Early Changes of the Standardized Uptake Values (SUVmax) Predict the Efficacy of Everolimus-Exemestane in Patients with Hormone Receptor-Positive Metastatic Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Results
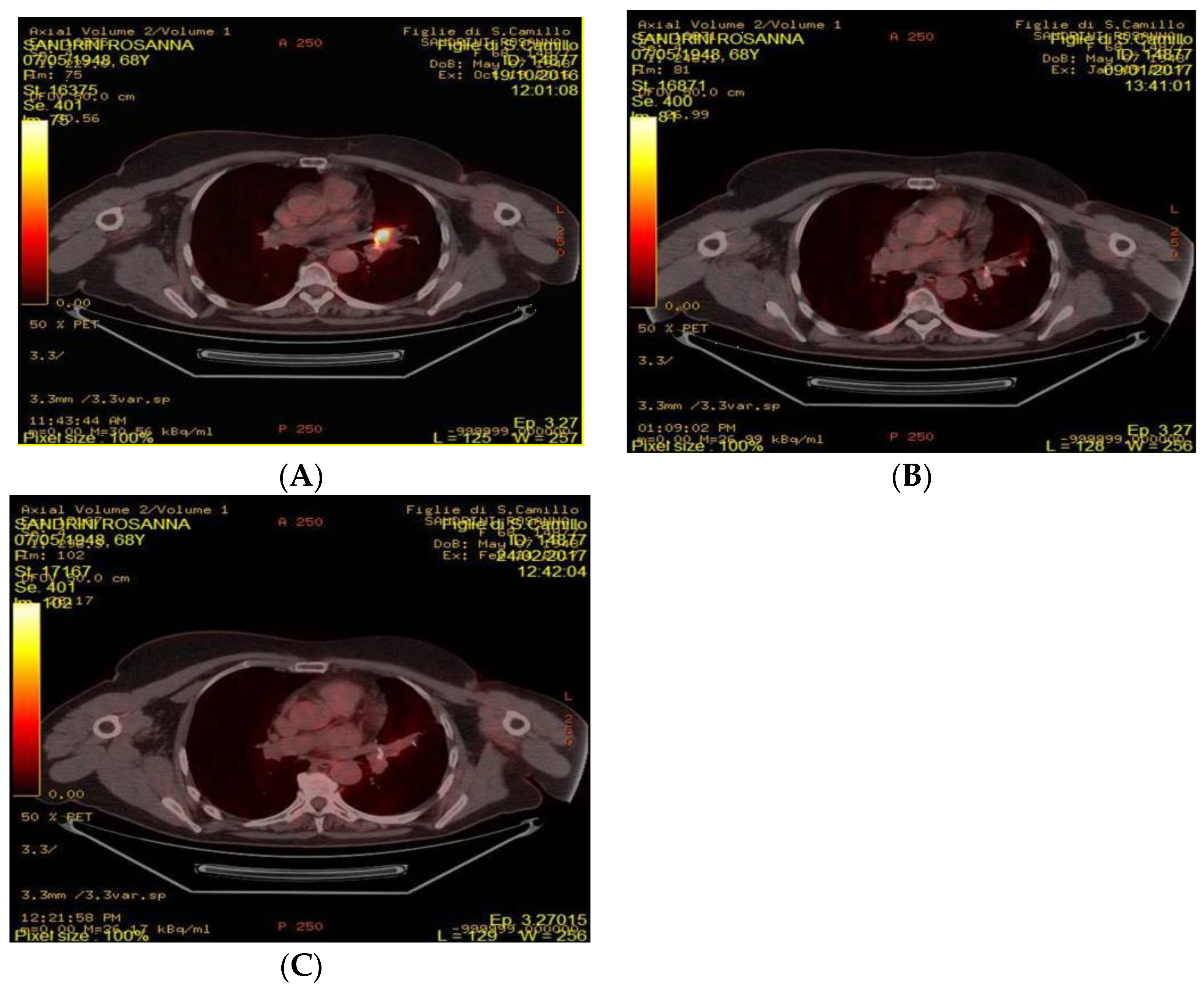
2.1. Patient Characteristics
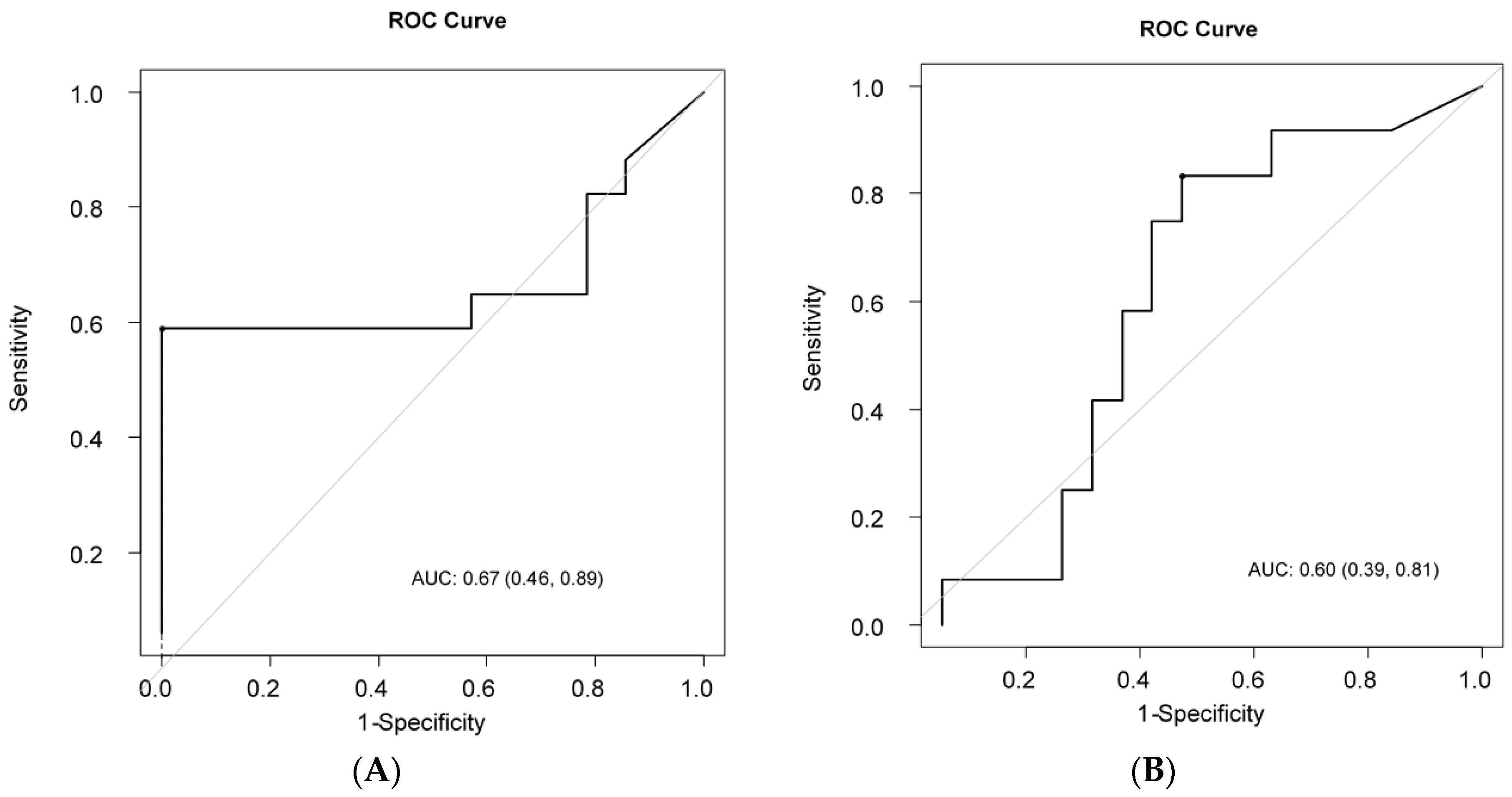
2.2. Determination of ΔSUV Cut-off Value to Discriminate LRs From Non-LRs and LSs From Non-LSs
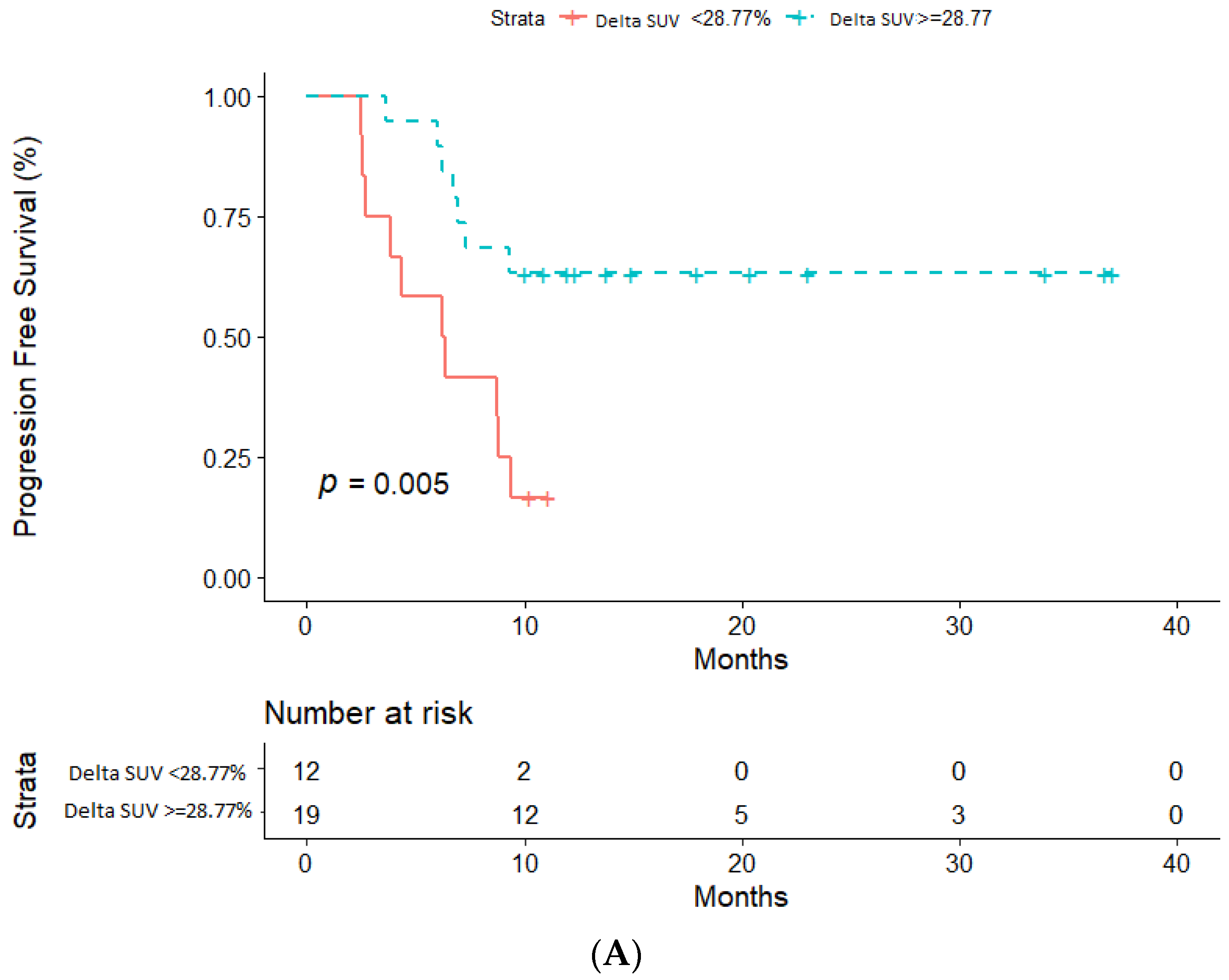
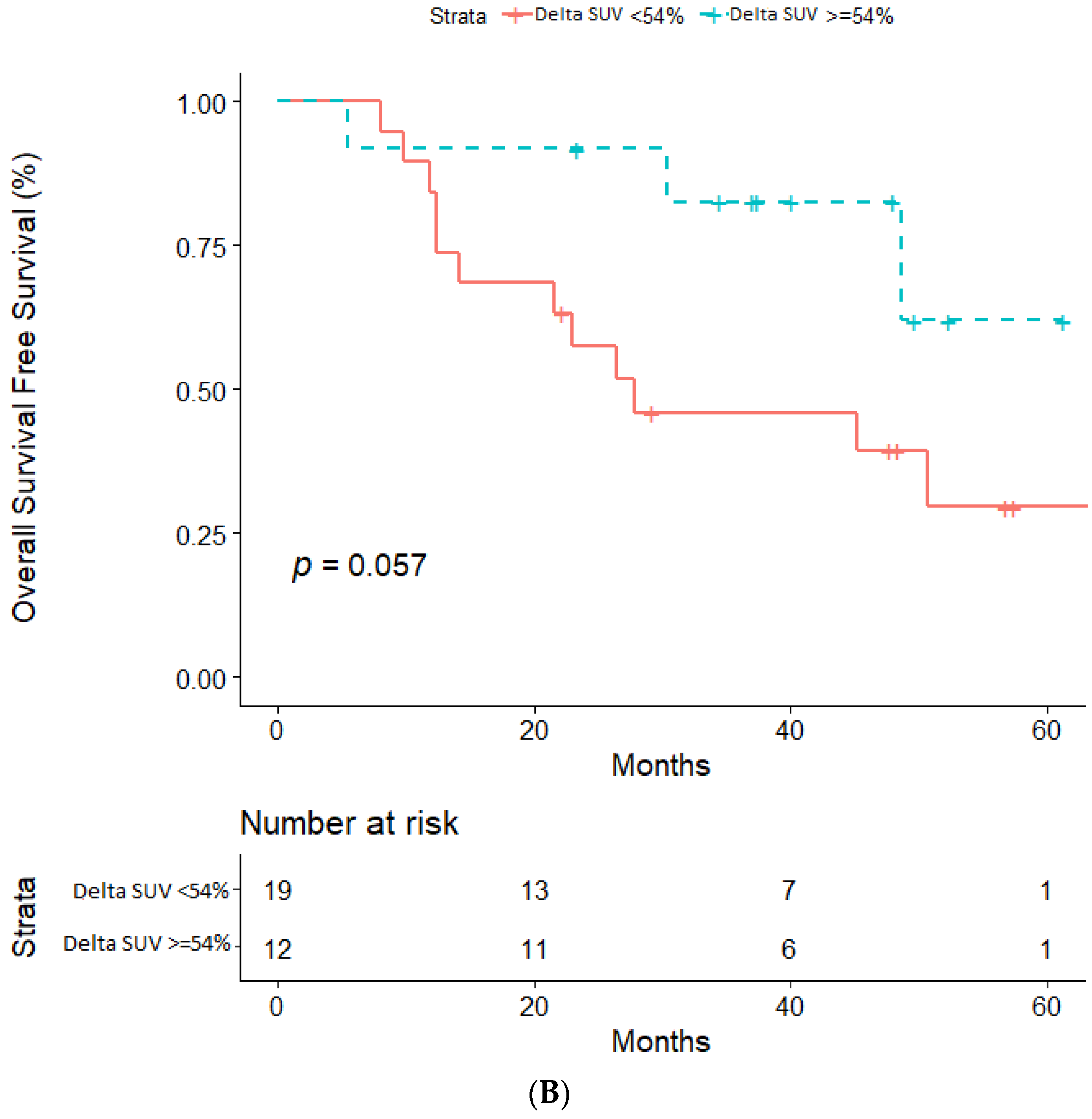
2.3. Impact of ΔSUV (%) on PFS and OS
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. FDG-PET/CT Acquisition and Analysis
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALK | Anaplastic lymphoma kinase |
| AKT | protein kinase B |
| AUC | area under the curve |
| EGFR | Estimated glomerular filtration rate |
| ET | endocrine therapy |
| ER | estrogen receptor |
| 18F-FDG PET/CT | 18Fludeoxyglucose-Positron Emission Tomography/Computed Tomorgraphy |
| HR | Hazard ratio |
| HR | hormone receptor-positive |
| HER2 | Human epidermal growth factor receptor 2 |
| LR | long responder |
| LS | long survival |
| mBC | metastatic breast cancer |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| NSAI | non-steroideal aromatase inhibitor |
| NSCLC | non small cell lung cancer |
| OS | overall survival |
| PFS | progression-free survival |
| PgR | Progesterone receptor |
| PI3K | phosphatidylinositol 3-kinase |
| PTEN | Phosphatase and tensin homolog |
