Will Next-Generation Immunotherapy Overcome the Intrinsic Diversity and Low Immunogenicity of Sarcomas to Improve Clinical Benefit?
Abstract
Simple Summary
Abstract
1. Introduction
2. Immunogenic Landscape of Sarcomas
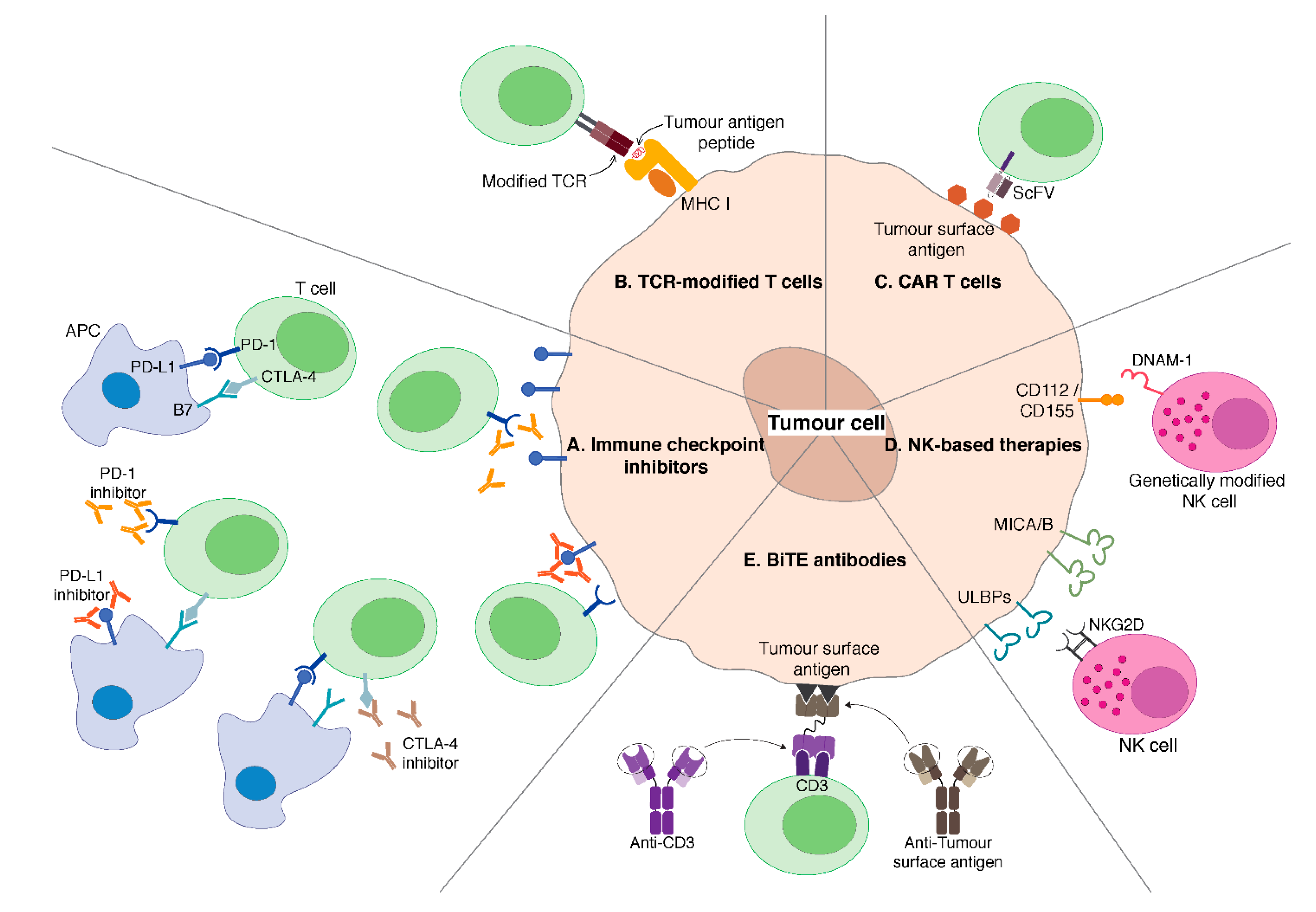
3. Immune Checkpoint Inhibitors
3.1. CTLA-4 Blockade
3.2. PD-1/PD-L1 Blockade
3.3. CTLA-4 + PD-1/PD-L1 Immune Checkpoint Inhibitors
4. Adoptive Transfer of Genetically Modified T Cells
4.1. T Cells Engineered to Express TAA-Specific TCRs
4.2. CAR T Cells
5. NK Cell-Based Therapies
6. Bispecific T Cell Engager (BiTE) Antibodies
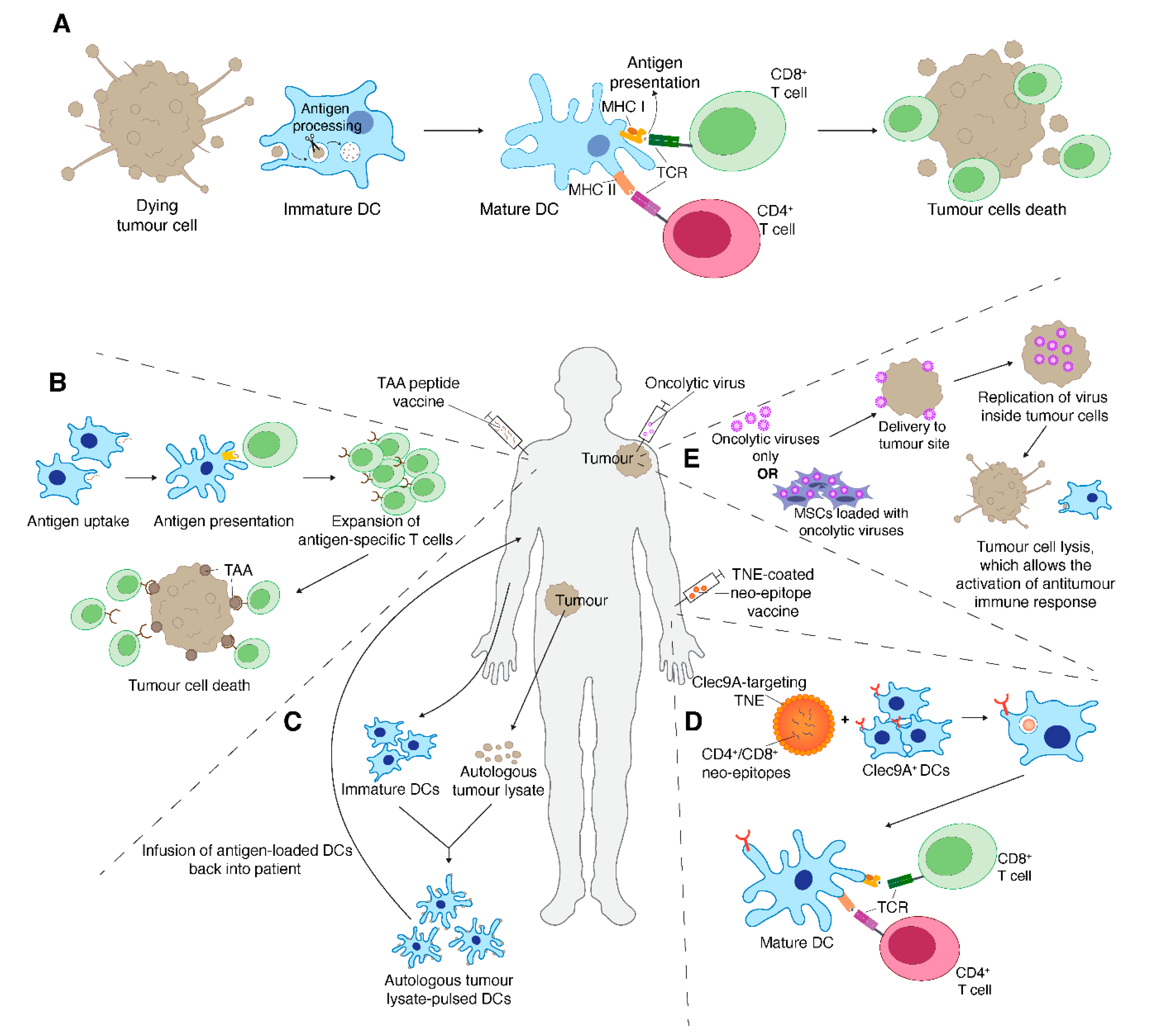
7. Therapeutic Cancer Vaccines
8. Oncolytic Virus Therapy
9. Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma Epidemiology and Etiology: Potential Environmental and Genetic Factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.G.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Denniston, K.A.; Lin, C.J.; Lin, C. A Comparison of Pediatric vs. Adult Patients with the Ewing Sarcoma Family of Tumors. Front. Oncol. 2017, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaid, T.; Wang, W.-L.; Somaiah, N.; Lazar, A.J.F. Molecular profiling of sarcomas: New vistas for precision medicine. Virchows Arch. 2017, 471, 243–255. [Google Scholar] [CrossRef]
- Helman, L.J.; Meltzer, P. Mechanisms of sarcoma development. Nat. Rev. Cancer 2003, 3, 685–694. [Google Scholar] [CrossRef]
- Taylor, B.S.; Barretina, J.; Maki, R.G.; Antonescu, C.R.; Singer, S.; Ladanyi, M. Advances in sarcoma genomics and new therapeutic targets. Nat. Rev. Cancer 2011, 11, 541–557. [Google Scholar] [CrossRef]
- Brien, G.L.; Stegmaier, K.; Armstrong, S.A. Targeting chromatin complexes in fusion protein-driven malignancies. Nat. Rev. Cancer 2019, 19, 255–269. [Google Scholar] [CrossRef]
- Tucker, M.A.; D’Angio, G.J.; Boice, J.D.; Strong, L.C.; Li, F.P.; Stovall, M.; Stone, B.J.; Green, D.M.; Lombardi, F.; Newton, W.; et al. Bone Sarcomas Linked to Radiotherapy and Chemotherapy in Children. N. Engl. J. Med. 1987, 317, 588–593. [Google Scholar] [CrossRef]
- Van Geel, A.N.; Pastorino, U.; Jauch, K.W.; Judson, I.R.; Van Coevorden, F.; Buesa, J.M.; Nielsen, O.S.; Boudinet, A.; Tursz, T.; Schmitz, P.I.M. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996, 77, 675–682. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities or Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets for Bone and Soft-Tissue Sarcomas. Int. J. Mol. Sci. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.P. Intrinsic radiation resistance of mesenchymal cancer stem cells and implications for treatment response in a murine sarcoma model. Dan. Med. J. 2012, 59, B4388. [Google Scholar] [PubMed]
- Xu-Monette, Z.Y.; Zhang, M.; Li, J.; Young, K.H. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front. Immunol. 2017, 8, 1597. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Hirota, S. Gain-of-Function Mutations of c-kit in Human Gastrointestinal Stromal Tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Hirota, S.; Ohashi, A.; Nishida, T.; Isozaki, K.; Kinoshita, K.; Shinomura, Y.; Kitamura, Y. Gain-of-function mutations of platelet-derived growth factor receptor α gene in gastrointestinal stromal tumors. Gastroenterology 2003, 125, 660–667. [Google Scholar] [CrossRef]
- Lasota, J.; Miettinen, M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008, 53, 245–266. [Google Scholar] [CrossRef]
- Reilley, M.J.; Bailey, A.; Subbiah, V.; Janku, F.; Naing, A.; Falchook, G.; Karp, D.; Piha-Paul, S.; Tsimberidou, A.; Fu, S.; et al. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J. Immunother. Cancer 2017, 5, 35. [Google Scholar] [CrossRef]
- Demetri, G.D.; Von Mehren, M.; Blanke, C.D.; Van den Abbeele, M.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Cavnar, M.J.; Zeng, S.; Bamboat, Z.M.; Ocuin, L.M.; Obaid, H.; Sorenson, E.C.; Popow, R.; Ariyan, E.C.; Rossi, F.; et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011, 17, 1094–1100. [Google Scholar] [CrossRef]
- Gasparotto, D.; Sbaraglia, M.; Rossi, S.; Baldazzi, D.; Brenca, M.; Mondello, A.; Nardi, F.; Racanelli, D.; Cacciatore, M.; Tos, A.P.D.; et al. Tumor genotype, location and malignant potential shape the immunogenicity of primary, untreated Gastrointestinal Stromal Tumors. JCI Insight 2020. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Pardoll, D. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Read, S.; Greenwald, R.; Izcue, A.; Robinson, N.; Mandelbrot, D.; Francisco, L.; Sharpe, A.H.; Powrie, F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006, 177, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Contardi, E.; Palmisano, G.L.; Tazzari, P.L.; Martelli, A.M.; Falà, F.; Fabbi, M.; Kato, T.; Lucarelli, E.; Donati, D.; Polito, L.; et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer 2005, 117, 538–550. [Google Scholar] [CrossRef]
