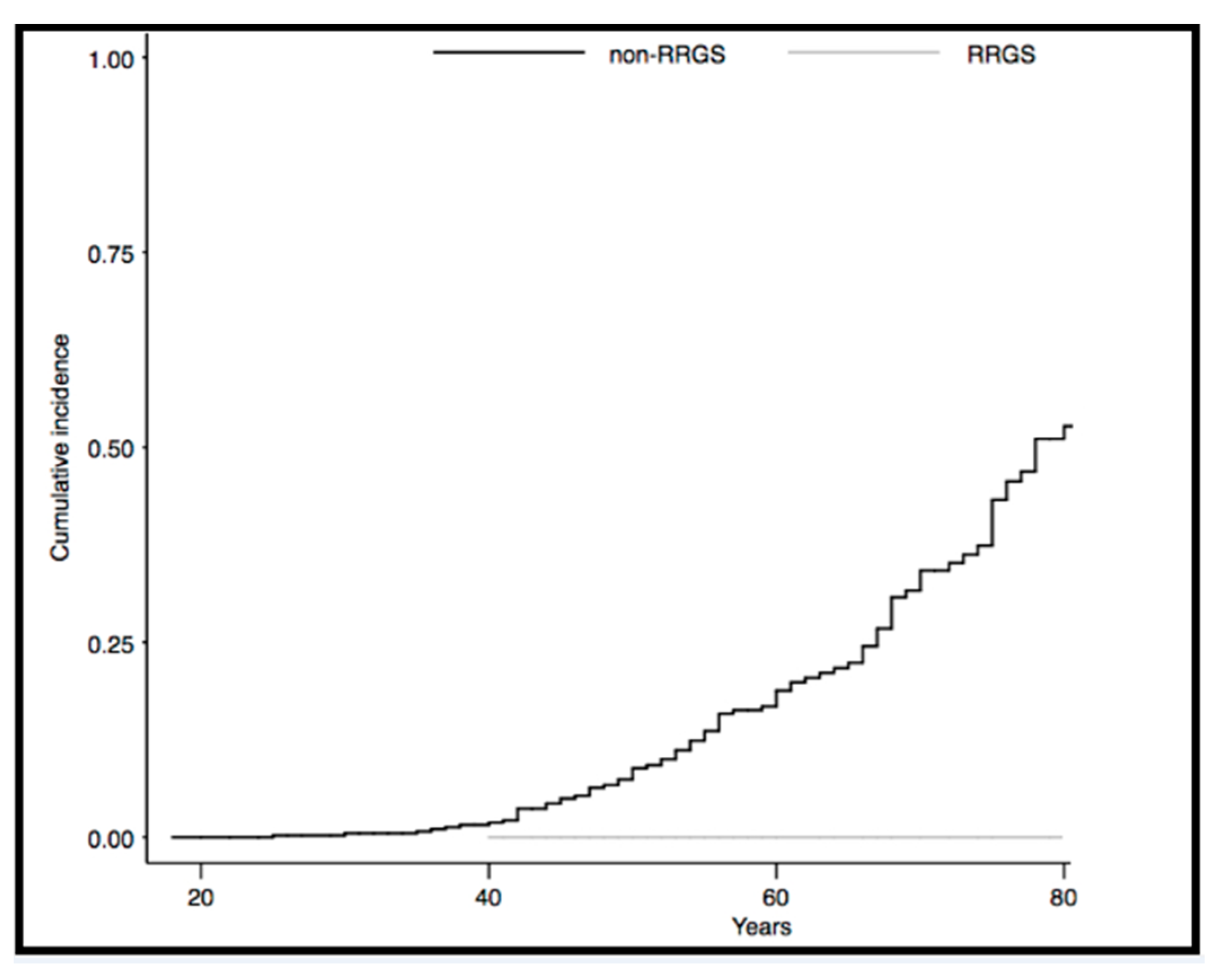
2.2.1. Incidence of Gynecological Cancer
2.2.2. Gynecological Cancer Mortality
3. Discussion
4. Materials and Methods
4.1. Study Sample
4.2. Data Collection
4.3. Colorectal Cancer Cohort
4.4. Gynecologicall Cancer Cohort
4.5. Definitions
4.6. Mutation Testing
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Moreira, L.; Balaguer, F.; Lindor, N.; De La Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of Lynch Syndrome Among Patients with Colorectal Cancer. JAMA 2012, 308, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Broeke, S.W.T.; Be, J.-P.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Correction: Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef] [PubMed]
- ten Broeke, S.W.; Van Der Klift, H.M.; Tops, C.M.; Aretz, S.; Bernstein, I.; Buchanan, D.D.; De La Chapelle, A.; Capella, G.; Clendenning, M.; Engel, C.; et al. Cancer Risks for PMS2-Associated Lynch Syndrome. J. Clin. Oncol. 2018, 36, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: A report from the prospective Lynch syndrome database. Gut 2017, 66, 1657–1664. [Google Scholar] [CrossRef]
- Vasen, H.; Abdirahman, M.; Brohet, R.; Langers, A.M.J.; Kleibeuker, J.H.; Van Kouwen, M.; Koornstra, J.J.; Boot, H.; Cats, A.; Dekker, E.; et al. One to 2-Year Surveillance Intervals Reduce Risk of Colorectal Cancer in Families with Lynch Syndrome. Gastroenterology 2010, 138, 2300–2306. [Google Scholar] [CrossRef]
- Järvinen, H.J.; Aarnio, M.; Mustonen, H.; Aktan–Collan, K.; Aaltonen, L.A.; Peltomäki, P.; De La Chapelle, A.; Mecklin, J.P. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000, 118, 829–834. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Ahadova, A.; Dominguez-Valentin, M.; Macrae, F.; Evans, D.G.; Therkildsen, C.; Sampson, J.; Scott, R.; Burn, J.; Möslein, G.; et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered. Cancer Clin. Pract. 2019, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, T.; in collaboration with The Mallorca Group; Pylvänäinen, K.; Evans, D.G.; Järvinen, H.; Renkonen-Sinisalo, L.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Lindblom, A.; et al. Colorectal cancer incidence in path_MLH1 carriers subjected to different follow-up protocols: A Prospective Lynch Syndrome Database report. Hered. Cancer Clin. Pract. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Vasen, H.F.; Seppälä, T.; Aretz, S.; Bigirwamungu-Bargeman, M.; De Boer, S.Y.; Bucksch, K.; Büttner, R.; Holinski-Feder, E.; Holzapfel, S.; et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy among 3 Countries with Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018, 155, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Seppälä, T.T.; Sampson, J.R.; Macrae, F.; Winship, I.; Evans, D.G.; Scott, R.J.; Burn, J.; Möslein, G.; Bernstein, I.; et al. Survival by colon cancer stage and screening interval in Lynch syndrome: A prospective Lynch syndrome database report. Hered. Cancer Clin. Pract. 