Patient-Reported Outcomes of Palbociclib Plus Exemestane with GnRH Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer: A Prospective, Open-Label, Randomized Phase ll Trial (KCSG-BR 15-10)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Study Treatments
2.3. Patient Reported Outcomes Assessment
2.4. Hormone Measurement
2.5. Statistical Analysis
3. Results
3.1. Baseline Patients and Disease Characteristics
3.2. Global Health Status/Quality of Life
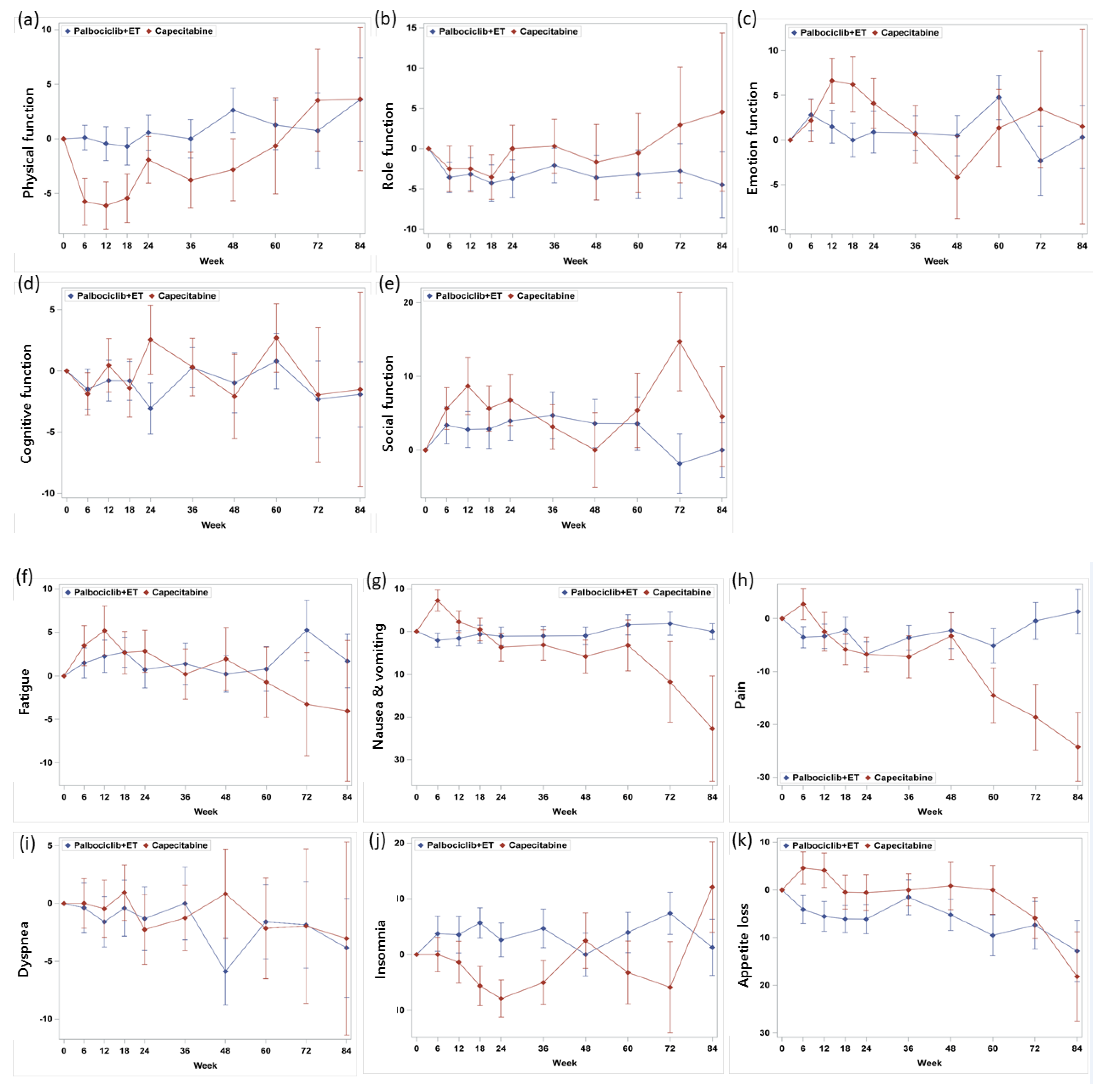
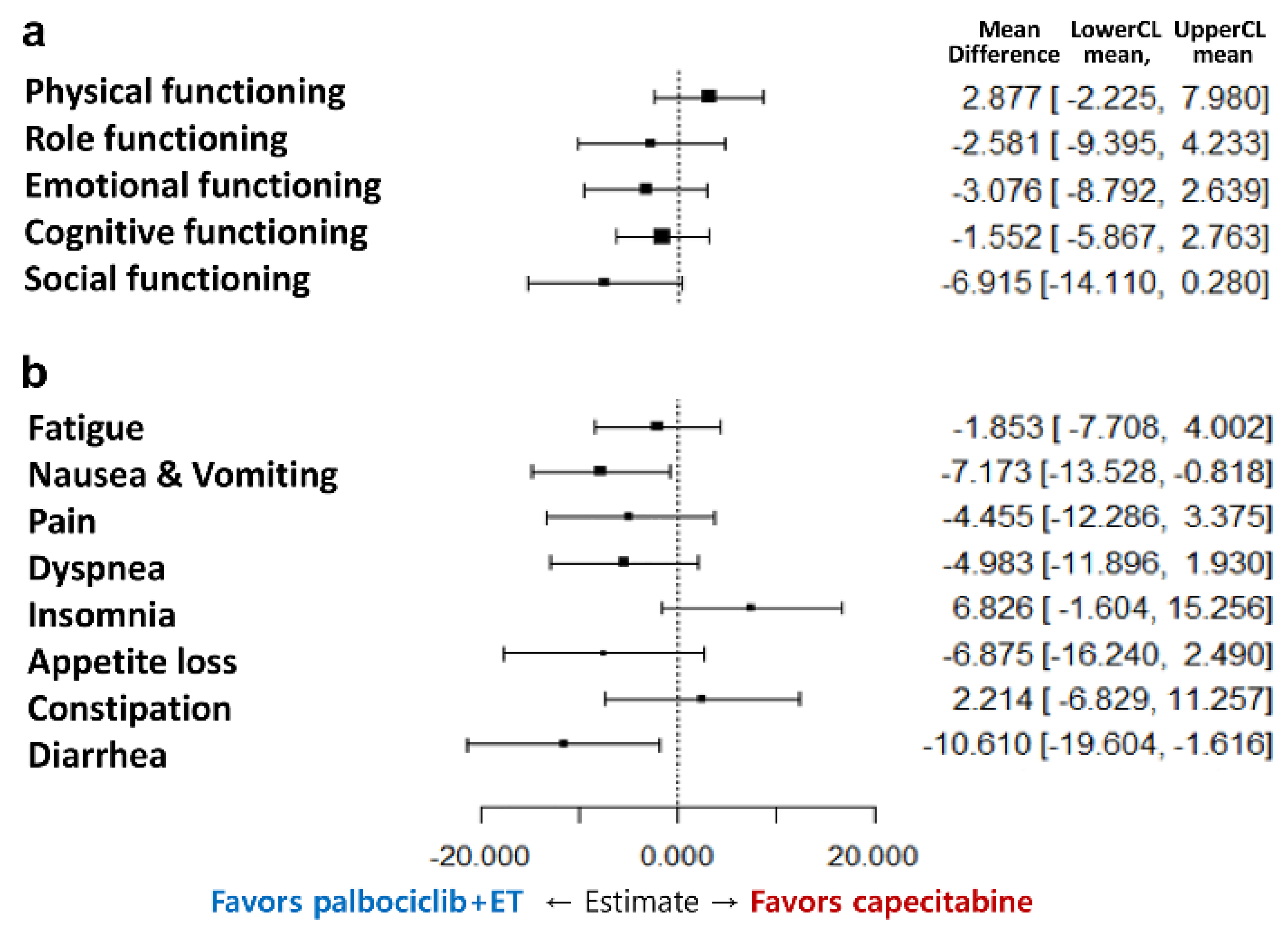
3.3. Functioning Scales
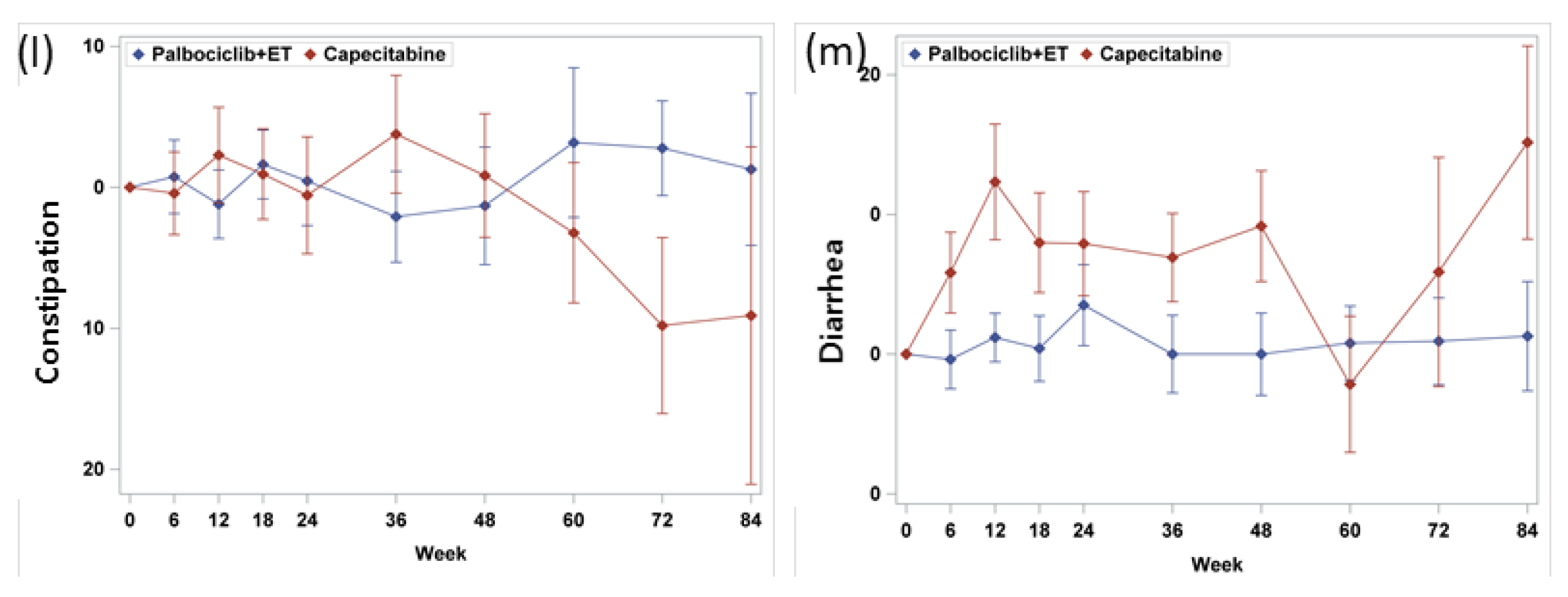
3.4. Symptom Scales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; Andre, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) dagger. Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Swallow, E.; Zhang, J.; Thomason, D.; Tan, R.D.; Kageleiry, A.; Signorovitch, J. Real-world patterns of endocrine therapy for metastatic hormone-receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2−) breast cancer patients in the United States: 2002–2012. Curr. Med. Res. Opin. 2014, 30, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, R.; Scazafave, M. Real-World Treatment Patterns for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer in Europe and the United States. Oncol. Ther. 2016, 4, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Huober, J.; Volz, B.; Nabieva, N.; Taran, F.A.; Schwitulla, J.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors—Data from the German PRAEGNANT breast cancer registry. Breast 2018, 37, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.; van Kampen, R.J.; Voogd, A.C.; Dercksen, M.W.; van den Berkmortel, F.; Smilde, T.J.; van de Wouw, A.J.; Peters, F.P.; van Riel, J.M.; Peters, N.A.; et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: A study of the Southeast Netherlands Breast Cancer Consortium. Ann. Oncol. 