Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer—A Secondary Analysis of the PET Plan Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Toxicity
2.3. Dose Modifications and Completion of Chemotherapy
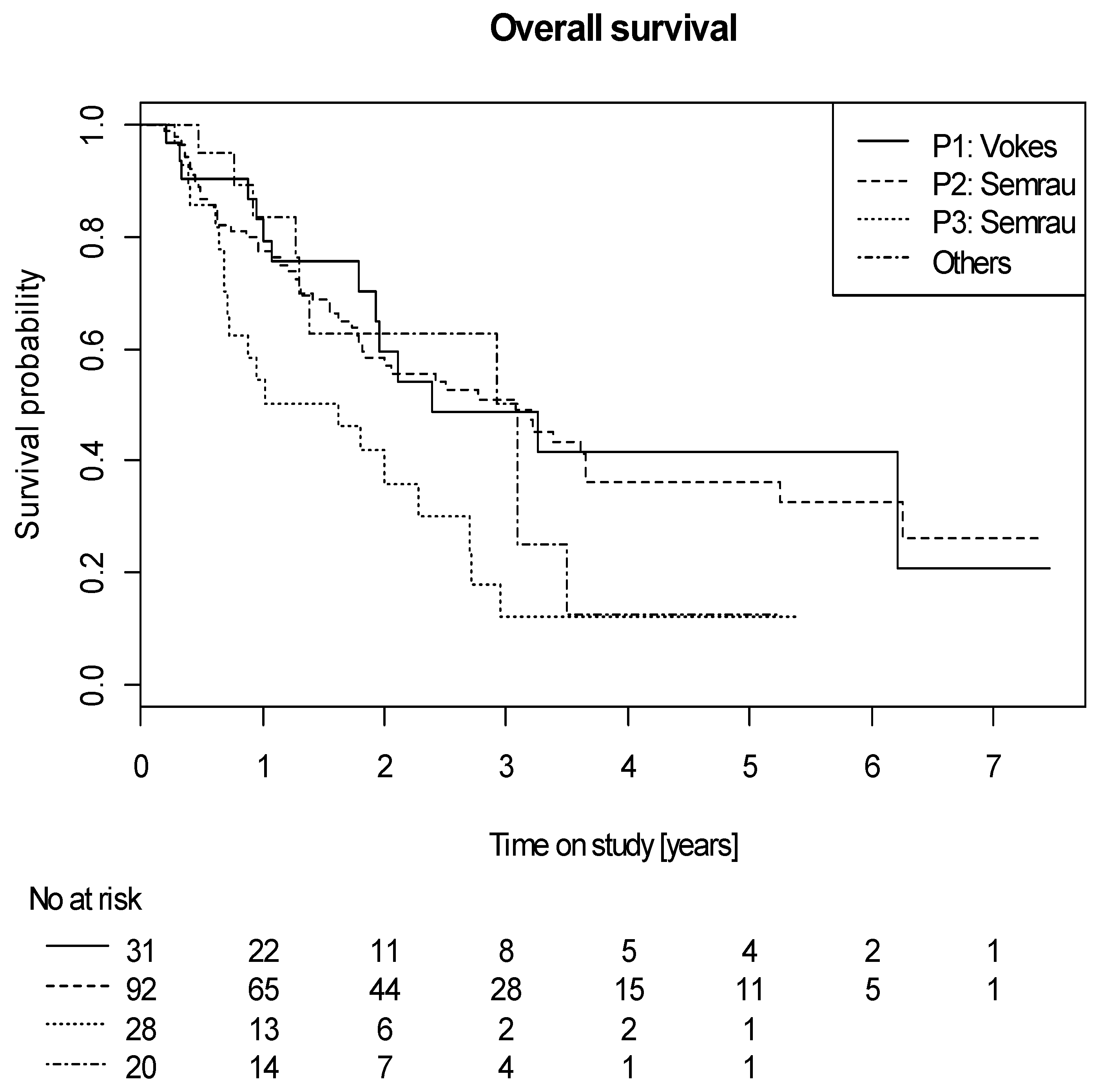
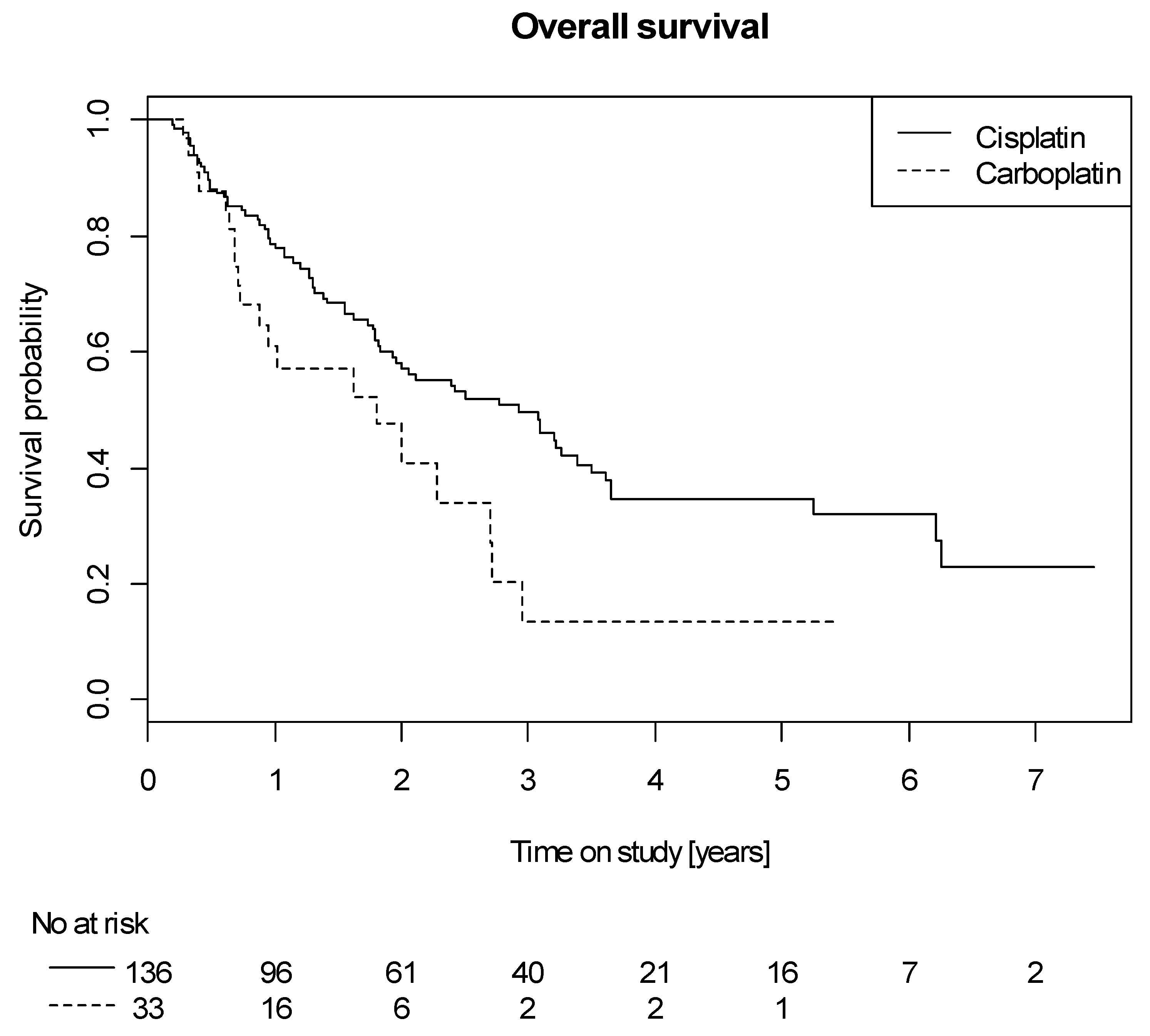
2.4. Efficacy
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Radiotherapy
4.3. Chemotherapy
- A
- Cisplatin 80 mg/m2/d (day 1 and 22) and vinorelbin 15 mg/m2/d (day 1 + 8 and 22 + 29) according to Vokes et al. [6] (Protocol 1, P1),
- B
- Cisplatin 20 mg/m2/d (day 1–5 and 29–33) and vinorelbin 12.5 mg/m2/d (day 1, 8, 15 and 29, 36, 43) according to Semrau et al. [26], (protocol 2, P2)
- C
- Carboplatin AUC1 (day 1–5 and 29–33) and vinorelbin 12.5 mg/m2/d (day 1, 8, 15 and 29, 36, 43) according to Semrau et al. [26], (protocol 3, P3)
- D
- Other platinum-based doublets such as cisplatin, 50 mg/m2/d (days 1, 8, 29, and 36) and etoposide, 50 mg/m2/d (days 1–5 and 29–33) according to Albain et al. [52], cisplatin 20 mg/m2/d and etoposide 50 mg/m2/d (days 1 to 5 and days 29 to 33) according to Fournel et al. [8], cisplatin and oral vinorelbine followed with maintenance with oral vinorelbine and cisplatin, analog to the GILT trial [25] or any other platinum-based doublet according to the institutional standard operating procedure of each (others).