2019, 8, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Haanstra, J.F.; Cappel, W.H.D.V.T.N.; Gopie, J.P.; Vecht, J.; Vanhoutvin, S.A.L.W.; Cats, A.; Van Der Zaag-Loonen, H.J.; Langers, A.M.J.; Bergmann, J.H.W.; Van De Meeberg, P.C.; et al. Quality of Life After Surgery for Colon Cancer in Patients with Lynch Syndrome. Dis. Colon Rectum 2012, 55, 653–659. [Google Scholar] [CrossRef]
- Urso, E.D.L.; Celotto, F.; Giandomenico, F.; Gavaruzzi, T.; Del Bianco, P.; Lotto, L.; Spolverato, G.; Pucciarelli, S.; Bao, Q.R.; Emanuele, D.U. Analysis of morbidity and mortality, quality of life and bowel function after total colectomy with ileorectal anastomosis versus right and left hemicolectomy: A study to optimise the treatment of lynch syndrome and attenuated polyposis coli. Eur. J. Surg. Oncol. 2020, 46, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.F.; Mecklin, J.-P.; Watson, P.; Utsunomiya, J.; Bertario, L.; Lynch, P.; Svendsen, L.B.; Cristofaro, G.; Müller, H.; Khan, M.P.; et al. Surveillance in hereditary nonpolyposis colorectal cancer. Dis. Colon Rectum 1993, 36, 425–429. [Google Scholar] [CrossRef] [PubMed]
- De Vos tot Nederveen Cappel, W.H.; Nagengast, F.M.; Griffioen, G.; Menko, F.H.; Taal, B.G.; Kleibeuker, J.H.; Vasen, H.F. Surveillance for Hereditary Nonpolyposis Colorectal Cancer. Dis. Colon Rectum 2002, 45, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.J.; Hong, Y.H.; Kim, B.C.; Chang, H.J.; Han, K.S.; Hong, C.W.; Sohn, D.K.; Park, S.C.; Lee, D.W.; Kim, B.; et al. Analysis of metachronous colorectal neoplasms and survival following segmental or extended resection in patients with hereditary non-polyposis colorectal cancer. Int. J. Color. Dis. 2020, 35, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Martin, S.T.; Winter, D.C. Segmental vs extended colectomy in the management of hereditary nonpolyposis colorectal cancer: A systematic review and meta-analysis. Color. Dis. 2015, 17, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Anele, C.; Adegbola, S.O.; Askari, A.; Rajendran, A.; Clark, S.K.; Latchford, A.; Faiz, O. Risk of metachronous colorectal cancer following colectomy in Lynch syndrome: A systematic review and meta-analysis. Color. Dis. 2017, 19, 528–536. [Google Scholar] [CrossRef]
- Kalady, M.F.; McGannon, E.; Vogel, J.D.; Manilich, E.; Fazio, V.W.; Church, J.M. Risk of colorectal adenoma and carcinoma after colectomy for colorectal cancer in patients meeting Amsterdam criteria. Ann. Surg. 2010, 252, 507–511. [Google Scholar] [CrossRef]