2016, 27, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.H.; Kim, Y.J.; Park, S.E.; Lee, H.S.; Lim, S.W.; Cho, J.H.; Kim, J.Y.; Ahn, J.S.; Im, Y.H.; et al. Does guideline non-adherence result in worse clinical outcomes for hormone receptor-positive and HER2-negative metastatic breast cancer in premenopausal women?: Result of an institution database from South Korea. BMC Cancer 2019, 19, e84. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [PubMed]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.; Ueno, T.; Lin, C.H.; Liu, Q.; Lee, K.H.; Leung, R.; Naito, Y.; Park, Y.H.; Im, S.A.; Li, H.; et al. Treating HR+/HER2− breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression. Breast Cancer Res. Treat. 2019, 177, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, T.Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef]
- Montazeri, A. Health-related quality of life in breast cancer patients: A bibliographic review of the literature from 1974 to 2007. J. Exp. Clin. Cancer Res. 2008, 27, e32. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Campone, M.; Bondarenko, I.; Sakaeva, D.; Krishnamurthy, S.; Roman, L.; Lebedeva, L.; Vedovato, J.C.; Aapro, M. Randomised phase III trial of vinflunine plus capecitabine versus capecitabine alone in patients with advanced breast cancer previously treated with an anthracycline and resistant to taxane. Ann. Oncol. 2018, 29, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Di Meglio, A.; Pistilli, B.; Gbenou, A.S.; El-Mouhebb, M.; Dauchy, S.; Charles, C.; Joly, F.; Everhard, S.; Lambertini, M.; et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: A prospective patient-reported outcomes analysis. Ann. Oncol. 