4.4. Response Evaluation and Toxicity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nakagawa, K.; Nishimura, Y.; Tsujino, K.; Satouchi, M.; Kudo, S.; Hida, T.; Kawahara, M.; Takeda, K.; Katakami, N.; et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3739–3745. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Kiura, K.; Takigawa, N.; Kamei, H.; Harita, S.; Hiraki, S.; Watanabe, Y.; Sugimoto, K.; Shibayama, T.; Yonei, T.; et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, Z.; Dang, J.; Li, G. Comparative efficacy and safety for different chemotherapy regimens used concurrently with thoracic radiation for locally advanced non-small cell lung cancer: A systematic review and network meta-analysis. Radiat. Oncol. 2019, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Martinez Aguillo, M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Vokes, E.E.; II, J.E.H.; Crawford, J.; Leopold, K.A.; Perry, M.C.; Miller, A.A.; Green, M.R. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non–small-cell lung cancer: Cancer and leukemia group B study 9431. J. Clin. Oncol. 2002, 20, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Blackstock, A.W.; Bogart, J.A.; Wang, X.; Munley, M.; Rosenman, J.; Gu, L.; Masters, G.A.; Ungaro, P.; Sleeper, A.; et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2457–2463. [Google Scholar] [CrossRef]
- Fournel, P.; Robinet, G.; Thomas, P.; Souquet, P.J.; Lena, H.; Vergnenegre, A.; Delhoume, J.Y.; Le Treut, J.; Silvani, J.A.; Dansin, E.; et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe lyon-saint-etienne d’oncologie thoracique-groupe francais de pneumo-cancerologie NPC 95-01 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5910–5917. [Google Scholar] [CrossRef]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460. [Google Scholar] [CrossRef]
- Kelly, K.; Chansky, K.; Gaspar, L.E.; Albain, K.S.; Jett, J.; Ung, Y.C.; Lau, D.H.; Crowley, J.J.; Gandara, D.R. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2450–2456. [Google Scholar] [CrossRef]
- Jalal, S.I.; Riggs, H.D.; Melnyk, A.; Richards, D.; Agarwala, A.; Neubauer, M.; Ansari, R.; Govindan, R.; Bruetman, D.; Fisher, W.; et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: Analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2012, 23, 1730–1738. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; Committee, E.G. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Botterweck, A.; Dirx, M.; Pijls-Johannesma, M.; Wanders, R.; Hochstenbag, M.; Dingemans, A.M.; Bootsma, G.; Geraedts, W.; Simons, J.; et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: A prospective, population-based study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2009, 20, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Lokich, J.; Anderson, N. Carboplatin versus cisplatin in solid tumors: An analysis of the literature. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 1998, 9, 13–21. [Google Scholar] [CrossRef]
- Muggia, F.M. Overview of carboplatin: Replacing, complementing, and extending the therapeutic horizons of cisplatin. Semin Oncol. 1989, 16, 7–13. [Google Scholar]
- Fossella, F.; Pereira, J.R.; von Pawel, J.; Pluzanska, A.; Gorbounova, V.; Kaukel, E.; Mattson, K.V.; Ramlau, R.; Szczesna, A.; Fidias, P.; et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3016–3024. [Google Scholar] [CrossRef]