| ROC | Receiver Operating Characteristic |
| SUV | standardized uptake volume |
| TKI | Tyrosine Kinase Inhibitor |
References
- Prat, A.; Brase, J.C.; Cheng, Y.; Nuciforo, P.; Paré, L.; Pascual, T.; Martínez, D.; Galván, P.; Vidal, M.; Adamo, B.; et al. Everolimus plus Exemestane for Hormone Receptor-Positive Advanced Breast Cancer: A PAM50 Intrinsic Subtype Analysis of BOLERO-2. Oncologist 2019. [Google Scholar] [CrossRef]
- Ma, C.X.; Reinert, T.; Chmielewska, I.; Ellis, M.J. Mechanisms of aromatase inhibitor resistance. Nat. Rev. Cancer 2015. [Google Scholar] [CrossRef]
- Keegan, N.M.; Gleeson, J.P.; Hennessy, B.T.; Morris, P.G. PI3K inhibition to overcome endocrine resistance in breast cancer. Expert Opin. Investig. Drugs 2018. [Google Scholar] [CrossRef]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Peng, Y.; Wei, X. Molecular Cancer volume 18, Article number: 26 (2019) Cite this article Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019. [Google Scholar] [CrossRef]
- Tserga, A.; Chatziandreou, I.; Michalopoulos, N.V.; Patsouris, E.; Saetta, A.A. Mutation of genes of the PI3K/AKT pathway in breast cancer supports their potential importance as biomarker for breast cancer aggressiveness. Virchows Arch. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, G.; Militello, L.; Steelman, L.S.; Cavallaro, A.; Basile, F.; Nicoletti, F.; Stivala, F.; McCubrey, J.A.; Libra, M. PIK3CA mutations in human solid tumors Role in sensitivity to various therapeutic approaches. Cell Cycle 2009. [Google Scholar] [CrossRef]
