Current Concepts in Multi-Modality Imaging of Solid Tumor Angiogenesis
Abstract
Simple Summary
Abstract
1. Introduction
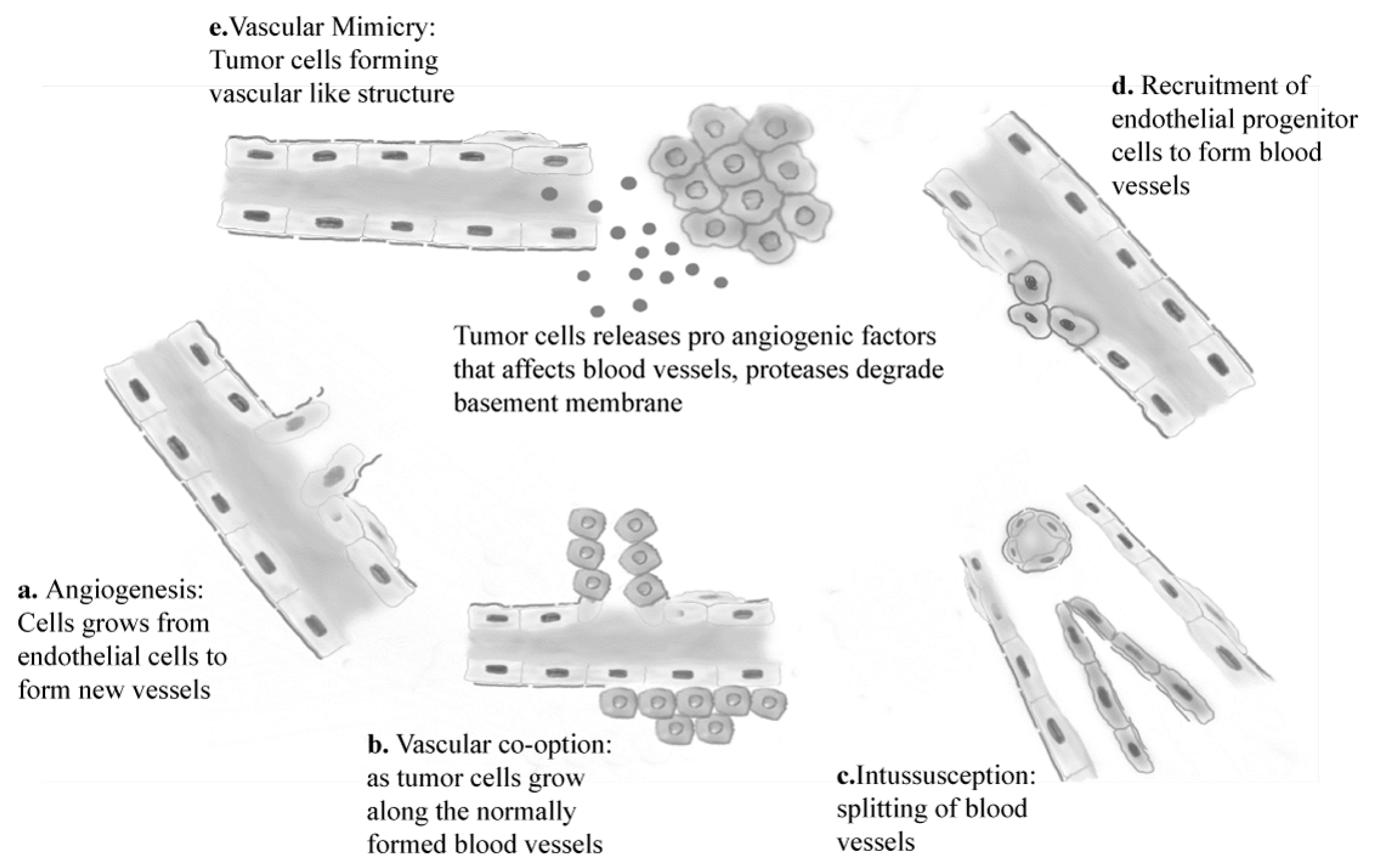
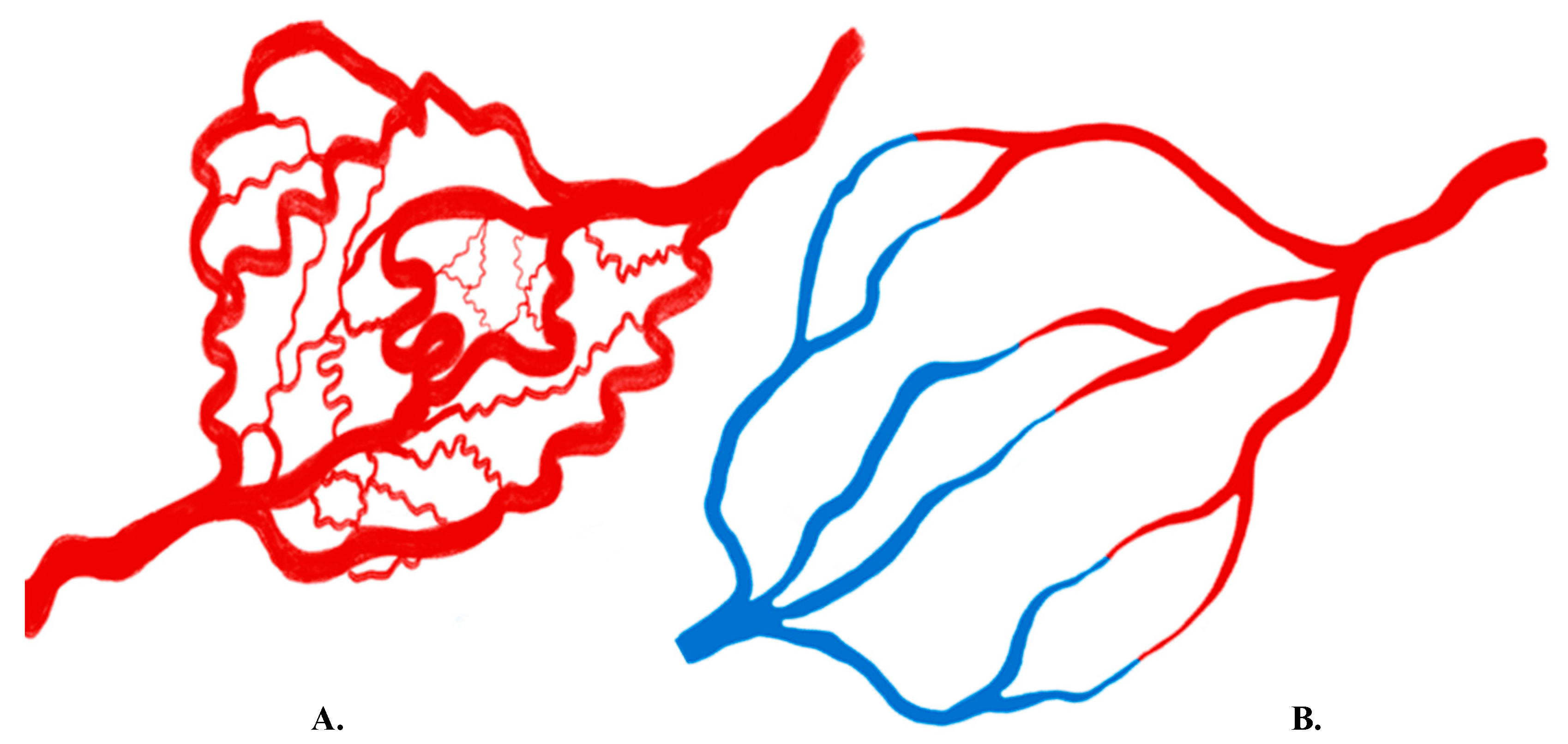
2. Molecular Basis of Angiogenesis
3. Overview of Imaging Angiogenesis
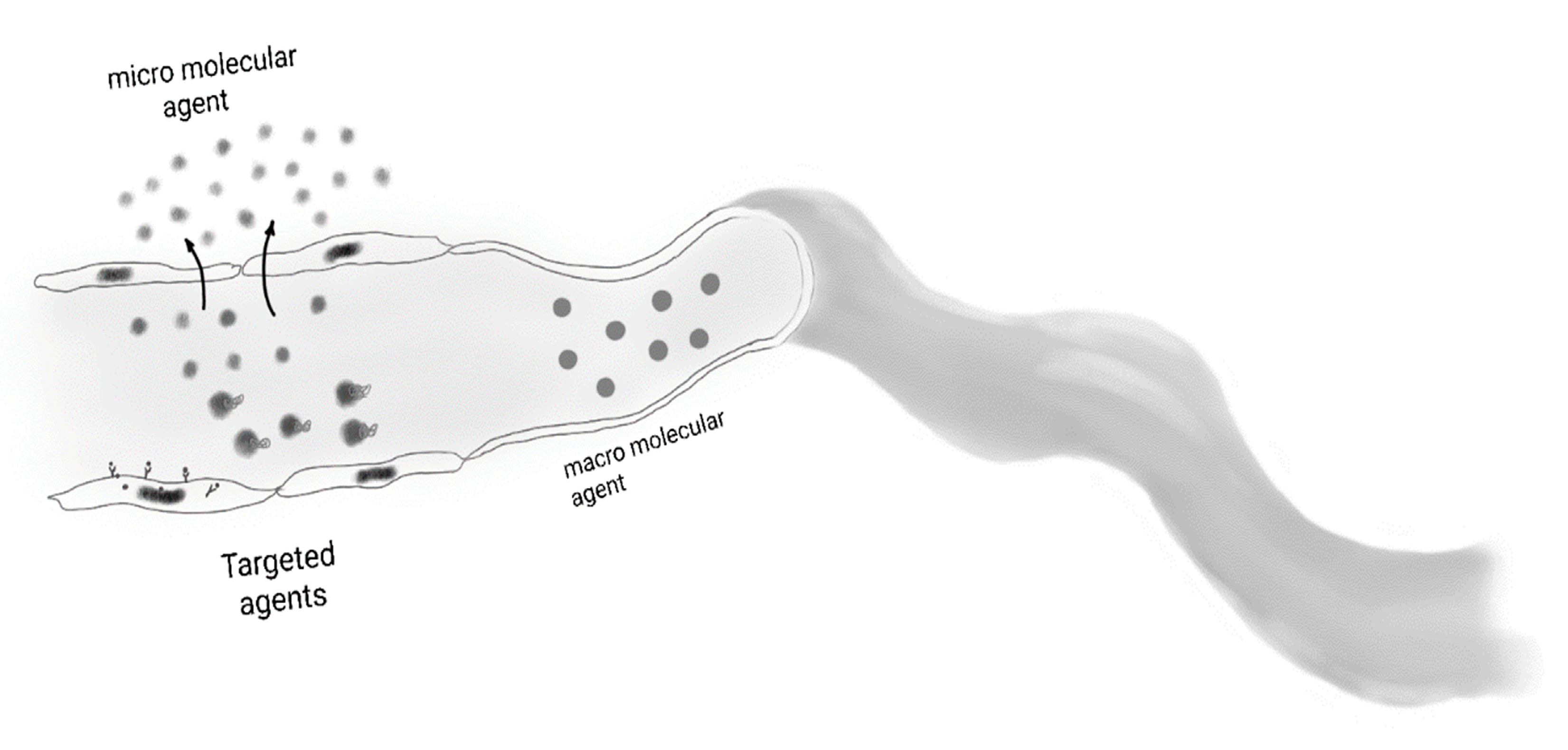
4. Invasive and Non-Invasive Imaging Methods
5. Choosing the Best Imaging Modality for Evaluating Angiogenesis
6. Parameters Derived from Imaging Data
6.1. Steady-State Imaging
6.2. Dynamic Imaging
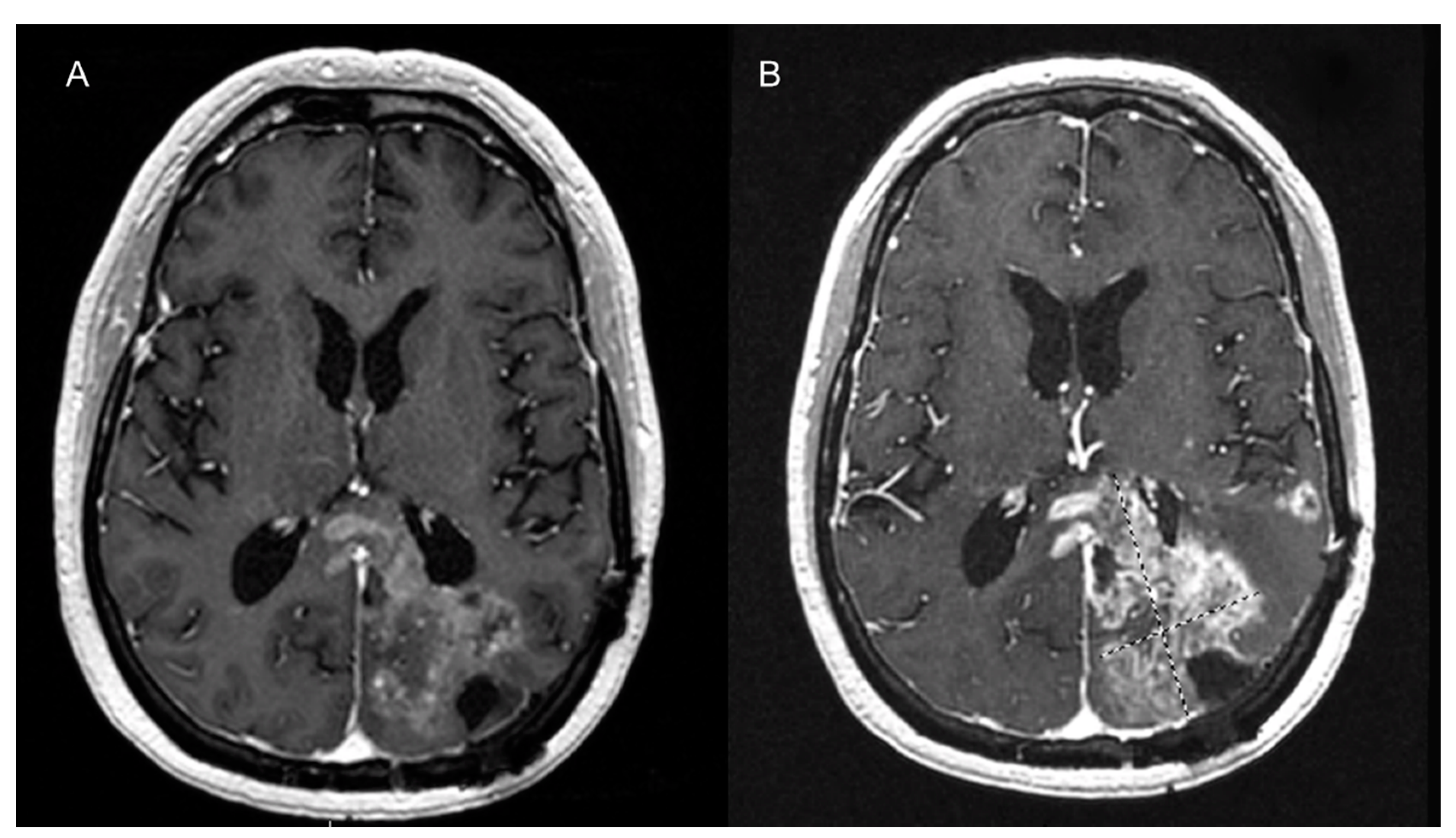
7. MR Imaging of Angiogenesis
7.1. Unenhanced MRI Imaging of Angiogenesis
7.2. Contrast-Enhanced MR Imaging of Angiogenesis
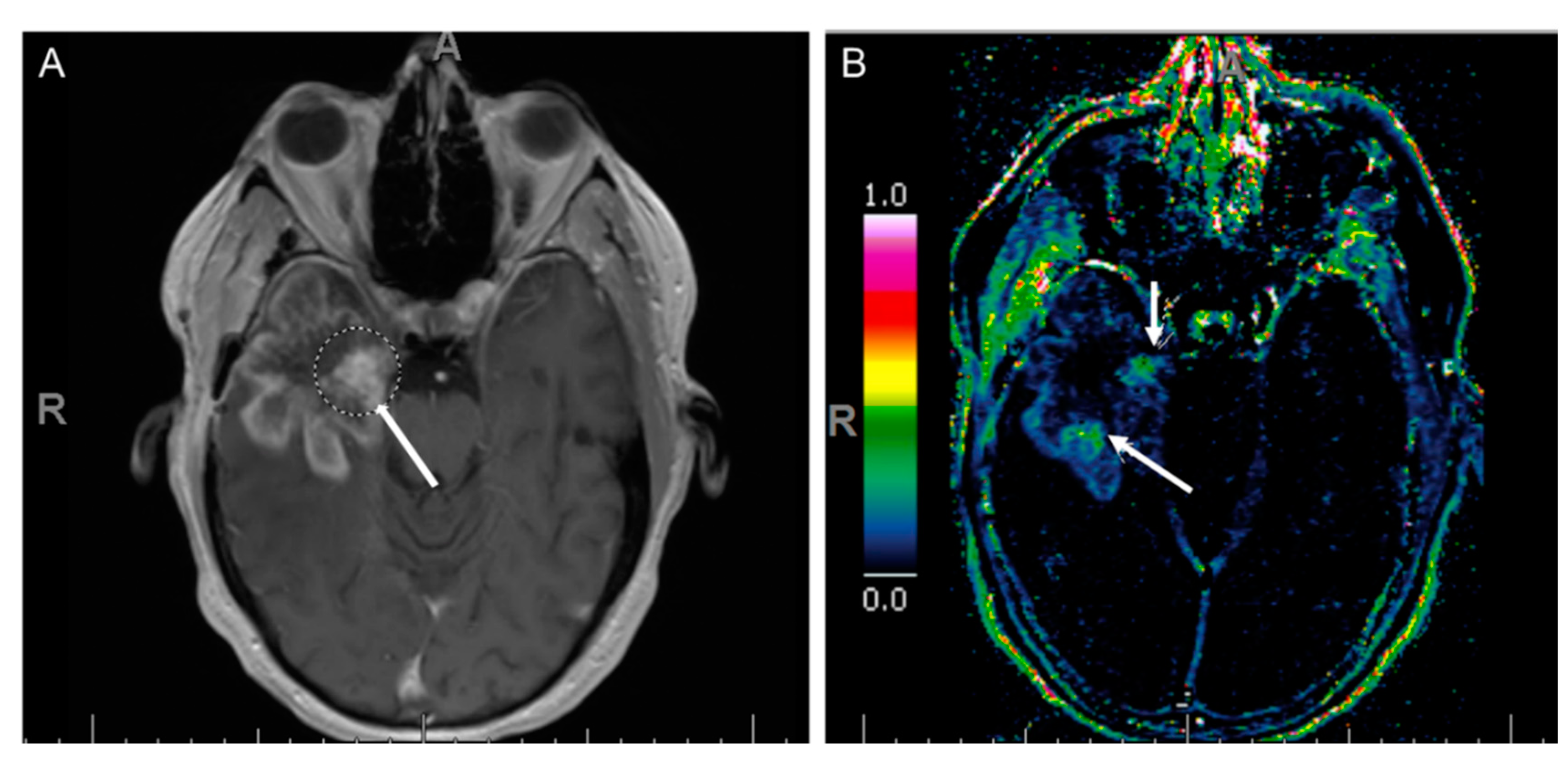
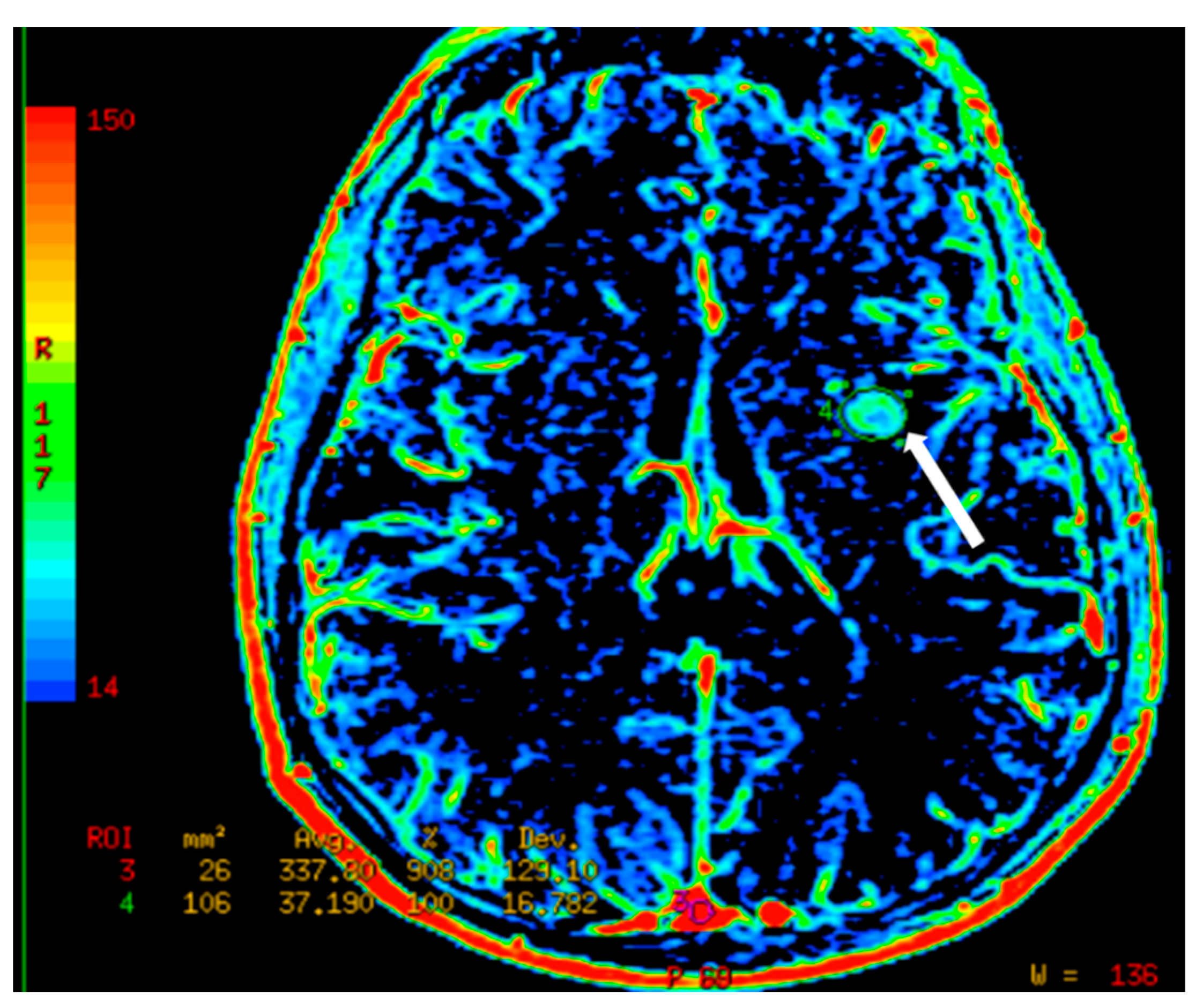
7.3. Dynamic Susceptibility Contrast (DSC) Perfusion MRI
7.4. Dynamic Contrast-Enhanced Perfusion MRI
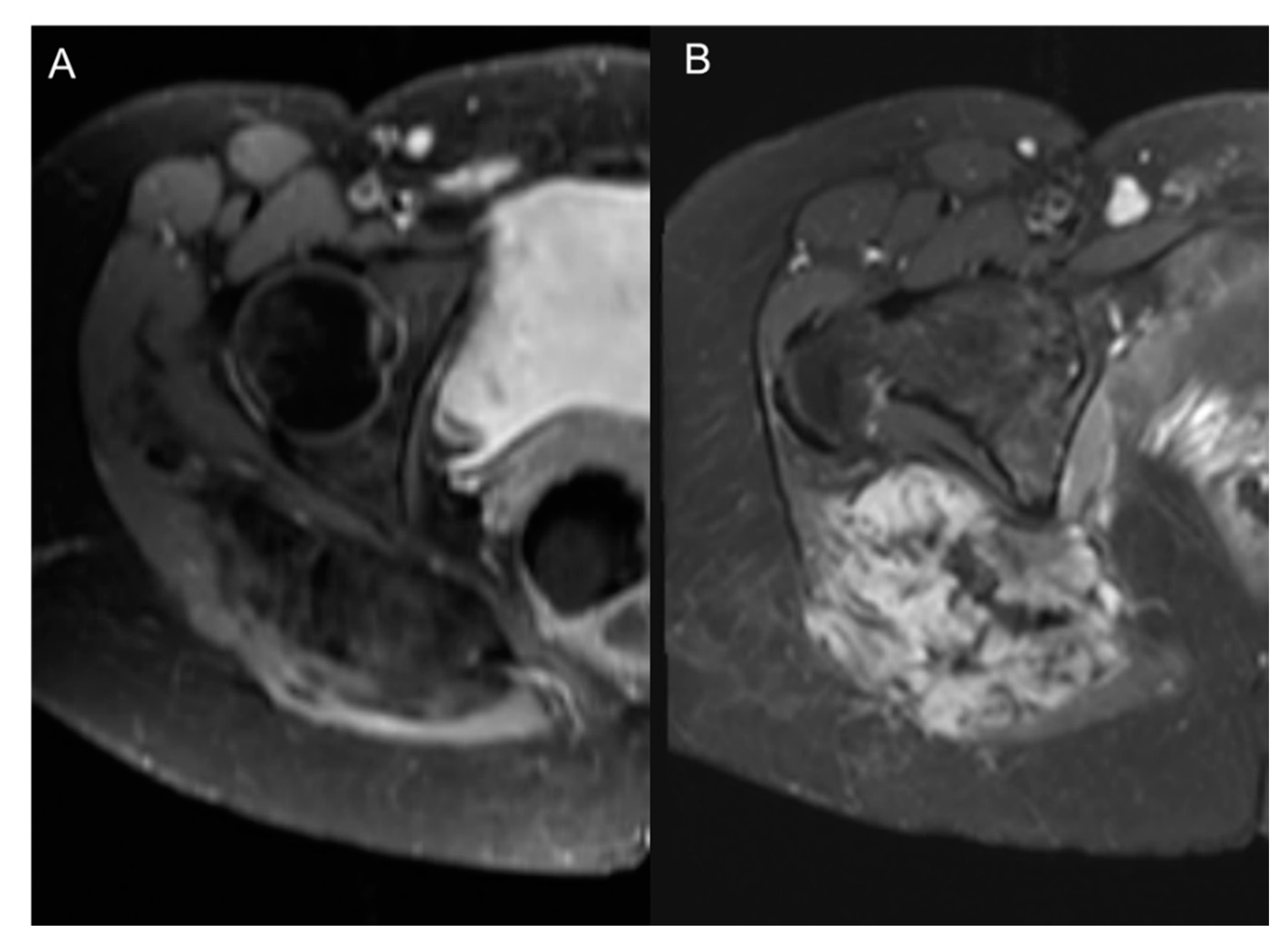
8. CT Imaging of Angiogenesis
8.1. Multi-Slice Computed Tomography (MSCT) Perfusion Imaging Technique
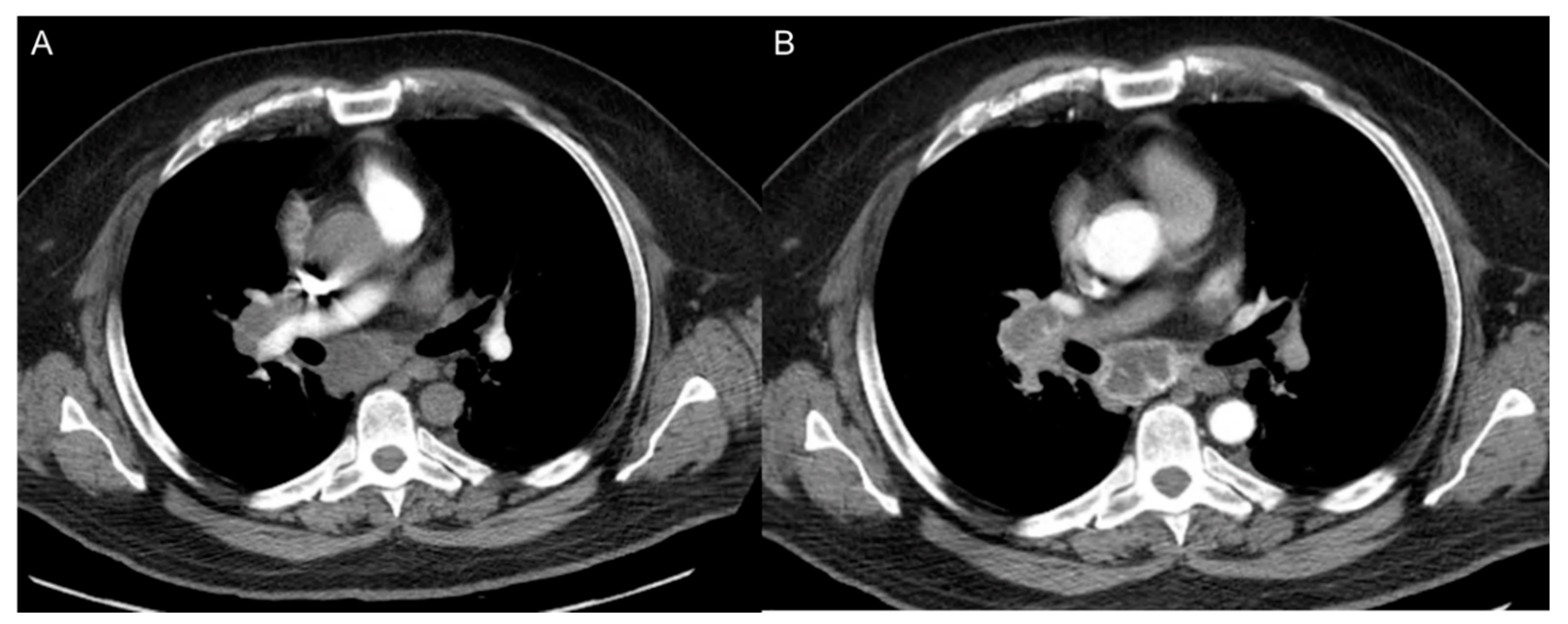
8.2. Clinical Applications of CT Perfusion
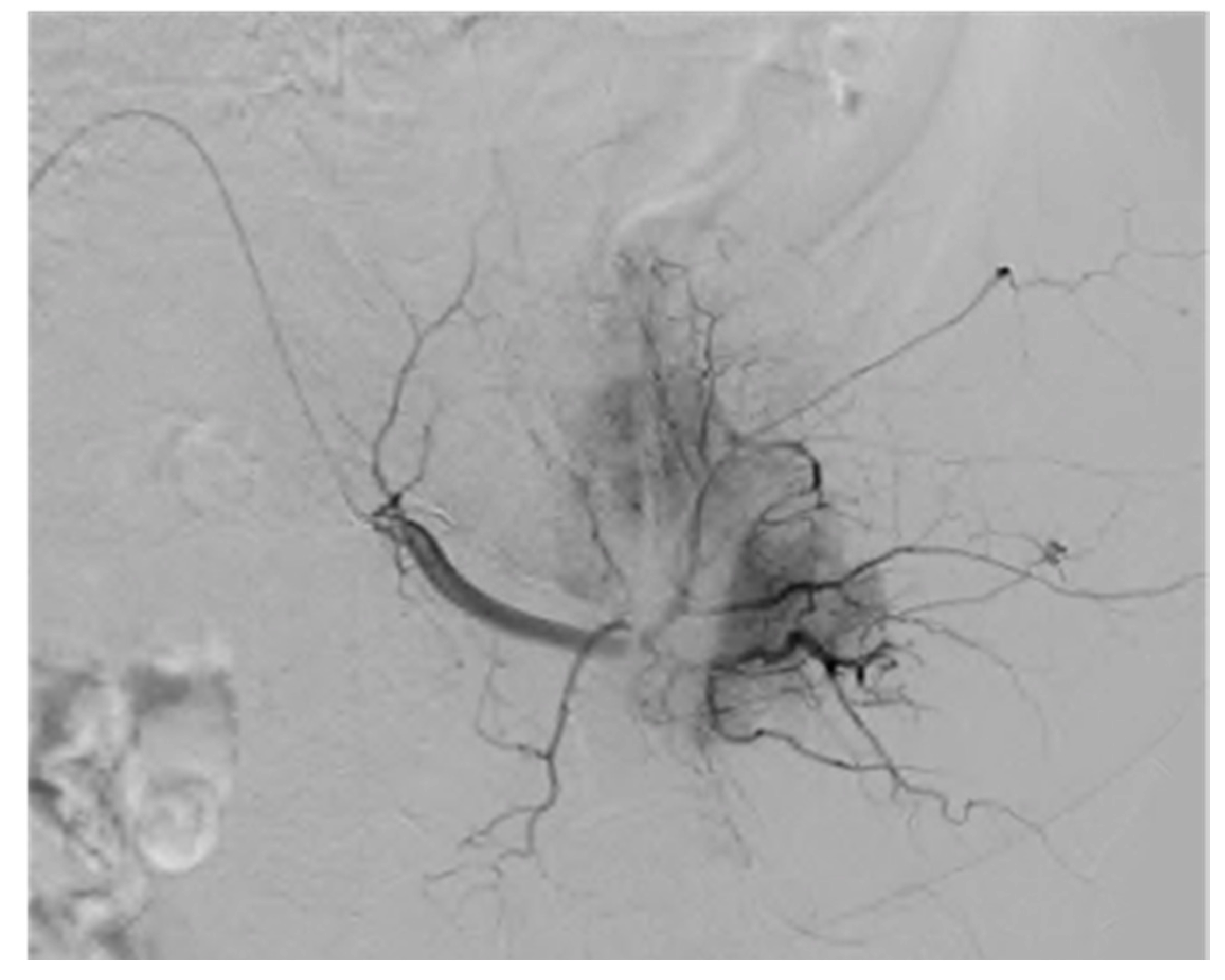
9. Ultrasound Imaging of Angiogenesis
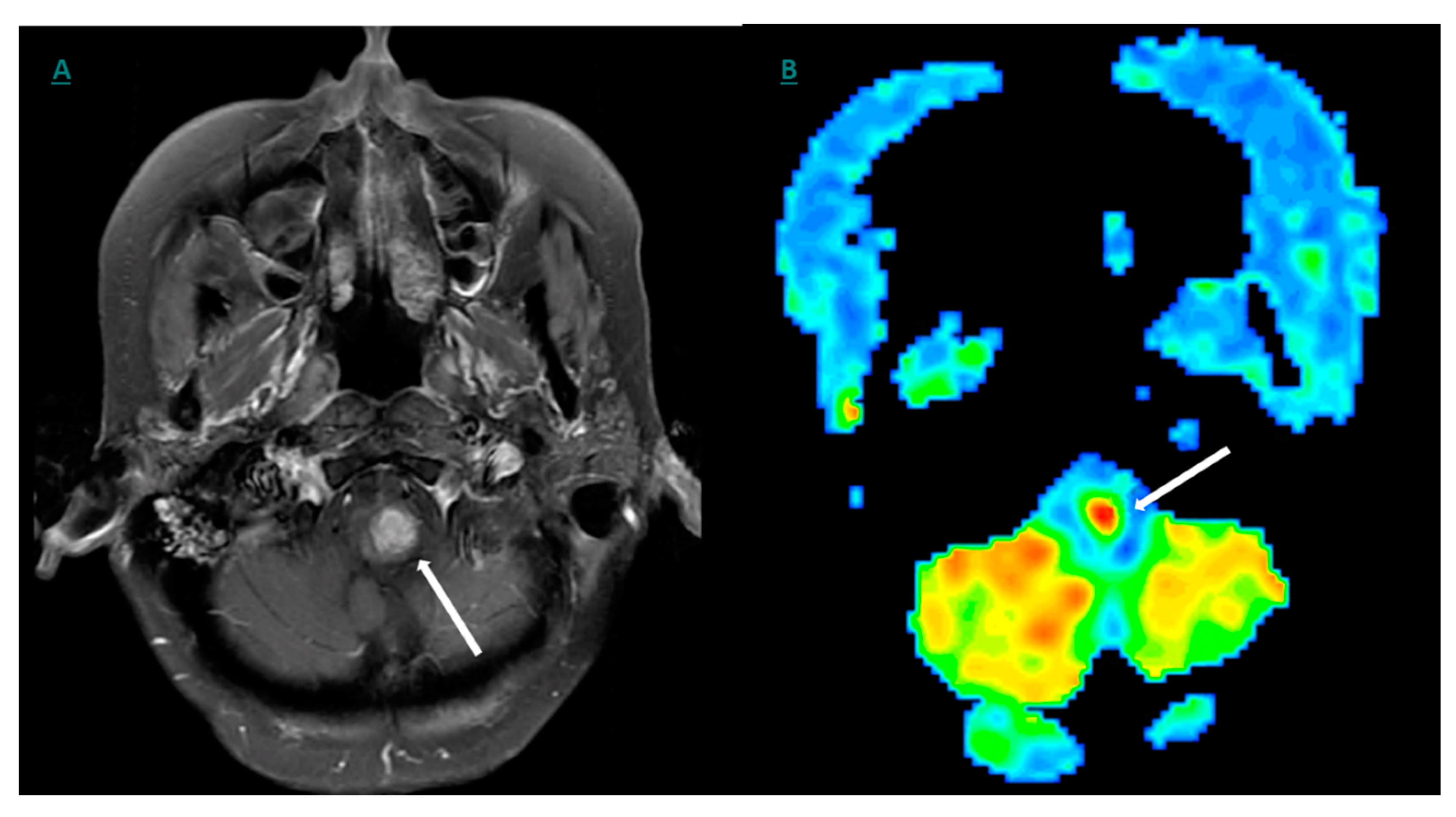
10. Positron Emission Tomography (PET) and Molecular Imaging
11. 18F-FDG- (Fluorine 18 Fluoro-Deoxy-Glucose)-Based Imaging
11.1. VEGF and VEGFR Targeting
11.2. Targeting Extracellular Matrix (ECM)
11.3. Other Potential Targets
12. Emerging Imaging Techniques for Angiogenesis
12.1. Ultrasound
12.2. Photoacoustic Imaging (PI)
12.3. X-ray Phase Contrast Imaging
13. Assessing the Response to Anti-Angiogenic Therapy
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.C.; Pien, H.H.; Sahani, D.; Sorensen, A.G.; Thrall, J.H. Imaging angiogenesis: Applications and potential for drug development. J. Natl. Cancer Inst. 2005, 97, 172–187. [Google Scholar] [CrossRef]
- Mulder, W.J.; Griffioen, A.W. Imaging of angiogenesis. Angiogenesis 2010, 13, 71–74. [Google Scholar] [CrossRef]
- Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, W.; Xu, K.; Yi, L.; Guo, Y.; Xie, T.; Tong, H.; Zhou, B.; Wang, S.; Li, Q.; et al. Dual inhibition of PFKFB3 and VEGF normalizes tumor vasculature, reduces lactate production, and improves chemotherapy in glioblastoma: Insights from protein expression profiling and MRI. Theranostics 2020, 10, 7245. [Google Scholar] [CrossRef]
- Kallinowski, F.; Schlenger, K.H.; Runkel, S.; Kloes, M.; Stohrer, M.; Okunieff, P.; Vaupel, P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989, 49, 3759–3764. [Google Scholar]
- Cai, W.; Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008, 49, 113S–128S. [Google Scholar] [CrossRef]
- McDonald, D.M.; Choyke, P.L. Imaging of angiogenesis: From microscope to clinic. Nat. Med. 2003, 9, 713–725. [Google Scholar] [CrossRef]
- Kong, Z.; Yan, C.; Zhu, R.; Wang, J.; Wang, Y.; Wang, Y.; Wang, R.; Feng, F.; Ma, W. Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas. Neuroimage Clin. 2018, 20, 51–60. [Google Scholar] [CrossRef]
