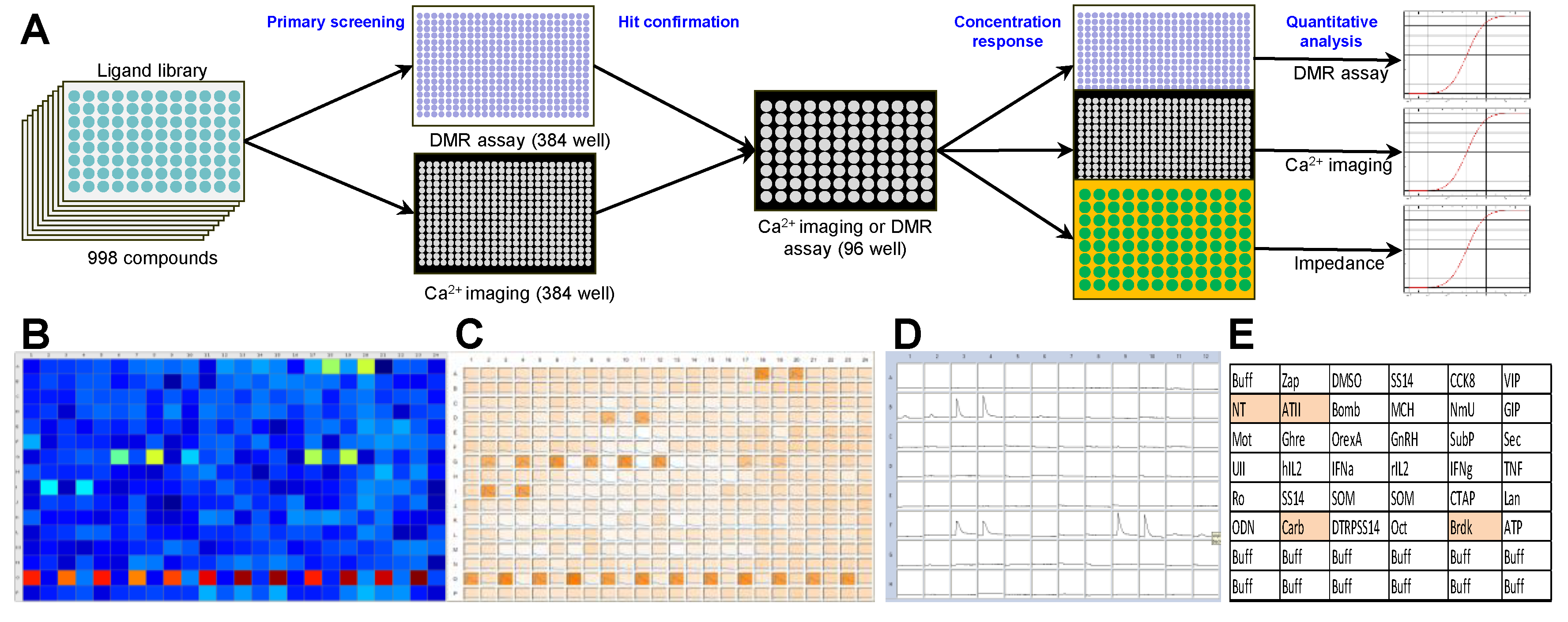
2. Results
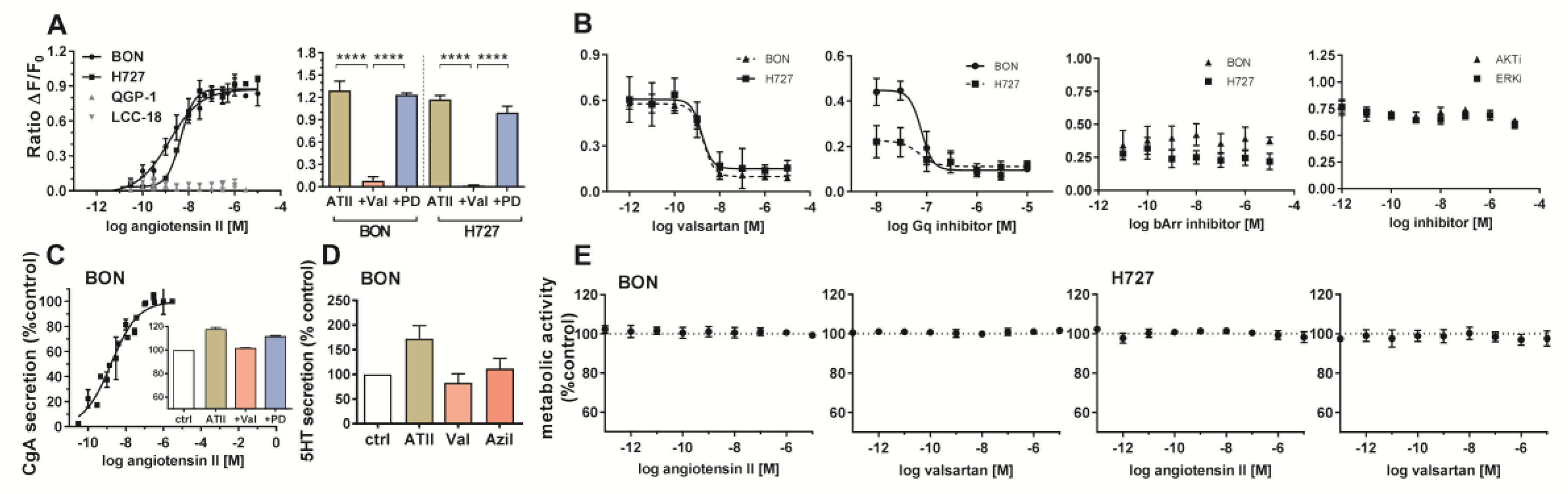
A 998-compound chemical library mainly consisting of peptide receptor ligands (
Table S1) was applied in a primary screening in the NEN cell line BON. Both a label-free dynamic mass redistribution (DMR) as well as an intracellular calcium mobilization assay were employed in 384-well format (
Figure 1A–C). A total of 89 hits were detected (8.9%), which were assayed again in BON cells in a 96-well format (
Figure 1D,E). Thirty primary hits, including structurally related compounds, were confirmed in 96-well format in the DMR assay, and 19 hits in the calcium assay, with 11 compounds confirmed in both assays (
Table S2). Angiotensin II (ATII) appeared consistently as a strong activator of intracellular signaling in BON cells. This was subsequently validated in three functional assays: DMR, calcium and impedance. Quantitative analysis showed a half maximal effective concentration (EC
50) for ATII of 0.88 ± 0.28 nM, 4.9 ± 5.2 nM and 0.45 ± 0.03 nM in DMR, calcium and impedance, respectively (
Figure 1F–H).
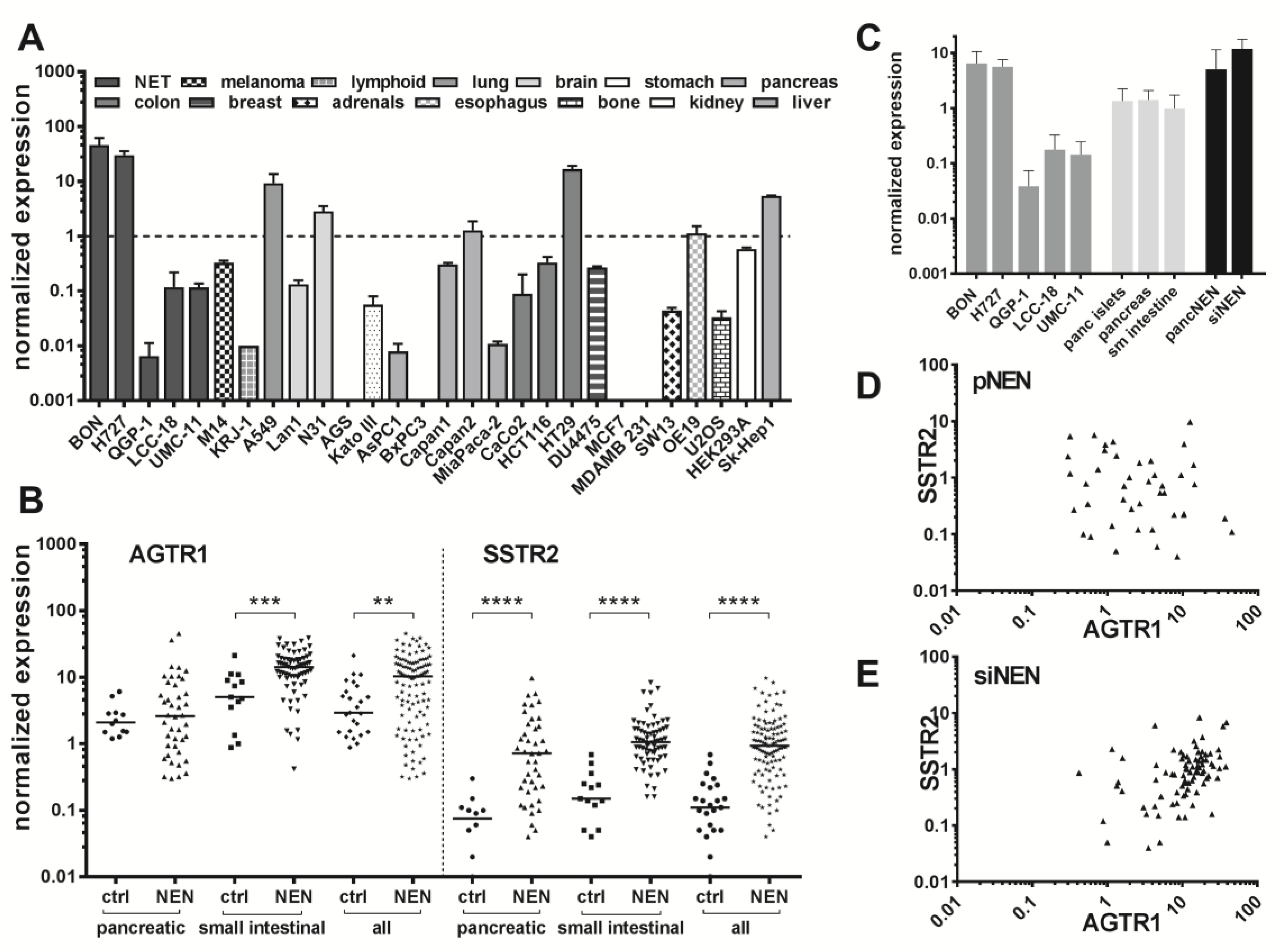
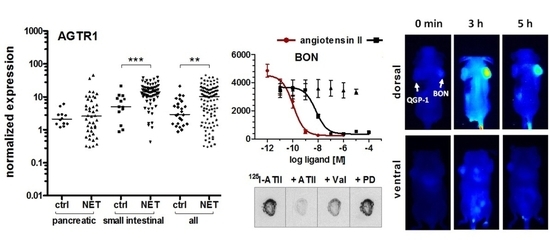