- Mazzanti, P.; Massacesi, C.; Rocchi, M.B.; Mattioli, R.; Lippe, P.; Trivisonne, R.; Buzzi, F.; De Signoribus, G.; Tuveri, G.; Rossi, G.; et al. Randomized, multicenter, phase II study of gemcitabine plus cisplatin versus gemcitabine plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer 2003, 41, 81–89. [Google Scholar] [CrossRef]
- Zatloukal, P.; Petruzelka, L.; Zemanova, M.; Kolek, V.; Skrickova, J.; Pesek, M.; Fojtu, H.; Grygarkova, I.; Sixtova, D.; Roubec, J.; et al. Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: A phase III randomized trial. Lung Cancer 2003, 41, 321–331. [Google Scholar] [CrossRef]
- Paccagnella, A.; Favaretto, A.; Oniga, F.; Barbieri, F.; Ceresoli, G.; Torri, W.; Villa, E.; Verusio, C.; Cetto, G.L.; Santo, A.; et al. Cisplatin versus carboplatin in combination with mitomycin and vinblastine in advanced non small cell lung cancer. A multicenter, randomized phase III trial. Lung Cancer 2004, 43, 83–91. [Google Scholar] [CrossRef]
- Belani, C.P.; Lee, J.S.; Socinski, M.A.; Robert, F.; Waterhouse, D.; Rowland, K.; Ansari, R.; Lilenbaum, R.; Natale, R.B. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2005, 16, 1069–1075. [Google Scholar] [CrossRef]
- Rudd, R.M.; Gower, N.H.; Spiro, S.G.; Eisen, T.G.; Harper, P.G.; Littler, J.A.; Hatton, M.; Johnson, P.W.; Martin, W.M.; Rankin, E.M.; et al. Gemcitabine plus carboplatin versus mitomycin, ifosfamide, and cisplatin in patients with stage IIIB or IV non-small-cell lung cancer: A phase III randomized study of the London Lung Cancer Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 142–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Booton, R.; Lorigan, P.; Anderson, H.; Baka, S.; Ashcroft, L.; Nicolson, M.; O’Brien, M.; Dunlop, D.; O’Byrne, K.; Laurence, V.; et al. A phase III trial of docetaxel/carboplatin versus mitomycin C/ifosfamide/cisplatin (MIC) or mitomycin C/vinblastine/cisplatin (MVP) in patients with advanced non-small-cell lung cancer: A randomised multicentre trial of the British Thoracic Oncology Group (BTOG1). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2006, 17, 1111–1119. [Google Scholar] [CrossRef]
- D’Addario, G.; Pintilie, M.; Leighl, N.B.; Feld, R.; Cerny, T.; Shepherd, F.A. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: A meta-analysis of the published literature. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- Nestle, U.; Schimek-Jasch, T.; Kremp, S.; Schaefer-Schuler, A.; Mix, M.; Kusters, A.; Tosch, M.; Hehr, T.; Eschmann, S.M.; Bultel, Y.P.; et al. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): A multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020, 21, 581–592. [Google Scholar] [CrossRef]
- Flentje, M.; Huber, R.M.; Engel-Riedel, W.; Andreas, S.; Kollmeier, J.; Staar, S.; Dickgreber, N.; Vaissiere, N.; De Almeida, C.; Edlich, B.; et al. GILT—A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther. Und Onkol. Organ. Dtsch. Rontgenges. 2016, 192, 216–222. [Google Scholar] [CrossRef]
- Semrau, S.; Klautke, G.; Virchow, J.C.; Kundt, G.; Fietkau, R. Impact of comorbidity and age on the outcome of patients with inoperable NSCLC treated with concurrent chemoradiotherapy. Respir. Med. 2008, 102, 210–218. [Google Scholar] [CrossRef][Green Version]
- Arriagada, R.; Auperin, A.; Burdett, S.; Higgins, J.P.; Johnson, D.H.; Le Chevalier, T.; Le Pechoux, C.; Parmar, M.K.; Pignon, J.P.; Souhami, R.L.; et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef]
- Marino, P.; Preatoni, A.; Cantoni, A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 1995, 76, 593–601. [Google Scholar] [CrossRef]