- Raatschen, H.J.; Simon, G.H.; Fu, Y.; Sennino, B.; Shames, D.M.; Wendland, M.F.; McDonald, D.M.; Brasch, R.C. Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats. Radiology 2008, 247, 391–399. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Cao, Y.; Langer, R. A review of Judah Folkman’s remarkable achievements in biomedicine. Proc. Natl. Acad. Sci. USA 2008, 105, 13203–13205. [Google Scholar] [CrossRef]
- Demené, C.; Payen, T.; Dizeux, A.; Barrois, G.; Gennisson, J.; Bridal, L.; Tanter, M. 3-D Longitudinal Imaging of Tumor Angiogenesis in Mice in Vivo Using Ultrafast Doppler Tomography. Ultrasound Med. Biol. 2019, 45, 1284–1296. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Niccoli Asabella, A.; Di Palo, A.; Altini, C.; Ferrari, C.; Rubini, G. Multimodality Imaging in Tumor Angiogenesis: Present Status and Perspectives. Int. J. Mol. Sci. 2017, 18, 1864. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R. Vascular endothelial growth factor: Much more than an angiogenesis factor. Mol. Biol. Cell 2010, 21, 377–379. [Google Scholar] [CrossRef]
- Iagaru, A.; Gambhir, S.S. Imaging tumor angiogenesis: The road to clinical utility. Am. J. Roentgenol. 2013, 201, W183–W191. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Jeswani, T.; Padhani, A.R. Imaging tumour angiogenesis. Cancer Imaging 2005, 5, 131–138. [Google Scholar] [CrossRef]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Garcia-Figueiras, R.; Goh, V.J.; Padhani, A.R.; Baleato-Gonzalez, S.; Garrido, M.; Leon, L.; Gómez-Caamaño, A. CT perfusion in oncologic imaging: A useful tool? Am. J. Roentgenol. 2013, 200, 8–19. [Google Scholar] [CrossRef]
- Lanza, G.M.; Winter, P.M.; Caruthers, S.D.; Hughes, M.S.; Cyrus, T.; Marsh, J.N.; Neubauer, A.M.; Partlow, K.C.; Wickline, S.A. Nanomedicine opportunities for cardiovascular disease with perfluorocarbon nanoparticles. Nanomedicine 2006, 1, 321–329. [Google Scholar] [CrossRef]
- Li, K.C.; Guccione, S.; Bednarski, M.D. Combined vascular targeted imaging and therapy: A paradigm for personalized treatment. J. Cell. Biochem. Suppl. 2002, 39, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Gutierrez, J.; Narang, J.; Scarpace, L.; Schultz, L.R.; Lemke, N.; Patel, S.C.; Mikkelsen, T.; Rock, J.P. In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. Am. J. Neuroradiol. 2011, 32, 388–394. [Google Scholar] [CrossRef]
- Santarosa, C.; Castellano, A.; Conte, G.M.; Cadioli, M.; Iadanza, A.; Terreni, M.R.; Franzin, A.; Bello, L.; Caulo, M.; Falini, A.; et al. Dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging for glioma grading: Preliminary comparison of vessel compartment and permeability parameters using hotspot and histogram analysis. Eur. J. Radiol. 2016, 85, 1147–1156. [Google Scholar] [CrossRef]
- Hilario, A.; Hernandez-Lain, A.; Sepulveda, J.M.; Lagares, A.; Perez-Nuñez, A.; Ramos, A. Perfusion MRI grading diffuse gliomas: Impact of permeability parameters on molecular biomarkers and survival. Neurocirugía 2019, 30, 11–18. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.; Euceda, L.R.; Meyer, D.E.; Langseth, K.; Bathen, T.F.; Moestue, S.A.; Huuse, E.M. Multiparametric characterization of response to anti-angiogenic therapy using USPIO contrast-enhanced MRI in combination with dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2018, 47, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J. Carbon Dioxide Angiography: Scientific Principles and Practice. Vasc. Spec. Int. 2015, 31, 67–80. [Google Scholar] [CrossRef]