- Hingorani, P.; Maas, M.L.; Gustafson, M.P.; Dickman, P.S.; Adams, R.H.; Watanabe, M.; Eshun, F.; Williams, J.; Seidel, M.J.; Dietz, A.B. Increased CTLA-4+ T cells and an increased ratio of monocytes with loss of class II (CD14+ HLA-DRlo/neg) found in aggressive pediatric sarcoma patients. J. Immunother. Cancer 2015, 3, 35. [Google Scholar] [CrossRef]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.B.; Bishop, R.J.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef]
- Maki, R.G.; Jungbluth, A.A.; Gnjatic, S.; Schwartz, G.K.; D’Adamo, D.R.; Keohan, M.L.; Wagner, M.J.; Scheu, K.; Chiu, R.; Ritter, E.; et al. A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma 2013, 2013, 168145. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, A.N.; Keohan, M.L.; Dickson, M.A.; Gounder, M.M.; Chi, P.; Loo, J.K.; Gaffney, L.; Schneider, L.; Patel, Z.; et al. Combined KIT and CTLA-4 Blockade in Patients with Refractory GIST and Other Advanced Sarcomas: A Phase Ib Study of Dasatinib plus Ipilimumab. Clin. Cancer Res. 2017, 23, 2972–2980. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Kim, J.R.; Moon, Y.J.; Kwon, K.S.; Bae, J.S.; Wagle, S.; Kim, K.M.; Park, H.S.; Lee, H.; Moon, W.S.; Chung, M.J.; et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS ONE 2013, 8, e82870. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.C.; Maclean, F.M.; Sioson, L.; Tran, D.; Bonar, F.; Mahar, A.; Cheah, A.L.; Russell, P.; Grimison, P.; Richardson, L.; et al. Prevalence of PD-L1 expression in matched recurrent and/or metastatic sarcoma samples and in a range of selected sarcomas subtypes. PLoS ONE 2020, 15, e0222551. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.-X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; He, Q.; Yearley, J.H.; Emerson, R.; Vignali, M.; Zhang, Y.; Redman, M.W.; Baker, K.K.; Cooper, S.; Donahue, B.; et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017, 123, 3291–3304. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.; Büclein, V.; Kampmann, E.; Knösel, T.; Nössner, E.; Subklewe, M.; Lindner, L. Dissecting the role of tumor-infiltrating lymphocytes (TIL) in patients with high-risk soft-tissue sarcoma (STS) receiving neo-adjuvant chemotherapy (NAC) with regional hyperthermia (RHT). Ann. Oncol. 2016, 27, vi488. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, M.; Sung, M.; Lee, S.E.; Kim, Y.J.; Choi, Y.-L. Status of programmed death-ligand 1 expression in sarcomas. J. Transl. Med. 2018, 16, 303. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; A Priebat, D.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Burgess, M.A.; Bolejack, V.; Schuetze, S.; Van Tine, B.A.; Attia, S.; Riedel, R.F.; Hu, J.S.; Davis, L.E.; Okuno, S.H.; Priebat, D.A.; et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J. Clin. Oncol. 2019, 37, 11015. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Barysauskas, C.M.; Solomon, S.; Tahlil, K.; Malley, R.; Hohos, M.; Polson, K.; Loucks, M.; Severgnini, M.; Patel, T.; et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer 2017, 123, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Attia, S.; Riedel, R.F.; et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, J.; Gao, Y.; Liao, Y.; Cote, G.M.; Choy, E.; Chebib, I.; Mankin, H.; Hornicek, F.; Duan, Z. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget 2015, 6, 11139–11149. [Google Scholar] [CrossRef]
- Zou, M.-X.; Peng, A.-B.; Lv, G.-H.; Wang, X.-B.; Li, J.; She, X.-L.; Jiang, Y. Expression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosis. Am. J. Transl. Res. 2016, 8, 3274–3287. [Google Scholar]
- Machado, I.; López-Guerrero, J.A.; Scotlandi, K.; Picci, P.; Llombart-Bosch, A. Immunohistochemical analysis and prognostic significance of PD-L1, PD-1, and CD8+ tumor-infiltrating lymphocytes in Ewing’s sarcoma family of tumors (ESFT). Virchows Arch. 2018, 472, 815–824. [Google Scholar] [CrossRef]
- Raj, S.; Bui, M.; Gonzales, R.; Letson, D.; Antonia, S. Impact of Pdl1 Expression on Clinical Outcomes in Subtypes of Sarcoma. Ann. Oncol. 2014, 25, iv498. [Google Scholar] [CrossRef]