- Natarajan, N.; Watson, P.; Silva-Lopez, E.; Lynch, H.T. Comparison of Extended Colectomy and Limited Resection in Patients with Lynch Syndrome. Dis. Colon Rectum 2010, 53, 77–82. [Google Scholar] [CrossRef]
- Parry, S.; Win, A.K.; Parry, B.; Macrae, F.A.; Gurrin, L.C.; Church, J.M.; Baron, J.A.; Giles, G.G.; Leggett, B.A.; Winship, I.; et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: The advantage of more extensive colon surgery. Gut 2011, 60, 950–957. [Google Scholar] [CrossRef]
- Stupart, D.; Goldberg, P.A.; Baigrie, R.J.; Algar, U.; Ramesar, R. Surgery for colonic cancer in HNPCC: Total vs segmental colectomy. Color. Dis. 2011, 13, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.; Holter, S.; Semotiuk, K.; Winter, L.; Pollett, A.; Gallinger, S.; Cohen, Z.; Gryfe, R. DNA Mismatch Repair Status Predicts Need for Future Colorectal Surgery for Metachronous Neoplasms in Young Individuals Undergoing Colorectal Cancer Resection. Dis. Colon Rectum 2015, 58, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kim, E.R.; Hong, S.N.; Kim, Y.-H.; Huh, J.W.; Park, Y.A.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; et al. Survival Outcome and Risk of Metachronous Colorectal Cancer After Surgery in Lynch Syndrome. Ann. Surg. Oncol. 2016, 24, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, M.J.; Casey, M.J.; Lynch, H.T.; Snyder, C.L.; Stacey, M.; Walters, R.W. Efficacy of proximal colectomy for surgical management of right-sided first colorectal cancer in Lynch Syndrome mutation carriers. Am. J. Surg. 2018, 216, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Renkonen-Sinisalo, L.; Seppälä, T.T.; Järvinen, H.J.; Mecklin, J.-P. Subtotal Colectomy for Colon Cancer Reduces the Need for Subsequent Surgery in Lynch Syndrome. Dis. Colon Rectum 2017, 60, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Allen, J.I.; Axilbund, J.E.; Boland, C.R.; Burke, C.A.; Burt, R.W.; Church, J.M.; Dominitz, J.A.; Johnson, D.A.; Kaltenbach, T.; et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome. Dis. Colon Rectum 2014, 109, 1159–1179. [Google Scholar] [CrossRef]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef]
- Stjepanovic, N.; Moreira, L.; Carneiro, F.; Balaguer, F.; Cervantes, A.; Balmaña, J.; Martinelli, E. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1558–1571. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; DeSouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2019, 69, 411–444. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Soares, A.S.; Jimenez-Rodriguez, R.; Sánchez-Guillén, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. BJS 2020. online ahead of print. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Ryan, N.A.J.; Arends, M.J.; Bosse, T.; Burn, J.; Cornes, J.M.; Crawford, R.; Eccles, D.; Frayling, I.M.; Ghaem-Maghami, S.; et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 2019, 21, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Helder-Woolderink, J.; De Bock, G.; Sijmons, R.; Hollema, H.; Mourits, M.; De Bock, G.H. The additional value of endometrial sampling in the early detection of endometrial cancer in women with Lynch syndrome. Gynecol. Oncol. 2013, 131, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Lynch, H.T.; Chen, L.; Munsell, M.F.; Soliman, P.T.; Clark, M.B.; Daniels, M.S.; White, K.G.; Boyd-Rogers, S.G.; Conrad, P.G.; et al. Prophylactic Surgery to Reduce the Risk of Gynecologic Cancers in the Lynch Syndrome. N. Engl. J. Med. 2006, 354, 261–269. [Google Scholar] [CrossRef]
- Van Der Groep, P.; Van Der Wall, E.; Van Diest, P.J. Pathology of hereditary breast cancer. Cell. Oncol. 2011, 34, 71–88. [Google Scholar] [CrossRef]
- Ahadova, A.; Gallon, R.; Gebert, J.; Ballhausen, A.; Endris, V.; Kirchner, M.; Stenzinger, A.; Burn, J.; Doeberitz, M.V.K.; Bläker, H.; et al. Three molecular pathways model colorectal carcinogenesis in Lynch syndrome. Int. J. Cancer 2018, 143, 139–150. [Google Scholar] [CrossRef]
- Ahadova, A.; Doeberitz, M.V.K.; Bläker, H.; Kloor, M. CTNNB1-mutant colorectal carcinomas with immediate invasive growth: A model of interval cancers in Lynch syndrome. Fam. Cancer 2016, 15, 579–586. [Google Scholar] [CrossRef]
- Engel, C.; Ahadova, A.; Seppälä, T.T.; Aretz, S.; Bigirwamungu-Bargeman, M.; Bläker, H.; Bucksch, K.; Büttner, R.; Cappel, W.T.D.V.T.N.; Endris, V.; et al. Associations of Pathogenic Variants in MLH1, MSH2, and MSH6 With Risk of Colorectal Adenomas and Tumors and With Somatic Mutations in Patients with Lynch Syndrome. Gastroenterology 2020, 158, 1326–1333. [Google Scholar] [CrossRef]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.-P.; Möslein, G.; McRonald, E.F.; Bertario, L.; Evans, D.G.; et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Rodríguez-Bigas, M.A.; Vasen, H.F.; Pekka-Mecklin, J.; Myrhøj, T.; Rozen, P.; Bertario, L.; Järvinen#, H.J.; Jass, J.R.; Kunitomo, K.; Nomizu, T.; et al. Rectal Cancer Risk in Hereditary Nonpolyposis Colorectal Cancer After Abdominal Colectomy. Ann. Surg. 1997, 225, 202–207. [Google Scholar] [CrossRef]
- Ghezzi, F.; Uccella, S.; Cromi, A.; Bogani, G.; Donadello, N.; Riva, C. Primary Peritoneal Cancer in Lynch Syndrome. Int. J. Gynecol. Pathol. 2013, 32, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Seppälä, T.T.; Engel, C.; Aretz, S.; Macrae, F.; Winship, I.; Capella, G.; Thomas, H.; Hovig, E.; Nielsen, M.; et al. Risk-Reducing Gynecological Surgery in Lynch Syndrome: Results of an International Survey from the Prospective Lynch Syndrome Database. J. Clin. Med. 2020, 158, 1326–1333. [Google Scholar]