- Gounis, M.J.; Lieber, B.B.; Webster, K.A.; Wakhloo, A.K. A novel angiographic methodology for the quantification of angiogenesis. IEEE Trans. Biomed. Eng. 2008, 55, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Marx, A.; Reyes-Aldasoro, C.C.; Schad, L.R.; Zöllner, F.G.; Weis, C.A. Continuous representation of tumor microvessel density and detection of angiogenic hotspots in histological whole-slide images. Oncotarget 2015, 6, 19163. [Google Scholar] [CrossRef]
- Miles, K.A. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: A review. Eur. J. Radiol. 1999, 30, 198–205. [Google Scholar] [CrossRef]
- Anderson, H.; Price, P.; Blomley, M.; Leach, M.O.; Workman, P. Measuring changes in human tumour vasculature in response to therapy using functional imaging techniques. Br. J. Cancer 2001, 85, 1085–1093. [Google Scholar] [CrossRef]
- Zormpas-Petridis, K.; Jerome, N.P.; Blackledge, M.D.; Carceller, F.; Poon, E.; Clarke, M.; McErlean, C.M.; Barone, G.; Koers, A.; Vaidya, S.J.; et al. MRI Imaging of the Hemodynamic Vasculature of Neuroblastoma Predicts Response to Antiangiogenic Treatment. Cancer Res. 2019, 79, 2978–2991. [Google Scholar] [CrossRef]
- Naish, J.H.; McGrath, D.M.; Bains, L.J.; Passera, K.; Roberts, C.; Watson, Y.; Cheung, S.; Taylor, M.B.; Logue, J.P.; Buckley, D.L.; et al. Comparison of dynamic contrast-enhanced MRI and dynamic contrast-enhanced CT biomarkers in bladder cancer. Magn. Reson. Med. 2011, 66, 219–226. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Jackson, A.; Parker, G.J.; Jayson, G.C. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br. J. Cancer 2007, 96, 189–195. [Google Scholar] [CrossRef]
- Anderson, H.L.; Yap, J.T.; Miller, M.P.; Robbins, A.; Jones, T.; Price, P.M. Assessment of pharmacodynamic vascular response in a phase I trial of combretastatin A4 phosphate. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 2823–2830. [Google Scholar] [CrossRef]
- Krix, M.; Kiessling, F.; Vosseler, S.; Farhan, N.; Mueller, M.M.; Bohlen, P.; Fusenig, N.E.; Delorme, S. Sensitive noninvasive monitoring of tumor perfusion during antiangiogenic therapy by intermittent bolus-contrast power Doppler sonography. Cancer Res. 2003, 63, 8264–8270. [Google Scholar]
- Barrett, T.; Brechbiel, M.; Bernardo, M.; Choyke, P.L. MRI of tumor angiogenesis. J. Magn. Reson. Imaging 2007, 26, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Kim, S.G.; Garwood, M. Imaging blood flow in brain tumors using arterial spin labeling. Magn. Reson. Med. 2000, 44, 169–173. [Google Scholar] [CrossRef]
- Williams, D.S. Quantitative perfusion imaging using arterial spin labeling. Methods Mol. Med. 2006, 124, 151–173. [Google Scholar]