- Zhu, Z.; Jin, Z.; Zhang, M.; Tang, Y.; Yang, G.; Yuan, X.; Yao, J.; Sun, D. Prognostic value of programmed death-ligand 1 in sarcoma: A meta-analysis. Oncotarget 2017, 8, 59570–59580. [Google Scholar] [CrossRef]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-Cell Immunity to Osteosarcoma Through Antibody Blockade of PD-1/PD-L1 Interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef]
- Koirala, P.; Roth, M.E.; Gill, J.; Piperdi, S.; Chinai, J.M.; Geller, D.S.; Hoang, B.H.; Park, A.; Fremed, M.A.; Zang, X.; et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016, 6, 30093. [Google Scholar] [CrossRef] [PubMed]
- Fritzsching, B.; Fellenberg, J.; Moskovszky, L.; Sápi, Z.; Krenacs, T.; Machado, I.; Poeschl, J.; Lehner, B.; Szendrõi, M.; Bosch, A.L.; et al. CD8+/FOXP3+-ratio in osteosarcoma microenvironment separates survivors from non-survivors: A multicenter validated retrospective study. OncoImmunology 2015, 4, e990800. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, A.; Versleijen-Jonkers, Y.M.H.; Hillebrandt-Roeffen, M.H.; Van Houdt, L.; Gorris, M.A.; Van Dam, L.S.; Mentzel, T.; Weidema, M.; Savci-Heijink, C.D.; Desar, I.M.; et al. Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8+ lymphocytes in primary sarcomas is subtype dependent. Oncotarget 2017, 8, 71371–71384. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Okamoto, M.; Sasaki, J.; Kuroda, C.; Ishida, H.; Ueda, K.; Okano, S.; Ideta, H.; Kamanaka, T.; Sobajima, A.; et al. Clinical outcome of osteosarcoma and its correlation with programmed death-ligand 1 and T cell activation markers. OncoTargets Ther. 2019, 12, 2513–2518. [Google Scholar] [CrossRef]
- Zheng, B.; Ren, T.; Huang, Y.; Sun, K.; Wang, S.; Bao, X.; Liu, K.; Guo, W. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J. Hematol. Oncol. 2018, 11, 16. [Google Scholar] [CrossRef]
- Wu, C.-C.; Beird, H.C.; Livingston, J.A.; Advani, S.; Mitra, A.; Cao, S.; Reuben, A.; Ingram, D.; Wang, W.-L.; Ju, Z.; et al. Immuno-genomic landscape of osteosarcoma. Nat. Commun. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Lussier, D.M.; Johnson, J.L.; Hingorani, P.; Blattman, J.N. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J. Immunother. Cancer 2015, 3, 21. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Juretić, E.; Spagnoli, G.C.; Schultz-Thater, E.; Sarcevic, B. Cancer/testis tumour-associated antigens: Immunohistochemical detection with monoclonal antibodies. Lancet Oncol. 2003, 4, 104–109. [Google Scholar] [CrossRef]
- Smith, S.M.; Iwenofu, O.H. NY-ESO-1: A promising cancer testis antigen for sarcoma immunotherapy and diagnosis. Chin. Clin. Oncol. 2018, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, A.A.; Antonescu, C.R.; Busam, K.J.; Iversen, K.; Kolb, D.; Coplan, K.; Chen, Y.T.; Stockert, E.; Ladanyi, M.; Old, L.J. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int. J. Cancer 2001, 94, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor Regression in Patients with Metastatic Synovial Cell Sarcoma and Melanoma Using Genetically Engineered Lymphocytes Reactive With NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.N.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A Pilot Trial Using Lymphocytes Genetically Engineered with an NY-ESO-1-Reactive T-cell Receptor: Long-term Follow-up and Correlates with Response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Melchiori, L.; Merchant, M.S.; Bernstein, D.; Glod, J.; Kaplan, R.N.; Grupp, S.; Tap, W.D.; Chagin, K.; Binder, G.K.; et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 c259T Cells in Synovial Sarcoma. Cancer Discov. 2018, 8, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, I.; Brett, S.; Domogala, A.; Patasic, L.; Kijewska, M.; Soor, K.; Georgouli, M.; Dopierala, J.; Fisher, P.; Jing, J.; et al. NY-ESO-1 and LAGE1A: An emerging target for cell therapies in solid tumours. Ann. Oncol. 2019, 30, v503. [Google Scholar] [CrossRef]
- Ramachandran, I.; Lowther, D.E.; Dryer-Minnerly, R.; Wang, R.; Fayngerts, S.; Nunez, D.; Betts, G.; Bath, N.; Tipping, A.J.; Melchiori, L.; et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J. Immunother. Cancer 2019, 7, 276. [Google Scholar] [CrossRef]
- Van Tine, B.; Butler, M.; Araujo, D.; Johnson, M.; Clarke, J.; Liebner, D.; Odunsi, K.; Olszanski, A.; Basu, S.; Brophy, F.; et al. ADP-A2M4 (MAGE-A4) in patients with synovial sarcoma. Ann. Oncol. 2019, 30, v684–v685. [Google Scholar] [CrossRef]
- Hong, D.S.; Van Tine, B.A.; Olszanski, A.J.; Johnson, M.L.; Liebner, D.A.; Trivedi, T.; Lin, Q.; Elefant, E.; Dryer-Minnerly, R.; Navenot, J.-M.; et al. Phase I dose escalation and expansion trial to assess the safety and efficacy of ADP-A2M4 SPEAR T cells in advanced solid tumors. J. Clin. Oncol. 2020, 38, 102. [Google Scholar] [CrossRef]