- Dannemann, N.; Hart, J.R.; Ueno, L.; Vogt, P.K. Phosphatidylinositol 4,5-bisphosphate (PIP 2)-specific AKT1 is oncogenic. Int. J. Cancer 2010, 127, 239–244. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Noh, W.C.; Kim, Y.H.; Kim, M.S.; Koh, J.S.; Kim, H.A.; Moon, N.M.; Paik, N.S. Activation of the mTOR signaling pathway in breast cancer and its correlation with the clinicopathologic variables. Breast Cancer Res. Treat. 2008. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors—The Cancer Genome Atlas Network. Supplementary information. Nature 2012. [Google Scholar] [CrossRef]
- Hosford, S.R.; Miller, T.W. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and Pi3K/AKT/mTOR pathways. Pharmgenomics. Pers. Med. 2014. [Google Scholar] [CrossRef]
- Hillmann, P.; Fabbro, D. PI3K/mTOR pathway inhibition: Opportunities in oncology and rare genetic diseases. Int. J. Mol. Sci. 2019, 20, 5792. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A.; Noguchi, S.; Pritchard, K.I.; Burris, H.A.; Baselga, J.; Gnant, M.; Hortobagyi, G.N.; Campone, M.; Pistilli, B.; Piccart, M.; et al. Everolimus plus exemestane in postmenopausal patients with HR+ breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lousberg, L.; Jerusalem, G. Safety, efficacy, and patient acceptability of everolimus in the treatment of breast cancer. Breast Cancer Basic Clin. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grüwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 2008. [Google Scholar] [CrossRef]