- Tempel, C.; Neeman, M. Spatial and temporal modulation of perfusion in the rat ovary measured by arterial spin labeling MRI. J. Magn. Reson. Imaging 1999, 9, 794–803. [Google Scholar] [CrossRef]
- Boss, A.; Martirosian, P.; Schraml, C.; Clasen, S.; Fenchel, M.; Anastasiadis, A.; Claussen, C.D.; Pereira, P.L.; Schick, F. Morphological, contrast-enhanced and spin labeling perfusion imaging for monitoring of relapse after RF ablation of renal cell carcinomas. Eur. Radiol. 2006, 16, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, K.; English, S.J.; Krishna, M.C.; Cook, J.A.; Sowers, A.L.; Mitchell, J.B.; Zhang, Y. Influence of body temperature on the BOLD effect in murine SCC tumors. Magn. Reson. Med. 2004, 51, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Doblas, S.; He, T.; Saunders, D.; Pearson, J.; Hoyle, J.; Smith, N.; Lerner, M.; Towner, R.A. Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. J. Magn. Reson. Imaging 2010, 32, 267–275. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Kang, B.J.; Song, B.J.; Kim, H.B.; Lee, H.; Jin, M.S.; Lee, A. Dynamic Contrast-Enhanced MRI Perfusion Parameters as Imaging Biomarkers of Angiogenesis. PLoS ONE 2016, 11, e0168632. [Google Scholar] [CrossRef]
- Padhani, A.R.; Dzik-Jurasz, A. Perfusion MR imaging of extracranial tumor angiogenesis. Top. Magn. Reson. Imaging 2004, 15, 41–57. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Wang, G.R.; Brenner, T.; Petrella, J.R.; Sorensen, A.G. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. Am. J. Roentgenol. 2002, 178, 711–716. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Tong, H.; Wang, S.; Yang, Y.; Liu, G.; Zhang, W. Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: Recent advances and current challenges. Contrast Media Mol. Imaging 2017, 2017, 7064120. [Google Scholar] [CrossRef] [PubMed]
- Choyke, P.L.; Dwyer, A.J.; Knopp, M.V. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J. Magn. Reson. Imaging 2003, 17, 509–520. [Google Scholar] [CrossRef]
- Essig, M.; Shiroishi, M.S.; Nguyen, T.B.; Saake, M.; Provenzale, J.M.; Enterline, D.; Anzalone, N.; Dörfler, A.; Rovira, A.; Wintermark, M.; et al. Perfusion MRI: The five most frequently asked technical questions. Am. J. Roentgenol. 2013, 200, 24–34. [Google Scholar] [CrossRef]
- Tofts, P.S. T1-weighted DCE imaging concepts: Modelling, acquisition and analysis. Signal 2010, 500, 400. [Google Scholar]
- Murase, K. Efficient method for calculating kinetic parameters using T1-weighted dynamic contrast-enhanced magnetic resonance imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2004, 51, 858–862. [Google Scholar] [CrossRef]
- D’Assignies, G.; Couvelard, A.; Bahrami, S.; Vullierme, M.; Hammel, P.; Hentic, O.; Sauvanet, A.; Bedossa, P.; Ruszniewski, P.; Vilgrain, V. Pancreatic endocrine tumors: Tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology 2009, 250, 407–416. [Google Scholar] [CrossRef]