- Suzuki, M.; Curran, K.J.; Cheung, N.K. Chimeric antigen receptors and bispecific antibodies to retarget T cells in pediatric oncology. Pediatr. Blood Cancer 2015, 62, 1326–1336. [Google Scholar] [CrossRef]
- Kowolik, C.M.; Topp, M.S.; Gonzalez, S.; Pfeiffer, T.; Olivares, S.; Gonzalez, N.; Smith, D.D.; Forman, S.J.; Jensen, M.C.; Cooper, L.J. CD28 Costimulation Provided through a CD19-Specific Chimeric Antigen Receptor Enhances In vivo Persistence and Antitumor Efficacy of Adoptively Transferred T Cells. Cancer Res. 2006, 66, 10995–11004. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009, 21, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef] [PubMed]
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Highfill, S.L.; Cui, Y.; Smith, J.P.; Walker, A.J.; Ramakrishna, S.; El-Etriby, R.; Galli, S.; Tsokos, M.G.; Orentas, R.J.; et al. Reduction of MDSCs with All-trans Retinoic Acid Improves CAR Therapy Efficacy for Sarcomas. Cancer Immunol. Res. 2016, 4, 869–880. [Google Scholar] [CrossRef]
- Chulanetra, M.; Morchang, A.; Sayour, E.; Eldjerou, L.; Milner, R.; Lagmay, J.; Cascio, M.; Stover, B.; Slayton, W.; Chaicumpa, W.; et al. GD2 chimeric antigen receptor modified T cells in synergy with sub-toxic level of doxorubicin targeting osteosarcomas. Am. J. Cancer Res. 2020, 10, 674–687. [Google Scholar]
- Ebb, D.H.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II Trial of Trastuzumab in Combination with Cytotoxic Chemotherapy for Treatment of Metastatic Osteosarcoma with Human Epidermal Growth Factor Receptor 2 Overexpression: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef]
- Ahmed, N.; Salsman, V.S.; Yvon, E.; Louis, C.U.; Perlaky, L.; Wels, W.S.; Dishop, M.K.; Kleinerman, E.E.; Pule, M.; Rooney, C.M.; et al. Immunotherapy for Osteosarcoma: Genetic Modification of T cells Overcomes Low Levels of Tumor Antigen Expression. Mol. Ther. 2009, 17, 1779–1787. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Hegde, M.; Derenzo, C.; Zhang, H.; Mata, M.; Gerken, C.; Shree, A.; Yi, Z.; Brawley, V.; Dakhova, O.; Wu, M.-F.; et al. Expansion of HER2-CAR T cells after lymphodepletion and clinical responses in patients with advanced sarcoma. J. Clin. Oncol. 2017, 35, 10508. [Google Scholar] [CrossRef]
- Hegde, M.; Joseph, S.K.; Pashankar, F.; DeRenzo, C.; Sanber, K.; Navai, S.; Byrd, T.T.; Hicks, J.; Xu, M.L.; Gerken, C.; et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 2020, 11, 3549. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yu, L.; Cooper, L.J.; Hollomon, M.; Huls, H.; Kleinerman, E.S. Genetically modified T cells targeting interleukin-11 receptor alpha-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 2012, 72, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 2016, 3, 16011. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kawaguchi, S.; Torigoe, T.; Asanuma, H.; Nakazawa, E.; Shimozawa, K.; Nabeta, Y.; Kimura, S.; Kaya, M.; Nagoya, S.; et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006, 97, 1374–1380. [Google Scholar] [CrossRef]
- Berghuis, D.; De Hooge, A.S.; Santos, S.J.; Horst, D.; Wiertz, E.J.; Van Eggermond, M.C.; Elsen, P.J.V.D.; Taminiau, A.H.; Ottaviano, L.; Schaefer, K.-L.; et al. Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: Implications for immune recognition. J. Pathol. 2009, 218, 222–231. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Markiewicz, K.; Zeman, K.; Kozar, A.; Golebiowska-Wawrzyniak, M.; Wozniak, W. Evaluation of selected parameters of cellular immunity in children with osteosarcoma at diagnosis. Med. Wieku Rozw. 2012, 16, 212–221. [Google Scholar]
- Buddingh, E.P.; Ruslan, S.E.; Berghuis, D.; Gelderblom, H.; Anninga, J.K.; Hogendoorn, P.C.; Egeler, R.M.; Schilham, M.W.; Lankester, A.C. Intact interferon signaling in peripheral blood leukocytes of high-grade osteosarcoma patients. Cancer Immunol. Immunother. 2012, 61, 941–947. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Schilham, M.W.; Ruslan, S.E.; Berghuis, D.; Szuhai, K.; Suurmond, J.; Taminiau, A.H.; Gelderblom, H.; Egeler, R.M.; Serra, M.; et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol. Immunother. 2011, 60, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Eslin, D.; Levy, A.; Roberson, J.; Giusti, V.; Sutphin, R. Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatr. Blood Cancer 2010, 55, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Luksch, R.; Perotti, D.; Cefalo, G.; Gambacorti Passerini, C.; Massimino, M.; Spreafico, F.; Casanova, M.; Ferrari, A.; Terenziani, M.; Polastri, D.; et al. Immunomodulation in a treatment program including pre- and post-operative interleukin-2 and chemotherapy for childhood osteosarcoma. Tumori 2003, 89, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, W.; Xu, P. NK cell and macrophages confer prognosis and reflect immune status in osteosarcoma. J. Cell Biochem. 2018, 120, 8792–8797. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; Webster, D.E.; DeSantes, K.B.; Durkin, E.T.; Shaaban, A.F. KIR receptor-ligand incompatibility predicts killing of osteosarcoma cell lines by allogeneic NK cells. Pediatr. Blood Cancer 2010, 55, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Tarozzi, A.; Meneghetti, A.; Cattini, L.; Facchini, A. Human osteosarcoma cell susceptibility to natural killer cell lysis depends on CD54 and increases after TNF alpha incubation. FEBS Lett. 1997, 406, 83–88. [Google Scholar] [CrossRef]
- Meneghetti, A.; Mariani, E.; Santi, S.; Riccio, M.; Cattini, L.; Paoletti, S.; Facchini, A. NK binding capacity and lytic activity depend on the expression of ICAM-1 on target bone tumours. Int. J. Oncol. 1999, 15, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Zauli, G.; Bavelloni, A.; Marmiroli, S.; Cataldi, A.; Weber, G.; Vitale, M. Tiazofurin induces a down-modulation of ICAM-1 expression on K562 target cells impairing NK adhesion and killing. Cell Immunol. 1995, 164, 100–104. [Google Scholar] [CrossRef]
- Cho, D.; Shook, D.R.; Shimasaki, N.; Chang, Y.-H.; Fujisaki, H.; Campana, D. Cytotoxicity of Activated Natural Killer Cells against Pediatric Solid Tumors. Clin. Cancer Res. 2010, 16, 3901–3909. [Google Scholar] [CrossRef]
- Leung, W.H.; Vong, Q.P.; Lin, W.; Janke, L.; Chen, T.; Leung, W. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRgamma activation. J. Exp. Med. 2013, 210, 2675–2692. [Google Scholar] [CrossRef]
- Fernandez, L.; Valentin, J.; Zalacain, M.; Leung, W.; Patino-Garcia, A.; Perez-Martinez, A. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. 2015, 368, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sayitoglu, E.C.; Georgoudaki, A.M.; Chrobok, M.; Ozkazanc, D.; Josey, B.J.; Arif, M.; Kusser, K.; Hartman, M.; Chinn, T.M.; Potens, R.; et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, M.; Guo, H.; Chen, Y.; Huse, M.; Cheung, N.-K.V. Retargeting T cells to GD2 pentasaccharide on human tumors using Bispecific humanized antibody. Cancer Immunol. Res. 2014, 3, 266–277. [Google Scholar] [CrossRef]
- Mitsis, D.; Francescutti, V.; Skitzki, J. Current Immunotherapies for Sarcoma: Clinical Trials and Rationale. Sarcoma 2016, 2016, 9757219. [Google Scholar] [CrossRef] [PubMed]
- Pender, A.; Jones, R.L.; Pollack, S. Optimising Cancer Vaccine Design in Sarcoma. Cancers 2018, 11, 1. [Google Scholar] [CrossRef]
- Pollack, S.M.; Loggers, E.T.; Rodler, E.T.; Yee, C.; Jones, R.L. Immune-based therapies for sarcoma. Sarcoma 2011, 2011, 438940. [Google Scholar] [CrossRef]
- Wilky, B.A.; Goldberg, J.M. Immunotherapy in sarcoma: A new frontier. Discov. Med. 2014, 17, 201–206. [Google Scholar]
- Jager, E.; Gnjatic, S.; Nagata, Y.; Stockert, E.; Jager, D.; Karbach, J.; Neumann, A.; Rieckenberg, J.; Chen, Y.T.; Ritter, G.; et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc. Natl. Acad. Sci. USA 2000, 97, 12198–12203. [Google Scholar] [CrossRef]
- Davis, I.D.; Chen, W.; Jackson, H.; Parente, P.; Shackleton, M.; Hopkins, W.; Chen, Q.; Dimopoulos, N.; Luke, T.; Murphy, R.; et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 10697–10702. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Agulnik, M.; Ryan, C.W.; Milhem, M.; George, S.; Jones, R.L.; Chmielowski, B.; Van Tine, B.A.; Tawbi, H.A.-H.; Elias, A.D.; et al. Trivalent ganglioside vaccine and immunologic adjuvant versus adjuvant alone in metastatic sarcoma patients rendered disease-free by surgery: A randomized phase 2 trial. J. Clin. Oncol. 2014, 32, 10520. [Google Scholar] [CrossRef]
- Clark, J.; Rocques, P.J.; Crew, A.J.; Gill, S.; Shipley, J.; Chan, A.M.; Gusterson, B.A.; Cooper, C.S. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat. Genet. 1994, 7, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Ida, K.; Kawaguchi, S.; Sato, Y.; Tsukahara, T.; Nabeta, Y.; Sahara, H.; Ikeda, H.; Torigoe, T.; Ichimiya, S.; Kamiguchi, K.; et al. Crisscross CTL Induction by SYT-SSX Junction Peptide and Its HLA-A*2402 Anchor Substitute. J. Immunol. 2004, 173, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nabeta, Y.; Tsukahara, T.; Hirohashi, Y.; Syunsui, R.; Maeda, A.; Sahara, H.; Ikeda, H.; Torigoe, T.; Ichimiya, S.; et al. Detection and Induction of CTLs Specific for SYT-SSX-Derived Peptides in HLA-A24+ Patients with Synovial Sarcoma. J. Immunol. 2002, 169, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Tsukahara, T.; Ida, K.; Kimura, S.; Murase, M.; Kano, M.; Emori, M.; Nagoya, S.; Kaya, M.; Torigoe, T.; et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: A study from the Japanese Musculoskeletal Oncology Group. Cancer Sci. 2012, 103, 1625–1630. [Google Scholar] [CrossRef]