- Steuer, C.E.; Behera, M.; Ernani, V.; Higgins, K.A.; Saba, N.F.; Shin, D.M.; Pakkala, S.; Pillai, R.N.; Owonikoko, T.K.; Curran, W.J.; et al. Comparison of concurrent use of thoracic radiation with either carboplatin-paclitaxel or cisplatin-etoposide for patients with stage III non-small-cell lung cancer: A systematic review. JAMA Oncol. 2017, 3, 1120–1129. [Google Scholar] [CrossRef]
- Santana-Davila, R.; Devisetty, K.; Szabo, A.; Sparapani, R.; Arce-Lara, C.; Gore, E.M.; Moran, A.; Williams, C.D.; Kelley, M.J.; Whittle, J. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: An analysis of Veterans Health Administration data. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 567–574. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; Ou, G.; Bi, N.; Li, W.; Ren, H.; Cao, J.; Liang, J.; Li, J.; Zhou, Z.; et al. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer 2012, 77, 89–96. [Google Scholar] [CrossRef]
- Liang, J.; Bi, N.; Wu, S.; Chen, M.; Lv, C.; Zhao, L.; Shi, A.; Jiang, W.; Xu, Y.; Zhou, Z.; et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: A multicenter randomized phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2017, 28, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Korol, E.E.; Kayaniyil, S.; Varol, N.; Ebner, T.; Goring, S.M. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer 2019, 135, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Boni, L.; Tiseo, M.; Fossella, F.V.; Schiller, J.H.; Paesmans, M.; Radosavljevic, D.; Paccagnella, A.; Zatloukal, P.; Mazzanti, P.; et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J. Natl. Cancer Inst. 2007, 99, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Matsuo, K.; Ueoka, H.; Kiura, K.; Tabata, M.; Tanimoto, M. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 3852–3859. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, X.; Zhou, X.; Huang, R.; Chu, Z. A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 2007, 57, 348–358. [Google Scholar] [CrossRef]
- Auperin, A.; Le Pechoux, C.; Pignon, J.P.; Koning, C.; Jeremic, B.; Clamon, G.; Einhorn, L.; Ball, D.; Trovo, M.G.; Groen, H.J.; et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2006, 17, 473–483. [Google Scholar] [CrossRef]
- Rosell, R.; Gatzemeier, U.; Betticher, D.C.; Keppler, U.; Macha, H.N.; Pirker, R.; Berthet, P.; Breau, J.L.; Lianes, P.; Nicholson, M.; et al. Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non-small-cell lung cancer: A cooperative multinational trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2002, 13, 1539–1549. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Eberhardt, W.E. Concurrent chemoradiotherapy in stage III non-small-cell lung cancer: What is the best regimen? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, R.E. Cisplatin versus carboplatin in NSCLC: Is there one “best” answer? Curr. Treat. Options Oncol. 2008, 9, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Ezer, N.; Smith, C.B.; Galsky, M.D.; Mhango, G.; Gu, F.; Gomez, J.; Strauss, G.M.; Wisnivesky, J. Cisplatin vs. carboplatin-based chemoradiotherapy in patients >65 years of age with stage III non-small cell lung cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 112, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Flieswasser, T.; Van Loenhout, J.; Freire Boullosa, L.; Van den Eynde, A.; De Waele, J.; Van Audenaerde, J.; Lardon, F.; Smits, E.; Pauwels, P.; Jacobs, J. Clinically relevant chemotherapeutics have the ability to induce immunogenic cell death in non-small cell lung cancer. Cells 2020, 9, 1474. [Google Scholar] [CrossRef]