- Antunovic, L.; de Sanctis, R.; Cozzi, L.; Kirienko, M.; Sagona, A.; Torrisi, R.; Tinterri, C.; Santoro, A.; Chiti, A.; Zelic, R.; et al. PET/CT radiomics in breast cancer: Promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef]
- Ueda, S.; Tsuda, H.; Asakawa, H.; Shigekawa, T.; Fukatsu, K.; Kondo, N.; Yamamoto, M.; Hama, Y.; Tamura, K.; Ishida, J.; et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in Primary Breast Cancer. Jpn. J. Clin. Oncol. 2008, 38, 250–258. [Google Scholar] [CrossRef]
- Mortazavi-Jehanno, N.; Giraudet, A.L.; Champion, L.; Lerebours, F.; Le Stanc, E.; Edeline, V.; Madar, O.; Bellet, D.; Pecking, A.P.; Alberini, J.L. Assessment of response to endocrine therapy using FDG PET/CT in metastatic breast cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2012. [Google Scholar] [CrossRef]
- Garcia Vicente, A.M.; Soriano Castrejón, A.; Amo-Salas, M.; Lopez Fidalgo, J.F.; Muñoz Sanchez, M.M.; Alvarez Cabellos, R.; Espinosa Aunion, R.; Muñoz Madero, V. Glycolytic activity in breast cancer using 18F-FDG PET/CT as prognostic predictor: A molecular phenotype approach. Rev. Esp. Med. Nucl. Imagen Mol. 2016. [Google Scholar] [CrossRef]