- Fields, R.C.; Shimizu, K.; Mule, J.J. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 9482–9487. [Google Scholar] [CrossRef]
- He, Y.T.; Zhang, Q.M.; Kou, Q.C.; Tang, B. In vitro generation of cytotoxic T lymphocyte response using dendritic cell immunotherapy in osteosarcoma. Oncol. Lett. 2016, 12, 1101–1106. [Google Scholar] [CrossRef]
- Miwa, S.; Nishida, H.; Tanzawa, Y.; Takeuchi, A.; Hayashi, K.; Yamamoto, N.; Mizukoshi, E.; Nakamoto, Y.; Kaneko, S.; Tsuchiya, H. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer 2017, 123, 1576–1584. [Google Scholar] [CrossRef]
- Geiger, J.D.; Hutchinson, R.J.; Hohenkirk, L.F.; McKenna, E.A.; Yanik, G.A.; Levine, J.E.; Chang, A.E.; Braun, T.M.; Mule, J.J. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001, 61, 8513–8519. [Google Scholar]
- Himoudi, N.; Wallace, R.; Parsley, K.L.; Gilmour, K.; Barrie, A.-U.; Howe, K.; Dong, R.; Sebire, N.J.; Michalski, A.; Thrasher, A.J.; et al. Lack of T-cell responses following autologous tumour lysate pulsed dendritic cell vaccination, in patients with relapsed osteosarcoma. Clin. Transl. Oncol. 2012, 14, 271–279. [Google Scholar] [CrossRef]
- Merchant, M.S.; Bernstein, D.; Amoako, M.; Baird, K.; Fleisher, T.A.; Morre, M.; Steinberg, S.M.; Sabatino, M.; Stroncek, D.F.; Venkatasan, A.M.; et al. Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas. Clin. Cancer Res. 2016, 22, 3182–3191. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Sznol, M.; Zhao, B.; Wang, D.; Carvajal, R.D.; Keohan, M.L.; Chuang, E.; Sanborn, R.E.; Lutzky, J.; Powderly, J.; et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014, 6, 232ra251. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Middelberg, A.P.; Gemiarto, A.; MacDonald, K.; Baxter, A.G.; Talekar, M.; Moi, D.; Tullett, K.M.; Caminschi, I.; Lahoud, M.H.; et al. Self-adjuvanting nanoemulsion targeting dendritic cell receptor Clec9A enables antigen-specific immunotherapy. J. Clin. Investig. 2018, 128, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Masterman, K.A.; Haigh, O.L.; Tullett, K.M.; Leal-Rojas, I.M.; Walpole, C.; Pearson, F.E.; Cebon, J.; Schmidt, C.; O’Brien, L.; Rosendahl, N.; et al. Human CLEC9A antibodies deliver NY-ESO-1 antigen to CD141(+) dendritic cells to activate naive and memory NY-ESO-1-specific CD8(+) T cells. J. Immunother. Cancer 2020, 8, e000691. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Itonaga, I.; Iwasaki, T.; Tsumura, H. Enhancement of antitumor immunity by combining anti-cytotoxic T lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed dendritic cells in murine osteosarcoma. Oncol. Rep. 2013, 29, 1001–1006. [Google Scholar] [CrossRef]
- Chawla, S.; Van Tine, B.A.; Pollack, S.; Ganjoo, K.; Elias, A.; Riedel, R.F.; Attia, S.; Choy, E.; Okuno, S.; Agulnik, M.; et al. A phase 2 study of CMB305 and atezolizumab in NY-ESO-1+ soft tissue sarcoma: Interim analysis of immunogenicity, tumor control and survival. Ann. Oncol. 2017, 28, v523. [Google Scholar] [CrossRef]
- Chawla, S.P.; Tine, B.A.V.; Pollack, S.; Ganjoo, K.N.; Elias, A.D.; Riedel, R.F.; Attia, S.; Choy, E.; Okuno, S.H.; Agulnik, M.; et al. A phase II randomized study of CMB305 and atezolizumab versus atezolizumab in NY-ESO-1+ soft tissue sarcoma: Analysis of immunogenicity, tumor control, and patient survival. J. Clin. Oncol. 2019, 37, 11011. [Google Scholar] [CrossRef]
- Lettieri, C.K.; Hingorani, P.; Kolb, E.A. Progress of oncolytic viruses in sarcomas. Expert Rev. Anticancer. Ther. 2012, 12, 229–242. [Google Scholar] [CrossRef]
- Abdelbary, H.; Brown, C.W.; Werier, J.; Bell, J. Using targeted virotherapy to treat a resistant Ewing sarcoma model: From the bedside to the bench and back. Sci. World J. 2014, 2014, 171439. [Google Scholar] [CrossRef]
- Morton, C.L.; Houghton, P.J.; Kolb, E.A.; Gorlick, R.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Keir, S.T.; Wu, J.; Smith, M.A. Initial testing of the replication competent Seneca Valley virus (NTX-010) by the pediatric preclinical testing program. Pediatr. Blood Cancer 2010, 55, 295–303. [Google Scholar] [CrossRef]