- Xu, N.; Lei, Z.; Li, X.L.; Zhang, J.; Li, C.; Feng, G.Q.; Li, D.N.; Liu, J.Y.; Wei, Q.; Bian, T.T.; et al. Clinical study of tumor angiogenesis and perfusion imaging using multi-slice spiral computed tomography for breast cancer. Asian Pac. J. Cancer Prev. 2013, 14, 429–433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, C.H.; Feng, S.; Xiao, P.; Peng, Z.P.; Pui, M.H.; Li, X.H.; Li, Z.P.; Meng, Q.F. Quantitative assessment of perirectal tumor infiltration with dynamic contrast-enhanced multi-detector row CT in rectal cancer. Eur. J. Radiol. 2011, 80, 279–283. [Google Scholar] [CrossRef]
- Mazzei, F.G.; Mazzei, M.A.; Cioffi Squitieri, N.; Pozzessere, C.; Righi, L.; Cirigliano, A.; Guerrini, S.; D’Elia, D.; Ambrosio, M.R.; Barone, A.; et al. CT perfusion in the characterisation of renal lesions: An added value to multiphasic CT. BioMed Res. Int. 2014, 2014, 135013. [Google Scholar] [CrossRef]
- Trojanowska, A.; Trojanowski, P.; Bisdas, S.; Staśkiewicz, G.; Drop, A.; Klatka, J.; Bobek-Billewicz, B. Squamous cell cancer of hypopharynx and larynx—Evaluation of metastatic nodal disease based on computed tomography perfusion studies. Eur. J. Radiol. 2012, 81, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bellomi, M.; Gatti, G.; Ping, X. Accuracy of computed tomography perfusion in assessing metastatic involvement of enlarged axillary lymph nodes in patients with breast cancer. Breast Cancer Res. 2007, 9, R40. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Ehling, J.; Lammers, T.; Kiessling, F. Non-invasive imaging for studying anti-angiogenic therapy effects. Thromb. Haemost. 2013, 109, 375–390. [Google Scholar] [CrossRef]
- Jugold, M.; Palmowski, M.; Huppert, J.; Woenne, E.C.; Mueller, M.M.; Semmler, W.; Kiessling, F. Volumetric high-frequency Doppler ultrasound enables the assessment of early antiangiogenic therapy effects on tumor xenografts in nude mice. Eur. Radiol. 2008, 18, 753–758. [Google Scholar] [CrossRef]
- Goodwin, A.P.; Nakatsuka, M.A.; Mattrey, R.F. Stimulus–responsive ultrasound contrast agents for clinical imaging: Motivations, demonstrations, and future directions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 111–123. [Google Scholar] [CrossRef][Green Version]
- Kiessling, F.; Huppert, J.; Palmowski, M. Functional and molecular ultrasound imaging: Concepts and contrast agents. Curr. Med. Chem. 2009, 16, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Flexman, M.L.; Vlachos, F.; Huang, J.; Hernandez, S.L.; Kim, H.K.; Johung, T.B.; Gander, J.W.; Reichstein, A.R.; Lampl, B.S.; et al. Contrast ultrasound imaging for identification of early responder tumor models to anti-angiogenic therapy. Ultrasound Med. Biol. 2012, 38, 1019–1029. [Google Scholar] [CrossRef]
- Pysz, M.A.; Foygel, K.; Rosenberg, J.; Gambhir, S.S.; Schneider, M.; Willmann, J.K. Antiangiogenic cancer therapy: Monitoring with molecular US and a clinically translatable contrast agent (BR55). Radiology 2010, 256, 519–527. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. PET Imaging of Angiogenesis. PET Clin. 2009, 4, 17–38. [Google Scholar] [CrossRef]
- O’Connor, J.P.B. Imaging angiogenesis: Role in measuring response to treatment. Antiangiogenesis Cancer Ther. 2014, 29–48. [Google Scholar] [CrossRef]