- Willemsen, A.E.; de Geus-Oei, L.F.; de Boer, M.; Tol, J.; Kamm, Y.; de Jong, P.C.; Jonker, M.A.; Vos, A.H.; Grootjans, W.; de Groot, J.W.B.; et al. Everolimus Exposure and Early Metabolic Response as Predictors of Treatment Outcomes in Breast Cancer Patients Treated with Everolimus and Exemestane. Target. Oncol. 2018. [Google Scholar] [CrossRef]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dckler, M.; Weber, W.A. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmound, T.; Noguchi, S.; Gmamt, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.; Singh, N.; Miles, K. Tumour response evaluation with fluorodeoxyglucose positron emission tomography: Research technique or clinical tool? Cancer Imaging 2010. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, M.; Ippolito, M.; Scarlattei, M.; Spadaro, P.; Cosentino, S.; Latteri, F.; Ruffini, L.; Bartolotti, M.; Bortesi, B.; Fumarola, C.; et al. Predictive and prognostic value of early response assessment using 18FDG-PET in advanced non-small cell lung cancer patients treated with erlotinib. Cancer Chemother. Pharmacol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, X.; Li, J.; Wang, Z.; Wang, Y. Prognostic value of early response assessment using (18F)FDG-PET in patients with advanced non-small cell lung cancer treated with tyrosine-kinase inhibitors. J. Investig. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Fukushima, T.; Gomi, D.; Koizumi, T.; Fukushima, T.; Gomi, D.; Kobayashi, T.; Sekiguchi, N.; Mamiya, K.; Tateishi, K.; et al. Correlation of early PET findings with tumor response to molecular targeted agents in patients with advanced driver-mutated non-small cell lung cancer. Med. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Schmid, J.S.; Higuchi, T.; Javadi, M.S.; Rowe, S.P.; Märkl, B.; Aulmann, C.; Gassnacht, M.; Kroiss, M.; Reiners, C.; et al. Predictive value of 18 F-FDG PET in patients with advanced medullary thyroid carcinoma treated with vandetanib. J. Nucl. Med. 2018. [Google Scholar] [CrossRef]
- Kirchner, G.I.; Meier-Wiedenbach, I.; Manns, M.P. Clinical Pharmacokinetics of Everolimus. Clin. Pharmacokinet. 2004. [Google Scholar] [CrossRef]
- Shankar, L.K.; Hoffman, J.M.; Bacharach, S.; Graham, M.M.; Karp, J.; Lammertsma, A.A.; Larson, S.; Mankoff, D.A.; Siegel, B.A.; Van den Abbeele, A.; et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J. Nucl. Med. 2006, 47, 1059–1066. [Google Scholar] [PubMed]

| Characteristic | Baseline Data |
|---|---|
| Age (y) | |
| Median (Range) | 65.3 (48.6; 80.3) |
| Baseline SUV | |
| Median (Range) | 6.0 (2.0; 17.2) |
| 3 months SUV | |
| Median (Range) | 3.4 (0.0; 14.0) |
| ΔSUV% 3 months | |
| Median (Range) | 39.2% (−375.0%; 100%) |
| Duration of Everolimus Treatment | |
| <10 months | 22 (71.0%) |
| ≥10 months | 9 (29.0%) |
| Follow-up (months) | |
| Median (Range) | 17.92 (12.96–21.69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirico, M.; Bernocchi, O.; Sobhani, N.; Giudici, F.; Corona, S.P.; Vernieri, C.; Nichetti, F.; Cappelletti, M.R.; Milani, M.; Strina, C.; et al. Early Changes of the Standardized Uptake Values (SUVmax) Predict the Efficacy of Everolimus-Exemestane in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancers 2020, 12, 3314. https://doi.org/10.3390/cancers12113314