- Kom, E.L.; Graubard, B.I.; Midthune, D. Time-to-Event Analysis of Longitudinal Follow-up of a Survey: Choice of the time-scale. Am. J. Epidemiol. 1997, 145, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M. A reference relative time-scale as an alternative to chronological age for cohorts with long follow-up. Emerg. Themes Epidemiol. 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Etchegary, H.; Dicks, E.; Watkins, K.; Alani, S.; Dawson, L. Decisions about prophylactic gynecologic surgery: A qualitative study of the experience of female Lynch syndrome mutation carriers. Hered. Cancer Clin. Pract. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.; Keating, S.; Clancy, T. The impact of risk-reducing gynaecological surgery in premenopausal women at high risk of endometrial and ovarian cancer due to Lynch syndrome. Fam. Cancer 2014, 14, 51–60. [Google Scholar] [CrossRef]
- Boland, C.R.; Shike, M. Report From the Jerusalem Workshop on Lynch Syndrome-Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 2010, 138, 2197.e1–2197.e7. [Google Scholar] [CrossRef]
- Thompson, B.A.; Insight, O.B.O.; Spurdle, A.B.; Plazzer, J.-P.; Greenblatt, M.S.; Akagi, K.; Al-Mulla, F.; Bapat, B.; Bernstein, I.; Capellá, G.; et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat. Genet. 2014, 46, 107–115. [Google Scholar] [CrossRef]
| All Individuals | Colorectal Cancer Cohort | Gynecological Cancer Cohort | |||
|---|---|---|---|---|---|
| Extended Surgery | Segmental Surgery | RRGS 1 | Non-RRGS 2 | ||
| TOTAL | n = 976 | n = 29 | n = 261 | n = 66 | n = 465 |
| Sex | |||||
| Male | 445 (45.6%) | 18 (62.1%) | 146 (55.9%) | ||
| Female | 531 (54.4%) | 11 (37.9%) | 115 (44.1%) | 66 (100%) | 465 (100%) |
| Mean age | 54.1 y 7 (18–95) | 56.4 y 7 (32–79) | 59.9 y 7 (29–96) | 57.3 y 7 (40–85) | 54.8 y 7 (18–95) |
| MMR gene | |||||
| MLH1 | 480 (49.2%) | 19 (65.5%) | 137 (52.5%) | 33 (50.0%) | 226 (48.6%) |
| MSH2 | 262 (26.8%) | 5 (17.2%) | 77 (29.5%) | 19 (28.8%) | 127 (27.3%) |
| MSH6 | 165 (16.9%) | 1 (3.4%) | 30 (11.5%) | 10 (15.2%) | 75 (16.1%) |
| PMS2 | 48 (4.9%) | 1 (3.4%) | 14 (5.4%) | 3 (4.5%) | 22 (4.7%) |
| EPCAM | 21 (2.2%) | 3 (10.3%) | 3 (1.1%) | 1 (1.5%) | 15 (3.2%) |
| Death | 221 (22.6%) | 4 (13.8%) | 41 (15.7%) | 0 (0%) | 98 (21.1%) |
| Mean age at death (range) | 58.4 y 7 (25–89) | 55.0 y 7 (44–71) | 50.8 y 7 (29–84) | 60.5 y 7 (25–89) | |
| First cancer diagnosis 3 | 678 (69.5%) | 29 (100%) | 261 (100%) | 33 (50.0%) | 277 (59.6%) |
| Colon | 384 (39.3%) | 26 (89.7%) | 214 (82.0%) | 22 (33.3%) | 119 (25.6%) |
| Endometrial | 97 (9.9%) | 1 (3.4%) | 17 (6.5%) | 4 (6.1%) | 86 (18.5%) |