- Hu, K.; Shang, J.; Xie, L.; Hanyu, M.; Zhang, Y.; Yang, Z.; Xu, H.; Wang, L.; Zhang, M.R. PET Imaging of VEGFR with a Novel 64Cu-Labeled Peptide. ACS Omega 2020, 5, 8508–8514. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.; Lagerweij, T.; Sewing, A.C.; Vugts, D.J.; van Vuurden, D.G.; Molthoff, C.F.; Caretti, V.; Veringa, S.J.; Petersen, N.; Carcaboso, A.M.; et al. Bevacizumab Targeting Diffuse Intrinsic Pontine Glioma: Results of 89Zr-Bevacizumab PET Imaging in Brain Tumor Models. Mol. Cancer Ther. 2016, 15, 2166–2174. [Google Scholar] [CrossRef]
- Zhu, Z.; Miao, W.; Li, Q.; Dai, H.; Ma, Q.; Wang, F.; Yang, A.; Jia, B.; Jing, X.; Liu, S.; et al. 99mTc-3PRGD2 for integrin receptor imaging of lung cancer: A multicenter study. J. Nucl. Med. 2012, 53, 716–722. [Google Scholar] [CrossRef]
- Kiugel, M.; Hellberg, S.; Käkelä, M.; Liljenbäck, H.; Saanijoki, T.; Li, X.G.; Tuomela, J.; Knuuti, J.; Saraste, A.; Roivainen, A. Evaluation of [68Ga]Ga-DOTA-TCTP-1 for the Detection of Metalloproteinase 2/9 Expression in Mouse Atherosclerotic Plaques. Molecules 2018, 23, 3168. [Google Scholar] [CrossRef]
- Rossin, R.; Berndorff, D.; Friebe, M.; Dinkelborg, L.M.; Welch, M.J. Small-animal PET of tumor angiogenesis using a (76)Br-labeled human recombinant antibody fragment to the ED-B domain of fibronectin. J. Nucl. Med. 2007, 48, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhu, Y.; Mu, D.; Fan, B.; Zhao, S.; Yang, G.; Ma, L.; Zheng, J.; Yu, J. Correlation of hypoxia as measured by fluorine-18 fluoroerythronitroimidazole (18F-FETNIM) PET/CT and overall survival in glioma patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.G.; Koczan, D.; Klippel, S.; Pan, L.; Cheng, C.; Willis, S.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Impact of angiogenesis-related gene expression on the tracer kinetics of 18F-FDG in colorectal tumors. J. Nucl. Med. 2008, 49, 1238–1244. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Borsi, L.; Balza, E.; Bestagno, M.; Castellani, P.; Carnemolla, B.; Biro, A.; Leprini, A.; Sepulveda, J.; Burrone, O.; Neri, D.; et al. Selective targeting of tumoral vasculature: Comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int. J. Cancer J. Int. Cancer 2002, 102, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef]
- Van Belle, P.A.; Elenitsas, R.; Satyamoorthy, K.; Wolfe, J.T.; Guerry, D.; Schuchter, L.; van Belle, T.J.; Albelda, S.; Tahin, P.; Herlyn, M.; et al. Progression-related expression of beta3 integrin in melanomas and nevi. Hum. Pathol. 1999, 30, 562–567. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, D.; Cheng, C.; Zhang, A.; Ma, C.; Wang, L.; Zhang, D.; Liu, H.; Jiang, H.; Wang, T.; et al. Targeting of MMP2 activity in malignant tumors with a 68Ga-labeled gelatinase inhibitor cyclic peptide. Nucl. Med. Biol. 2015, 42, 939–944. [Google Scholar] [CrossRef]
- Matter, A. Tumor angiogenesis as a therapeutic target. Drug Discov. Today 2001, 6, 1005–1024. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001, 97, 652–659. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar]