- Vasconcellos, V.F.; Marta, G.N.; da Silva, E.M.; Gois, A.F.; de Castria, T.B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 1, CD009256. [Google Scholar] [CrossRef]
- Naidoo, J.; Nishino, M.; Patel, S.P.; Shankar, B.; Rekhtman, N.; Illei, P.; Camus, P. Immune-related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage III non-small-cell lung cancer. Clin. Lung Cancer 2020, 21, e435–e444. [Google Scholar] [CrossRef]
- Palma, D.A.; Senan, S.; Tsujino, K.; Barriger, R.B.; Rengan, R.; Moreno, M.; Bradley, J.D.; Kim, T.H.; Ramella, S.; Marks, L.B.; et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 444–450. [Google Scholar] [CrossRef]
- Maggiore, R.J.; Zahrieh, D.; McMurray, R.P.; Feliciano, J.L.; Samson, P.; Mohindra, P.; Chen, H.; Wong, M.L.; Lafky, J.M.; Jatoi, A.; et al. Toxicity and survival outcomes in older adults receiving concurrent or sequential chemoradiation for stage III non-small cell lung cancer in Alliance trials (Alliance A151812). J. Geriatr. Oncol. 2020. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; Pottgen, C.; Gauler, T.C.; Friedel, G.; Veit, S.; Heinrich, V.; Welter, S.; Budach, W.; Spengler, W.; Kimmich, M.; et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4194–4201. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Crowley, J.J.; Turrisi, A.T., 3rd; Gandara, D.R.; Farrar, W.B.; Clark, J.I.; Beasley, K.R.; Livingston, R.B. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up††FootnotesApproved by the ESMO Guidelines Committee: February 2002, last update September 2018. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]

| P1 (n = 31) | P2 (n = 92) | P3 (n = 28) | Others * (n = 20) | |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 61 (57–65) | 65(58–72) | 70 (63–73) | 62 (59–70) |
| Sex | ||||
| Male | 24 (77%) | 68 (74%) | 22 (79%) | 13 (65%) |
| Female | 7 (23%) | 24 (26%) | 6 (21%) | 7 (35%) |
| ECOG performance status | ||||
| 0 | 5 (16%) | 12 (13%) | 6 (21%) | 6 (30%) |
| 1 | 25 (81%) | 70 (76%) | 19 (68%) | 12 (60%) |
| 2 | 1 (3%) | 10 (11%) | 3 (11%) | 2 (10%) |
| UICC (7th edition) stage at study inclusion | ||||
| IIA | 0 (0%) | 3 (3%) | 2 (7%) | 0 (0%) |
| IIB | 6 (19%) | 1 (1%) | 0 (0%) | 1 (5%) |
| IIIA | 9 (29%) | 39 (42%) | 11 (39%) | 3 (15%) |
| IIIB | 16 (52%) | 49 (53%) | 15 (54%) | 16 (80%) |
| Histology | ||||
| Squamous cell carcinoma | 21 (68%) | 49 (53%) | 19 (68%) | 11 (55%) |
| Adenocarcinoma | 9 (29%) | 31 (34%) | 6 (21%) | 9 (45%) |
| Large cell carcinoma | 0 (0%) | 2 (2%) | 2 (7%) | 0 (0%) |
| NOS or other subtypes | 1 (3%) | 9 (10%) | 1 (4%) | 0 (0%) |
| Missing | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) |
| GTV primary, mL | ||||
| Median (IQR) | 52 (35–113) | 73 (38–127) | 62 (27–76) | 98 (43–237) |
| PTV, mL | ||||
| Median (IQR) | 428 (326–627) | 525 (369–726) | 528 (343–695) | 682 (452–830) |
| Number of PET-positive lymph node stations | ||||
| Median (min–max) | 2 (0–7) | 3 (0–9) | 3.5 (1–7) | 3 (0–9) |
| Ejection fraction at baseline§ | ||||
| median (min–max) | 60 (50–76) | 55 (35–92) | 60 (50–79) | 60 (45–75) |
| missing | 10 (32%) | 4 (4%) | 1 (4%) | 0 (0%) |
| Creatinine in mg/dL at baseline | ||||
| Median (min–max) | 0.77 (0.42–1.25) | 0.86 (0.5–1.39) | 1.1 (0.6–1.77) | 0.84 (0.59–1.7) |