| Ovarian | 28 (2.9%) | 1 (3.4%) | 3 (1.1%) | 0 (0%) | 25 (5.4%) |
| Rectum | 26 (2.7%) | 0 (0%) | 19 (7.3%) | 3 (4.5%) | 9 (1.9%) |
| Other GI 4 | 26 (2.7%) | 0 (0%) | 2 (0.8%) | 0 (0%) | 10 (2.2%) |
| Urologic 5 | 14 (1.4%) | 0 (0%) | 0 (0%) | 2 (3.0%) | 5 (1.1%) |
| Other non-LS | 103 (10.6%) | 1 (3.4%) | 6 (2.3%)) | 2 (3.0%) | 23 (4.9%) |
| Mean age at first cancer diagnosis (range) | 47.6 y 7 (18–86) | 46.3 y 7 (25–79) | 45.9 y 7 (18–83) | 46.2 y 7 (28–66) | 49.0 y 7 (18–86) |
| Mean age at surgery of study (range) 6 | 46.0 y 7 (25–79) | 46.9 y 7 (18–83) | 49.1 y 7 (36–72) | 50.1 y 7 (28–80) | |
| Characteristics | TOTAL n = 425 (100%) | MLH1 n = 239 (56.2%) | MSH2 n = 112 (26.4%) | MSH6 n = 50 (11.8%) | PMS2 n = 17 (4%) | EPCAM n = 7 (1.6%) |
|---|---|---|---|---|---|---|
| Number of CRC | ||||||
| One | 312 (73.4%) | 181 (75.7%) | 77 (68.8%) | 38 (76.0%) | 14 (82.4%) | 2 (28.6%) |
| 2 or more | 113 (26.6%) | 58 (24.3%) | 35 (31.2%) | 12 (24.0%) | 3 (17.6%) | 5 (71.4%) |
| Mean age at first CRC 2 diagnosis (range) | 47.6 y 1 (18–86) | 45.2 y 1 (18–86) | 46.7 y 1 (21–83) | 56.3 y 1 (33–78) | 58.8 y 1 (38–72) | 44.8 y 1 (33–61) |
| Type of second CRC 2 | ||||||
| Synchronous | 42 (9.9%) | 21 (8.8%) | 9 (8.0%) | 7 (14%) | 2 (11.8%) | 3 (42.9%) |
| Metachronous | 71 (16.7%) | 37 (15.5%) | 26 (23.2%) | 5 (10%) | 1 (5.9%) | 2 (28.6%) |
| Characteristics | TOTAL n = 290 (29 1/261 2) | MLH1 n = 156 (19 1/137 2) | MSH2 n = 83 (5 1/78 2) | MSH6 n = 31 (1 1/30 2) | PMS2 n = 14 (1 1/13 2) | EPCAM n = 6 (3 1/3 2) |
|---|---|---|---|---|---|---|
| ONE CRC n = 192 | ||||||
| Extended surgery | 16/29 (55.2%) | 13/19 (68.4%) | 3/5 (60%) | 0/1 (0%) | 0/1 (0%) | 0/3 (0%) |
| Segmental surgery | 176/261 (67.4%) | 92/137 (67.2%) | 50/78 (64.1%) | 21/30 (70%) | 12/13 (92.4%) | 1/3 (33.3%) |
| SYNCHRONOUS CANCER n = 35 | ||||||
| Extended surgery | 12/29 (41.4%) | 6/19 (31.6%) | 2/5 (40.0%) | 1/1 (100%) | 1/1 (100%) | 2/3 (66.7%) |
| Segmental surgery | 23/261 (8.8%) | 11/137 (8.0%) | 7/78 (9.0%) | 4/30 (13.3%) | 0/13 (0%) | 1/3 (33.3%) |
| METACHRONOUS CANCER n = 63 | ||||||
| Extended surgery | 1/29 (3.4%) | 0/19 (0%) | 0/5 (0%) | 0/1 (0%) | 0/1 (0%) | 1/3 (33.3%) |
| Segmental surgery | 62/261 (23.8%) | 34/138 (24.8%) | 21/78 (26.9%) | 5/30 (16.7%) | 1/13 (7.7%) | 1/3 (33.3%) |
| Characteristics | TOTAL 150/531 (28.2%) | MLH1 52/259 (20.1%) | MSH2 58/146 (39.7%) | MSH6 32/85 (37.6%) | PMS2 6/25 (24.0%) | EPCAM 2/16 (1.3%) |
|---|---|---|---|---|---|---|
| Localization of gynecological cancer | ||||||
| Endometrial | 114 (76.0%) | 40 (76.9%) | 45 (77.6%) | 24 (75.0%) | 4 (66.7%) | 1 (50.0%) |
| Ovarian | 27 (18.0%) | 9 (17.3%) | 10 (17.2%) | 5 (15.6%) | 2 (33.3%) | 1 (50.0%) |