- Chen, K.; Ma, W.; Li, G.; Wang, J.; Yang, W.; Yap, L.P.; Hughes, L.D.; Park, R.; Conti, P.S. Synthesis and evaluation of 64Cu-labeled monomeric and dimeric NGR peptides for MicroPET imaging of CD13 receptor expression. Mol. Pharm. 2013, 10, 417–427. [Google Scholar] [CrossRef]
- Ma, W.; Kang, F.; Wang, Z.; Yang, W.; Li, G.; Ma, X.; Li, G.; Chen, K.; Zhang, Y.; Wang, J. (99m)Tc-labeled monomeric and dimeric NGR peptides for SPECT imaging of CD13 receptor in tumor-bearing mice. Amino Acids 2013, 44, 1337–1345. [Google Scholar] [CrossRef]
- Leow, C.H.; Bush, N.L.; Stanziola, A.; Braga, M.; Shah, A.; Hernandez-Gil, J.; Long, N.J.; Aboagye, E.O.; Bamber, J.C.; Tang, M.X. 3-D Microvascular Imaging Using High Frame Rate Ultrasound and ASAP without Contrast Agents: Development and Initial In Vivo Evaluation on Nontumor and Tumor Models. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 939–948. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Korpisalo, P.; Karvinen, H.; Liimatainen, T.; Laidinen, S.; Grohn, O.H.; Ylä-Herttuala, S. High-resolution ultrasound perfusion imaging of therapeutic angiogenesis. JACC Cardiovasc. Imaging 2008, 1, 83–91. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Horiguchi, A.; Shinchi, M.; Nakamura, A.; Wada, T.; Ito, K.; Asano, T.; Shinmoto, H.; Tsuda, H.; Ishihara, M. Pilot Study of Prostate Cancer Angiogenesis Imaging Using a Photoacoustic Imaging System. Urology 2017, 108, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef]
- Lundström, U.; Larsson, D.H.; Burvall, A.; Takman, P.A.; Scott, L.; Brismar, H.; Hertz, H.M. X-ray phase contrast for CO2 microangiography. Phys. Med. Biol. 2012, 57, 2603–2617. [Google Scholar] [CrossRef]
- Lundström, U.; Westermark, U.K.; Larsson, D.H.; Burvall, A.; Arsenian Henriksson, M.; Hertz, H.M. X-ray phase contrast with injected gas for tumor microangiography. Phys. Med. Biol. 2014, 59, 2801–2811. [Google Scholar] [CrossRef]
- Zhang, W.; Ran, S.; Sambade, M.; Huang, X.; Thorpe, P.E. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis 2002, 5, 35–44. [Google Scholar] [CrossRef]
- Hlatky, L.; Hahnfeldt, P.; Folkman, J. Clinical Application of Antiangiogenic Therapy: Microvessel Density, What It Does and Doesn’t Tell Us. J. Natl. Cancer Inst. 2002, 94, 883–893. [Google Scholar] [CrossRef]
- Elice, F.; Rodeghiero, F.; Falanga, A.; Rickles, F.R. Thrombosis associated with angiogenesis inhibitors. Best Pract. Res. Clin. Haematol. 2009, 22, 115–128. [Google Scholar] [CrossRef]
| Symbol | Measures | Significance |
|---|---|---|
| Cp (arterial concentration) | Concentration of tracer in arterial blood (input function in CT and MRI) |
|
| Ca (arterial concentration) | Concentration of tracer in arterial blood (input function in PET) | (as above) |
| F (flow) | Blood flow: represents the amount of blood passing per unit of time per tissue mass | Decreased under antiangiogenic therapy |
| P (permeability) | Capillary wall permeability surface area | Testing the integrity of the blood vessel mesh |
| PS (permeability-surface area product) | Capillary wall permeability surface area per unit mass of tissue (in practice, P and PS are not clearly separated) | Testing the integrity of the blood vessels mesh in relation to tissue volume |