- Paglino, J.C.; van den Pol, A.N. Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: Rare resistance is overcome by blocking interferon pathways. J. Virol. 2011, 85, 9346–9358. [Google Scholar] [CrossRef]
- Takakuwa, H.; Goshima, F.; Nozawa, N.; Yoshikawa, T.; Kimata, H.; Nakao, A.; Nawa, A.; Kurata, T.; Sata, T.; Nishiyama, Y. Oncolytic viral therapy using a spontaneously generated herpes simplex virus type 1 variant for disseminated peritoneal tumor in immunocompetent mice. Arch. Virol. 2003, 148, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Antonescu, C.R.; Bowler, T.; Munhoz, R.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Dholakia, R.; et al. Objective Response Rate Among Patients with Locally Advanced or Metastatic Sarcoma Treated with Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hadrys, A.; Sochanik, A.; McFadden, G.; Jazowiecka-Rakus, J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur. J. Pharmacol. 2020, 874, 172991. [Google Scholar] [CrossRef] [PubMed]
- Mahasa, K.J.; de Pillis, L.; Ouifki, R.; Eladdadi, A.; Maini, P.; Yoon, A.R.; Yun, C.O. Mesenchymal stem cells used as carrier cells of oncolytic adenovirus results in enhanced oncolytic virotherapy. Sci. Rep. 2020, 10, 425. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Martinez, M.; Moon, E.K. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front. Immunol. 2019, 10, 128. [Google Scholar] [CrossRef]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014, 6, 237ra267. [Google Scholar] [CrossRef]
- Deng, J.; Zeng, W.; Kong, W.; Shi, Y.; Mou, X. The Study of Sarcoma Microenvironment Heterogeneity Associated with Prognosis Based on an Immunogenomic Landscape Analysis. Front. Bioeng. Biotechnol. 2020, 8, 1003. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reynies, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougouin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Dufresne, A.; Lesluyes, T.; Menetrier-Caux, C.; Brahmi, M.; Darbo, E.; Toulmonde, M.; Italiano, A.; Mir, O.; Le Cesne, A.; Le Guellec, S.; et al. Specific immune landscapes and immune checkpoint expressions in histotypes and molecular subtypes of sarcoma. Oncoimmunology 2020, 9, 1792036. [Google Scholar] [CrossRef]
- Menetrier-Caux, C.; Montmain, G.; Dieu, M.C.; Bain, C.; Favrot, M.C.; Caux, C.; Blay, J.Y. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: Role of interleukin-6 and macrophage colony-stimulating factor. Blood 1998, 92, 4778–4791. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, S.W.; Kilvaer, T.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T. Prognostic impact of lymphocytes in soft tissue sarcomas. PLoS ONE 2011, 6, e14611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, J.; Zhang, J.; Wang, L.; Zhang, Q.; Ge, J.; Guo, Y.; Wang, B.; Huang, Y.; Yang, T.; et al. SDF-1/CXCR4 axis facilitates myeloid-derived suppressor cells accumulation in osteosarcoma microenvironment and blunts the response to anti-PD-1 therapy. Int. Immunopharmacol. 2019, 75, 105818. [Google Scholar] [CrossRef] [PubMed]
| Trial ID | Phase | Treatment | Sarcoma Type | Status |
|---|---|---|---|---|
| NCT03074318 | I/II | Avelumab and Trabectedin | Advanced liposarcoma and leiomyosarcoma | Active, not recruiting |
| NCT03006848 | II | Avelumab | Recurrent or progressive osteosarcoma | Active, not recruiting |
| NCT02834013 | II | Niovlumab and ipilimumab | Advanced angiosarcoma | Recruiting |
| NCT03474640 | I | Toripalimab | Advanced soft tissue sarcoma and chondrosarcoma | Recruiting |
| NCT04140526 | I | ONC-392 with/without pembrolizumab | Advanced soft tissue sarcoma | Recruiting |
| NCT02304458 | I/II | Nivolumab with/without ipilimumab | Recurrent/refractory sarcoma: Ewing sarcoma, osteosarcoma, rhabdomyosarcoma | Active, not recruiting |
| NCT04095208 | II | Nivolumab with/without relatlimab | Advanced or metastatic soft tissue sarcoma | Recruiting |
| NCT03899805 | II | Eribulin and pembrolizumab | Refractory liposarcoma, leiomyosarcoma, undifferentiated pleomorphic sarcoma | Recruiting |
| NCT03141684 | II | Atezolizumab | Advanced alveolar soft part sarcoma | Recruiting |
| NCT03338959 | I/II | Pembrolizumab with radiation therapy | Intermediate or high-grade soft tissue sarcoma | Recruiting |
| NCT04458922 | II | Atezolizumab | Newly diagnosed/unresectable/metastatic chondrosarcoma, clear cell sarcoma | Recruiting |
| NCT03307616 | II | Neoadjuvant nivolumab, nivolumab and ipilimumab, nivolumab and radiation therapy, nivolumab and ipilimumab and radiation therapy | Recurrent or resectable undifferentiated pleomorphic sarcoma or dedifferentiated liposarcoma | Active, not recruiting |