| Endometrial + ovarian | 9 (6.0%) | 3 (5.8%) | 3 (5.2%) | 3 (9.4%) | 0 (0.0%) | 0 (0.0%) |
| Mean age at gynecological cancer diagnosis (range) | 49.9 y 1 (28–80) | 47.6 y 1 (31–78) | 46.4 y 1 (28–80) | 51.0 y 1 (38–79) | 53.5 y 1 (42–66) | 38.0 y 1 (38–38) |
| Characteristics | TOTAL n = 531 (66 1/465 2) | MLH1 n = 259 (33 1/226 2) | MSH2 n = 146 (19 1/127 2) | MSH6 n = 85 (10 1/75 2) | PMS2 n = 25 (3 1/22 2) | EPCAM n = 16 (1 1/15 2) |
|---|---|---|---|---|---|---|
| ENDOMETRIAL CANCER n = 123 | ||||||
| RRGS 1 | 6 (9.1%) | 4 (12.1%) | 2 (10.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| non-RRGS 2 | 117 (25.2%) | 39 (17.3%) | 46 (36.2%) | 27 (36.0%) | 4 (18.2%) | 1 (6.7%) |
| OVARIAN CANCER n = 36 | ||||||
| RRGS 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| non-RRGS 2 | 36 (7.7%) | 12 (5.3%) | 13 (10.2%) | 8 (10.7%) | 2 (9.1%) | 1 (6.7%) |
| Author | Year | Collected Data/Type of Study | n (ES 1/SS 2) | Population | Follow-Up (Years) | Rate of mCRC 3 (ES 1/SS 2) | 10 Years Overall Survival (ES 1/SS 2) |
|---|---|---|---|---|---|---|---|
| Vasen [13] | 1993 | Retrospective Multicentric International | 54 (17 1/37 2) | Ams 4 | 5.8 (1–10) | 11.8% 1 vs. 21.6% 2 (p = 0.394) | n.r. 6 |
| De Vos tot Nederveen WH [14] | 2002 | Retrospective Multicentric National | 97 (29 1/68 2) | LS 5 (MLH1, MSH2, MSH6) | ES 1: 5 (1–15) SS 2: 6.8 (0–15) | 3.5 1 vs. 11.8% 2 (p > 0.05) | n.r. 6 |
| Kalady MF [18] | 2010 | Retrospective Single-institution | 296 (43 1/253 2) | Ams 4 (11 LS 5 confirmed) | 8,7 (n.r) | 8.0 1 vs. 25.0% 2 (p = 0.016) | n.r. 6 |
| Natarajan N [19] | 2010 | Retrospective Single-institution | 106 (37 1,7/69 2) | LS 5 (MLH1, MSH2) | 12 (5–20) | 10.8 1 vs. 33.3%2 (p = 0.006) | 86.5 1 vs. 76.8% 2 (p = 0.239) |
| Parry S [20] | 2011 | Retrospective Multicentric International | 382 (50 1/332 2) | LS 5 (MLH1, MSH2, MSH6, PMS2) | ES 1: 8 (1–30) SS 2: 9 (1–40) | 0 vs. 22.3% 2 (p = 0.019) | 98 vs. 97% (p = 0.692) |
| Stupart DA [21] (Stupart et al., 2011) | 2011 | Retrospective Single-institution | 60 (21 1/39 2) | LS 5 (MLH1, MSH2) | ES 1: 8 (0–34) SS 2: 6 (1–30) | 9.5 1 vs. 20.5% 2 (p = 0.346) | 76 1 vs. 62% 2 (p = 0.222) |
| Aronson M [22] | 2015 | Retrospective Single-institution | 105 (29 1/76 2) | LS 5 (MLH1, MSH2, MSH6, PMS2) | 6.2 (0–55) | 10.3 1 vs 28.9% 2 (p = 0.071) | n.r. 6 |
| Kim TJ [23] | 2017 | Retrospective Single-institution | 106 (30 1/76 2) | LS 5 (MLH1, MSH2, MSH6, EPCAM) | ES 1: 5.7 (1–13) SS 2: 6.4 (0–14) | 0 1 vs. 17.1% 2 (p = 0.038) | 82.9 1 vs. 83.3% 2 (p = 0.659) 9 |
| Hiatt MJ [24] | 2017 | Retrospective Single-institution | 64 8 (16 1/48 2) | LS 5 (MLH1, MSH2, MSH6, EPCAM) | n.r. 6 | 6.3 1 vs. 27.0% 2 (p n.r. 6) | 81.0 1 vs. 82.8% 2 (p = 0.471) |