Sirico M, Bernocchi O, Sobhani N, Giudici F, Corona SP, Vernieri C, Nichetti F, Cappelletti MR, Milani M, Strina C, et al. Early Changes of the Standardized Uptake Values (SUVmax) Predict the Efficacy of Everolimus-Exemestane in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancers. 2020; 12(11):3314. https://doi.org/10.3390/cancers12113314
Chicago/Turabian StyleSirico, Marianna, Ottavia Bernocchi, Navid Sobhani, Fabiola Giudici, Silvia P. Corona, Claudio Vernieri, Federico Nichetti, Maria Rosa Cappelletti, Manuela Milani, Carla Strina, and et al. 2020. "Early Changes of the Standardized Uptake Values (SUVmax) Predict the Efficacy of Everolimus-Exemestane in Patients with Hormone Receptor-Positive Metastatic Breast Cancer" Cancers 12, no. 11: 3314. https://doi.org/10.3390/cancers12113314
APA StyleSirico, M., Bernocchi, O., Sobhani, N., Giudici, F., Corona, S. P., Vernieri, C., Nichetti, F., Cappelletti, M. R., Milani, M., Strina, C., Cervoni, V., Barbieri, G., Ziglioli, N., Dester, M., Bianchi, G. V., De Braud, F., & Generali, D. (2020). Early Changes of the Standardized Uptake Values (SUVmax) Predict the Efficacy of Everolimus-Exemestane in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancers, 12(11), 3314. https://doi.org/10.3390/cancers12113314