| Missing | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Weight loss † | ||||
| <5% | 18 (58%) | 62 (67%) | 21 (75%) | 13 (65%) |
| 5–10% | 2 (7%) | 9 (10%) | 4 (14%) | 1 (5%) |
| >10% | 6 (19%) | 16 (17%) | 1 (4%) | 5 (25%) |
| Missing | 5 (16%) | 5 (6%) | 2 (7%) | 1 (5%) |
| Complete administration of chemotherapy | ||||
| Cycle 1 | 26 (84%) | 74 (80%) | 26 (93%) | 12 (60%) |
| Cycle 2 | 25 (81%) | 66 (72%) | 23 (82%) | 10 (50%) |
| Cisplatin (n = 136) | Carboplatin (n = 33) | |
|---|---|---|
| Age, years | ||
| Median (IQR) | 64 (58–71) | 68 (61–73) |
| Sex | ||
| Male | 101 (74%) | 25 (76%) |
| Female | 35 (26%) | 8 (24%) |
| ECOG performance status | ||
| 0 | 21 (15%) | 8 (24%) |
| 1 | 104 (76%) | 21 (64%) |
| 2 | 11 (8%) | 4 (12%) |
| UICC (7th edition) stage at study inclusion | ||
| IIA | 3 (2%) | 2 (6%) |
| IIB | 8 (6%) | 0 (0%) |
| IIIA | 49 (36%) | 13 (39%) |
| IIIB | 76 (56%) | 18 (55%) |
| Histology | ||
| Squamous cell carcinoma | 76 (56%) | 22 (67%) |
| Adenocarcinoma | 47 (35%) | 8 (24%) |
| Large cell carcinoma | 2 (1%) | 2 (6%) |
| NOS or other subtypes | 10 (7%) | 1 (3%) |
| Missing | 1 (1%) | 0 (0%) |
| GTV(Primary) mL | ||
| Median (IQR) | 68.8 (36–127) | 66 (28–150) |
| PTV, mL | ||
| Median (IQR) | 514 (343–699) | 542 (357–737) |
| Number of PET-positive lymph node stations | ||
| Median (min–max) | 3 (0–9) | 3 (1–7) |
| Ejection fraction at baseline § | ||
| Median (min–max) | 60 (35–92) | 59 (45–79) |
| Missing | 14 (10%) | 1 (3%) |
| Creatinine at baseline | ||
| Median (min–max) mg/dL | 0.82(0.42–1.39) | 1.03 (0.6–1.77) |
| Missing | 2 (1%) | 0 (0%) |
| Weight loss in 6 months before inclusion | ||
| <5% | 87 (64%) | 26 (79%) |
| 5–10% | 12 (9%) | 4 (12%) |
| >10% | 26 (19%) | 1 (3%) |
| Missing | 11 (8%) | 2 (6%) |
| Complete administration of chemotherapy | ||
| Cycle 1 | 108 (79%) | 29 (88%) |
| Cycle 2 | 97 (71%) | 26 (79%) |
| P1 (n = 31) | P2 (n = 92) | P3 (n = 28) | Other * (n = 20) | |
|---|---|---|---|---|
| Leukopenia | ||||
| Grade 0 | 10 (32%) | 22 (24%) | 15 (54%) | 3 (15%) |
| Grade 1–2 | 12 (39%) | 53 (58%) | 12 (43%) | 10 (50%) |
| Grade 3 | 5 (16%) | 14 (15%) | 1 (4%) | 5 (25%) |
| Grade 4 | 2 (6%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Missing | 2 (6%) | 3 (3%) | 0 (0%) | 1 (5%) |
| Anemia | ||||
| Grade 0 | 17 (55%) | 48 (52%) | 16 (57%) | 8 (40%) |
| Grade 1–2 | 11 (35%) | 38 (41%) | 11 (39%) | 10 (50%) |
| Grade 3 | 0 (0%) | 3 (3%) | 1 (4%) | 1 (5%) |
| Grade 4 | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 2 (6%) | 3 (3%) | 0 (0%) | 1 (5%) |
| Thrombocytopenia | ||||
| Grade 0 | 23 (74%) | 81 (88%) | 24 (86%) | 14 (70%) |
| Grade 1–2 | 3 (10%) | 8 (9%) | 4 (14%) | 5 (25%) |
| Grade 3 | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Grade 4 | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 2 (6%) | 3 (3%) | 0 (0%) | 1 (5%) |
| Cardiac toxicity | ||||
| Grade 0 | 24 (77%) | 76 (83%) | 21 (75%) | 19 (95%) |
| Grade 1–2 | 0 (0%) | 10 (11%) | 6 (21%) | 0 (0%) |
| Grade 3 | 2 (6%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Grade 4 | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Missing | 5 (16%) | 4 (4%) | 0 (0%) | 1 (5%) |
| Pneumonitis | ||||
| Grade 0 | 20 (65%) | 84 (91%) | 25 (89%) | 20 (100%) |
| Grade 1–2 | 7 (23%) | 4 (4%) | 3 (11%) | 0 (0%) |
| Grade 3 | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Grade 4 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 4 (13%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Renal toxicity | ||||