| Renkonen- Sinisalo L [25] | 2017 | Retrospective Multicentric National | 242 (98 1/144 2) | LS 5 (MLH1, MSH2, MSH6) | 15.0 (0–32) | 5.1 1 vs. 25.0% 2 (p < 0.001) | 47.2 1 vs. 41.1% 2 (p = 0.83) 10 |
| Roh SJ [15] | 2020 | Retrospective Single-institution | 87 (51 1/36 2) | Ams 4 | ES 1: 7.7 (n.r) SS 2: 6.6 (n.r) | 5.9 1 vs. 2.8% 2 (p = 0.637) | n.r. 6 |
| Heneghan HM [16] | 2015 | Meta-analysis | 948 (168 1/780 2) | LS 5 + Ams 4 | 8.9 (5–12) | 6.8 1 vs. 23.5% 2 (p < 0.005) | 89.8 1 vs. 90.7% 2 (p = 0.085) |
| Anele CC [17] | 2017 | Meta-analysis | 871 (166 1/705 2) | LS 5 | 7.6 (6–12) | 6 1 vs. 22.8% 2 (p < 0.0001) | n.r. 6 |
| CURRENT REPORT | 2020 | Retrospective Single-institution | 293 (29 1/264 2) | LS 5 (MLH1, MSH2, MSH6, PMS2, EPCAM) | ES 1: 10.9 (0–28) SS 2: 14.7 (0–47) | 3.4 1 vs. 23.8% 2 (p < 0.0001) | n.r. 6 |
| Author | Year | Collected Data/Type of Study | n (RRGS 1/Non-RRGS 2) | Follow-Up (Years) (RRGS 1/Non-RRGS 2) | Rate EC 3 (RRGS 1/Non-RRGS 2) | Rate OC 4 (RRGS 1/Non-RRGS 2) | 10 Years Overall Survival (RRGS 1/Non-RRGS 2) |
|---|---|---|---|---|---|---|---|
| Schmeler KM [34] | 2006 | Retrospective Multicentric National (USA) | 315 (61 1/254 2) | 13.3 1 (0.5–38) 7.4 2 (0.1–35) | 0 1 vs. 33.0% 2 (p < 0.001) | 0 1 vs. 5.5% 2 (p = 0.09) | n.r. 6 |
| CURRENT REPORT | 2020 | Retrospective Single-institution | 531 (66 1/465 2) | 8.7 1 (0–43) 10.4 2 (0–45) | 9.1 1 vs. 25.2% 2 (p < 0.001) | 0 1 vs. 7.7% 2 (p N/A 5) | n.r. 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dueñas, N.; Navarro, M.; Teulé, À.; Solanes, A.; Salinas, M.; Iglesias, S.; Munté, E.; Ponce, J.; Guardiola, J.; Kreisler, E.; et al. Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals. Cancers 2020, 12, 3419. https://doi.org/10.3390/cancers12113419
Dueñas N, Navarro M, Teulé À, Solanes A, Salinas M, Iglesias S, Munté E, Ponce J, Guardiola J, Kreisler E, et al. Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals. Cancers. 2020; 12(11):3419. https://doi.org/10.3390/cancers12113419
Chicago/Turabian StyleDueñas, Nuria, Matilde Navarro, Àlex Teulé, Ares Solanes, Mònica Salinas, Sílvia Iglesias, Elisabet Munté, Jordi Ponce, Jordi Guardiola, Esther Kreisler, and et al. 2020. "Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals" Cancers 12, no. 11: 3419. https://doi.org/10.3390/cancers12113419
APA StyleDueñas, N., Navarro, M., Teulé, À., Solanes, A., Salinas, M., Iglesias, S., Munté, E., Ponce, J., Guardiola, J., Kreisler, E., Carballas, E., Cuadrado, M., Matias-Guiu, X., de la Ossa, N., Lop, J., Lázaro, C., Capellá, G., Pineda, M., & Brunet, J. (2020). Assessing Effectiveness of Colonic and Gynecological Risk Reducing Surgery in Lynch Syndrome Individuals. Cancers, 12(11), 3419. https://doi.org/10.3390/cancers12113419