2019, 30, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Dieras, V.; Gelmon, K.A.; Finn, R.S.; Slamon, D.J.; Martin, M.; Neven, P.; Shparyk, Y.; Mori, A.; Lu, D.R.; et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann. Oncol. 2018, 29, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Iyer, S.; Turner, N.; Cristofanilli, M.; Ro, J.; Andre, F.; Loi, S.; Verma, S.; Iwata, H.; Bhattacharyya, H.; et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: Patient-reported outcomes from the PALOMA-3 trial. Ann. Oncol. 2016, 27, 1047–1054. [Google Scholar] [CrossRef] [PubMed]



| Domain/Scale | Palbociclib + Exemestane + GnRH Agonist (N = 92) Mean (SD) | Capecitabine (N = 86) Mean (SD) | p-Value | Reference Values b Mean (SD) |
|---|---|---|---|---|
| EORTC QLQ-C30 global health status/QoL c | ||||
| Global health status/QoL | 65.2 (20.8) | 57.0 (22.3) | 0.0223 | 60.2 (25.5) |
| EORTC QLQ-C30 functional scales c | ||||
| Physical functioning | 79.7 (18.3) | 78.4 (20.0) | 0.8244 | 81.6 (18.7) |
| Role functioning | 82.3 (22.4) | 74.3 (27.1) | 0.0411 | 67.4 (31.1) |
| Emotional functioning | 74.8 (18.7) | 70.2 (21.6) | 0.1266 | 65.9 (24.6) |
| Cognitive functioning | 83.9 (15.7) | 81.7 (18.0) | 0.5060 | 80.5 (23.2) |
| Social functioning | 77.0 (23.6) | 66.3 (28.1) | 0.0085 | 74.2 (28.4) |
| EORTC QLQ-C30 symptom scales d | ||||
| Fatigue | 30.8 (20.1) | 34.6 (21.7) | 0.2685 | 36.3 (27.0) |
| Nausea/vomiting | 8.2 (17.0) | 12.6 (23.5) | 0.1710 | 10.3 (19.7) |
| Pain | 23.9 (24.1) | 30.0 (23.6) | 0.0487 | 30.9 (29.6) |
| Dyspnea | 16.3 (22.9) | 17.7 (22.4) | 0.5714 | 20.4 (28.2) |
| Insomnia | 29.0 (27.2) | 31.7 (32.0) | 0.7879 | 33.1 (32.6) |
| Appetite loss | 19.2 (25.3) | 20.2 (28.7) | 0.9096 | 21.7 (31.0) |
| Constipation c | 16.3 (26.0) | 18.9 (26.3) | 0.6044 | 19.2 (28.8) |