| NCT02500797 | II | Nivolumab with/without ipilimumab | Metastatic/unresectable bone sarcoma, liposarcoma, undifferentiated pleomorphic sarcoma | Active, not recruiting |
| NCT03463408 | Early I | Neoadjuvant nivolumab and ipilimumab and radiation therapy | Resectable soft tissue sarcoma | Recruiting |
| NCT03116529 | I/II | Neoadjuvant durvalumab and tremelimumab and radiation therapy | High risk soft tissue sarcoma | Recruiting |
| NCT02815995 | II | Durvalumab and tremelimumab | Advanced/metastatic sarcoma | Active, not recruiting |
| NCT03138161 | I/II | Trabectedin and ipilimumab and nivolumab | Advanced/metastatic soft tissue sarcoma | Recruiting |
| NCT02992743 | II | Autologous NY-ESO-1 genetically modified T cells (NY-ESO-1c259T) | Advanced myxoid/round cell liposarcoma | Recruiting |
| NCT04044768 | II | Autologous ADP-A2M4 genetically modified T cells | Advanced synovial sarcoma or myxoid/ round cell liposarcoma | Recruiting |
| NCT03450122 | I | Autologous NY-ESO-1 genetically modified T cells and chemotherapy and aldesleukin with/without immunostimulatory agents: CMB305 and/or antigen-specific vaccine (ID-LV305) | Advanced or recurrent synovial sarcoma, myxoid liposarcoma, NY-ESO-1 positive sarcoma | Recruiting |
| NCT02650986 | I/II | Autologous NY-ESO-1 genetically modified T cells with/without decitabine | Advanced/metastatic/unresectable synovial sarcoma | Recruiting |
| NCT03250325 | I/II | Autologous NY-ESO-1 genetically modified T cells | Unresectable NY-ESO-1 positive synovial sarcoma | Active, not recruiting |
| NCT04556669 | I | Autologous CD22 CAR genetically modified T cells or TILs with scFv fragment of anti-PD-L1 monoclonal antibody | Sarcoma | Recruiting |
| NCT03635632 | I | Autologous GD2 CAR genetically modified T cells | Relapsed GD2 positive osteosarcoma, Ewing sarcoma, rhabdomyosarcoma | Recruiting |
| NCT03635632 | I/II | Autologous sarcoma specific (CD133, GD2, Muc1, CD117 or other marker) CAR genetically modified T cells | Advanced/ recurrent sarcoma | Recruiting |
| NCT03638206 | I/II | Autologous NY-ESO-1 CAR genetically modified T cells | Synovial sarcoma | Recruiting |
| NCT00902044 | I | Autologous HER2-CD28 CAR genetically modified T cells | Refractory HER2 positive sarcoma, metastatic HER2 positive osteosarcoma | Active, not recruiting |
| NCT04483778 | I | Autologous B7H3 CAR or bispecific B7H3 and CD19 CAR genetically modified T cells | Osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, synovial sarcoma, clear cell sarcoma, soft tissue sarcoma | Recruiting |
| NCT04433221 | I/II | Autologous sarcoma specific (GD2, HER2, PSMA, CD276 or other marker) CAR genetically modified T cells | Advanced/recurrent sarcoma | Recruiting |
| NCT02100891 | II | HLA-haploidentical haematopoietic cell transplantation and donor NK cell infusion | Advanced/recurrent Ewing sarcoma, rhabdomyosarcoma, osteosarcoma | Active, not recruiting |
| NCT02409576 | I/II | Expanded and activated allogenic NK cells | Advanced/metastatic/relapsed Ewing sarcoma, rhabdomyosarcoma | Recruiting |
| NCT03860207 | I/II | Humanised 3F8 bispecific antibody (Hu3F8-BsAb) | Relapsed/refractory GD2 positive osteosarcoma | Recruiting |
| NCT01803152 | I | Autologous tumour lysate with dendritic cell vaccine with/without myeloid derived suppressor cells inhibition | Relapsed sarcoma | Active, not recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, H.Y.; Chan, V.; Simpson, F.; Dolcetti, R. Will Next-Generation Immunotherapy Overcome the Intrinsic Diversity and Low Immunogenicity of Sarcomas to Improve Clinical Benefit? Cancers 2020, 12, 3392. https://doi.org/10.3390/cancers12113392
Chew HY, Chan V, Simpson F, Dolcetti R. Will Next-Generation Immunotherapy Overcome the Intrinsic Diversity and Low Immunogenicity of Sarcomas to Improve Clinical Benefit? Cancers. 2020; 12(11):3392. https://doi.org/10.3390/cancers12113392
Chicago/Turabian StyleChew, Hui Yi, Victor Chan, Fiona Simpson, and Riccardo Dolcetti. 2020. "Will Next-Generation Immunotherapy Overcome the Intrinsic Diversity and Low Immunogenicity of Sarcomas to Improve Clinical Benefit?" Cancers 12, no. 11: 3392. https://doi.org/10.3390/cancers12113392
APA StyleChew, H. Y., Chan, V., Simpson, F., & Dolcetti, R. (2020). Will Next-Generation Immunotherapy Overcome the Intrinsic Diversity and Low Immunogenicity of Sarcomas to Improve Clinical Benefit? Cancers, 12(11), 3392. https://doi.org/10.3390/cancers12113392