| E (extraction fraction) | Extraction fraction of oxygen | Ratio of blood oxygen that a tissue takes up from blood flow |
| Ktrans (volume transfer coefficient) | Volume transfer constant from plasma to the extravascular extracellular compartment |
|
| Kep (equilibrium product constant) | Rate constant between the extravascular extracellular compartment and the plasma (efflux of tracer back to the vascular space) |
|
| Ki (net influx rate) | Unidirectional influx constant | |
| Vp (plasma blood volume) | Fractional blood plasma volume | |
| Va (arterial blood volume) | Fractional blood volume | |
| Ve (extravascular extracellular volume fraction) | Volume of extracellular extravascular per tissue unit volume | |
| IAUC (initial area under the curve) | Area under the curve in contrast concentration against time curve |
|
| rBV (relative blood volume) | Percentage of blood volume in relation to tissue volume |
|
| Perfusion | Amount of blood flow within the capillary system (blood flow rate over organ mass) |
|
| MTT (Mean transient time) | Time a contrast agent has to pass through the tissue of interest from the arterial side to the venous draining side (blood volume over blood flow) |
|
| Vascular permeability | Depends on various parameters like tracer properties, vascular surface area, leakiness and the extracellular extravascular distribution space | Tumors possess immature capillary mesh so are characterized by higher-than-normal vascular permeability |
| Radiolabeled Substance | Example Radiotracer | Tracer Modality * | Significance |
|---|---|---|---|
| VEGF/VEGFR | [64Cu]VEGF125-136 [68] | PET |
|
| Bevacizumab | 89Zr-bevacizumab [69] | PET |
|
| Integrins (αvβ3) | 99mTc-3PRGD2 [70] | SPECT |
|
| Matrix metalloproteinases (MMP) | 68Ga-DOTA-TCTP-1 (MMP-2) [71] | PET |
|
| Fibronectin | 76Br-L19-SIP (ED-B) [72] | PET |
|
| Nitroimidazole | 18F-FETNIM [73] | PET |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, M.A.; Guccione, J.; Reiter, A.M.; Moawad, A.W.; Etchison, A.; Kamel, S.; Khatchikian, A.D.; Elsayes, K.M. Current Concepts in Multi-Modality Imaging of Solid Tumor Angiogenesis. Cancers 2020, 12, 3239. https://doi.org/10.3390/cancers12113239
Soliman MA, Guccione J, Reiter AM, Moawad AW, Etchison A, Kamel S, Khatchikian AD, Elsayes KM. Current Concepts in Multi-Modality Imaging of Solid Tumor Angiogenesis. Cancers. 2020; 12(11):3239. https://doi.org/10.3390/cancers12113239
Chicago/Turabian StyleSoliman, Moataz A., Jeffrey Guccione, Anna M. Reiter, Ahmed W. Moawad, Ashley Etchison, Serageldin Kamel, Aline D. Khatchikian, and Khaled M. Elsayes. 2020. "Current Concepts in Multi-Modality Imaging of Solid Tumor Angiogenesis" Cancers 12, no. 11: 3239. https://doi.org/10.3390/cancers12113239
APA StyleSoliman, M. A., Guccione, J., Reiter, A. M., Moawad, A. W., Etchison, A., Kamel, S., Khatchikian, A. D., & Elsayes, K. M. (2020). Current Concepts in Multi-Modality Imaging of Solid Tumor Angiogenesis. Cancers, 12(11), 3239. https://doi.org/10.3390/cancers12113239