| Diarrhea c | 8.7 (16.3) | 12.8 (22.7) | 0.3321 | 5.8 (15.2) |
| Total score | 80.4 (13.8) | 76.3 (16.7) | 0.1172 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Im, S.-A.; Kim, G.M.; Jung, K.H.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; et al. Patient-Reported Outcomes of Palbociclib Plus Exemestane with GnRH Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer: A Prospective, Open-Label, Randomized Phase ll Trial (KCSG-BR 15-10). Cancers 2020, 12, 3265. https://doi.org/10.3390/cancers12113265
Lee S, Im S-A, Kim GM, Jung KH, Kang SY, Park IH, Kim JH, Lee KE, Ahn HK, Lee MH, et al. Patient-Reported Outcomes of Palbociclib Plus Exemestane with GnRH Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer: A Prospective, Open-Label, Randomized Phase ll Trial (KCSG-BR 15-10). Cancers. 2020; 12(11):3265. https://doi.org/10.3390/cancers12113265
Chicago/Turabian StyleLee, Soohyeon, Seock-Ah Im, Gun Min Kim, Kyung Hae Jung, Seok Yun Kang, In Hae Park, Jee Hyun Kim, Kyoung Eun Lee, Hee Kyung Ahn, Moon Hee Lee, and et al. 2020. "Patient-Reported Outcomes of Palbociclib Plus Exemestane with GnRH Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer: A Prospective, Open-Label, Randomized Phase ll Trial (KCSG-BR 15-10)" Cancers 12, no. 11: 3265. https://doi.org/10.3390/cancers12113265
APA StyleLee, S., Im, S.-A., Kim, G. M., Jung, K. H., Kang, S. Y., Park, I. H., Kim, J. H., Lee, K. E., Ahn, H. K., Lee, M. H., Kim, H.-J., Kim, H. J., Lee, J. I., Koh, S.-J., & Park, Y. H. (2020). Patient-Reported Outcomes of Palbociclib Plus Exemestane with GnRH Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer: A Prospective, Open-Label, Randomized Phase ll Trial (KCSG-BR 15-10). Cancers, 12(11), 3265. https://doi.org/10.3390/cancers12113265