| Grade 0 | 20 (65%) | 54 (59%) | 13 (46%) | 12 (60%) |
| Grade 1–2 | 6 (19%) | 35 (38%) | 14 (50%) | 7 (35%) |
| Grade 3 | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Grade 4 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 5 (16%) | 3 (3%) | 0 (0%) | 1 (5%) |
| Cisplatin (n = 136) | Carboplatin (n = 33) | |
|---|---|---|
| Leukopenia | ||
| Grade 0 | 34 (25%) | 16 (48%) |
| Grade 1–2 | 69 (51%) | 16 (48%) |
| Grade 3 | 24 (18%) | 1 (3%) |
| Grade 4 | 3 (2%) | 0 (0%) |
| Missing | 6 (4%) | 0 (0%) |
| Anemia | ||
| Grade 0 | 69 (51%) | 19 (58%) |
| Grade 1–2 | 57 (42%) | 12 (36%) |
| Grade 3 | 3 (2%) | 2 (6%) |
| Grade 4 | 1 (1%) | 0 (0%) |
| Missing | 6 (4%) | 0 (0%) |
| Thrombocytopenia | ||
| Grade 0 | 112 (82%) | 29 (88%) |
| Grade 1–2 | 15 (11%) | 4 (12%) |
| Grade 3 | 2 (1%) | 0 (0%) |
| Grade 4 | 1 (1%) | 0 (0%) |
| Missing | 6 (4%) | 0 (0%) |
| Cardiac toxicity | ||
| Grade 0 | 112 (82%) | 26 (79%) |
| Grade 1–2 | 10 (7%) | 6 (18%) |
| Grade 3 | 4 (3%) | 0 (0%) |
| Grade 4 | 0 (0%) | 1 (3%) |
| Missing | 10 (7%) | 0 (0%) |
| Pneumonitis | ||
| Grade 0 | 117 (86%) | 30 (91%) |
| Grade 1–2 | 11 (8%) | 3 (9%) |
| Grade 3 | 2 (1%) | 0 (0%) |
| Grade 4 | 0 (0%) | 0 (0%) |
| Missing | 6 (4%) | 0 (0%) |
| Renal toxicity | ||
| Grade 0 | 82 (60%) | 16 (48%) |
| Grade 1–2 | 45 (33%) | 16 (48%) |
| Grade 3 | 0 (0%) | 1 (3%) |
| Grade 4 | 0 (0%) | 0 (0%) |
| Missing | 9 (7%) | 0 (0%) |
| Hazard Ratio | 95% CI | p | |
|---|---|---|---|
| Carboplatin vs. Cisplatin | 1.44 | 0.76–2.61 | 0.26 |
| Age at randomization | 1.007 | 0.98–1.037 | 0.63 |
| ECOG at baseline | 0.94 | ||
| 0 | 1.00 | ||
| 1 | 1.00 | 0.56–1.92 | |
| 2 | 1.13 | 0.48–2.58 | |
| Ejection fraction (%) at baseline § | 0.996 | 0.973–1.018 | 0.71 |
| Chemotherapy complete (Yes vs. No) | 0.72 | 0.45–1.15 | 0.17 |
| Creatinine cut-off 1.17 (≥1.17 vs. <1.17) | 1.69 | 0.84–3.20 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkika, E.; Lenz, S.; Schimek-Jasch, T.; Waller, C.F.; Kremp, S.; Schaefer-Schuler, A.; Mix, M.; Küsters, A.; Tosch, M.; Hehr, T.; et al. Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer—A Secondary Analysis of the PET Plan Trial. Cancers 2020, 12, 3359. https://doi.org/10.3390/cancers12113359
Gkika E, Lenz S, Schimek-Jasch T, Waller CF, Kremp S, Schaefer-Schuler A, Mix M, Küsters A, Tosch M, Hehr T, et al. Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer—A Secondary Analysis of the PET Plan Trial. Cancers. 2020; 12(11):3359. https://doi.org/10.3390/cancers12113359
Chicago/Turabian StyleGkika, Eleni, Stefan Lenz, Tanja Schimek-Jasch, Cornelius F. Waller, Stephanie Kremp, Andrea Schaefer-Schuler, Michael Mix, Andreas Küsters, Marco Tosch, Thomas Hehr, and et al. 2020. "Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer—A Secondary Analysis of the PET Plan Trial" Cancers 12, no. 11: 3359. https://doi.org/10.3390/cancers12113359
APA StyleGkika, E., Lenz, S., Schimek-Jasch, T., Waller, C. F., Kremp, S., Schaefer-Schuler, A., Mix, M., Küsters, A., Tosch, M., Hehr, T., Eschmann, S. M., Bultel, Y.-P., Hass, P., Fleckenstein, J., Thieme, A. H., Stockinger, M., Dieckmann, K., Miederer, M., Holl, G., ... Nestle, U. (2020). Efficacy and Toxicity of Different Chemotherapy Protocols for Concurrent Chemoradiation in Non-Small Cell Lung Cancer—A Secondary Analysis of the PET Plan Trial. Cancers, 12(11), 3359. https://doi.org/10.3390/cancers12113359