Endogenous expression of the ATII receptor AGTR1 was assessed in five NEN cell lines by RT-qPCR and compared to mRNA levels of 23 other tumor and non-tumor cell lines (
Figure 2A). NEN cell lines BON and H727 exhibited the highest mRNA levels of all tested, indicating an AGTR1 overexpression in NEN. The NEN cell line QGP-1 showed an at least 1000-fold lower AGTR1 mRNA expression, while LCC-18 and UMC-11 had intermediate expression levels. Pancreatic (
n = 42) and small-intestinal (
n = 71) tumor tissues of NEN patients were analyzed for their AGTR1 mRNA levels. To allow a direct comparison with their organs of origin, normal control tissues (
n = 25) were also included. Small-intestinal NEN (siNEN) demonstrated significantly (
p < 0.001) increased AGTR1 transcript levels, with an overall 2.8-fold higher median value in comparison to healthy control tissues, whereas pancreatic tissues showed no significant difference (
Figure 2B). Taking the median value of the siNEN samples as a cut-off, 50% of tumor samples yet only 7.7% of controls were above that value. When all NENs were compared to all controls, differences were also significant (3.6-fold higher median,
p < 0.01). No correlation of AGTR1 mRNA levels with stage, grade or Ki67 value were found (see
Figure S1 for correlation analysis with Ki67). To allow a direct comparison, AGTR1 mRNA levels in NEN cell lines, normal controls and NEN tumor tissue were analyzed and plotted. Normal pancreas, pancreatic islets and small intestine showed similar mRNA levels. Higher levels in pNEN and siNEN samples matched well those of NEN cell lines BON and H727, while the other NEN cell lines were found to have lower mRNA levels (
Figure 2C). The same clinical samples were also analyzed for expression of the established NEN target SSTR2, which was found to be significantly upregulated in both pancreatic and siNEN tissues, as expected. The ratio of NEN to control median values was higher for SSTR2 (8.5 versus 3.6 for AGTR1). However, AGTR1 was detected at an approximately 10-fold higher expression level than SSTR2. Correlation analysis confirmed a significant positive correlation between AGTR1 and SSTR2 expression for small-intestinal NENs (Pearson
r = 0.37,
p < 0.001,
r2 = 0.14), whereas no significant correlation was found for pancreatic samples (
r = −0.09) (
Figure 2D,E).
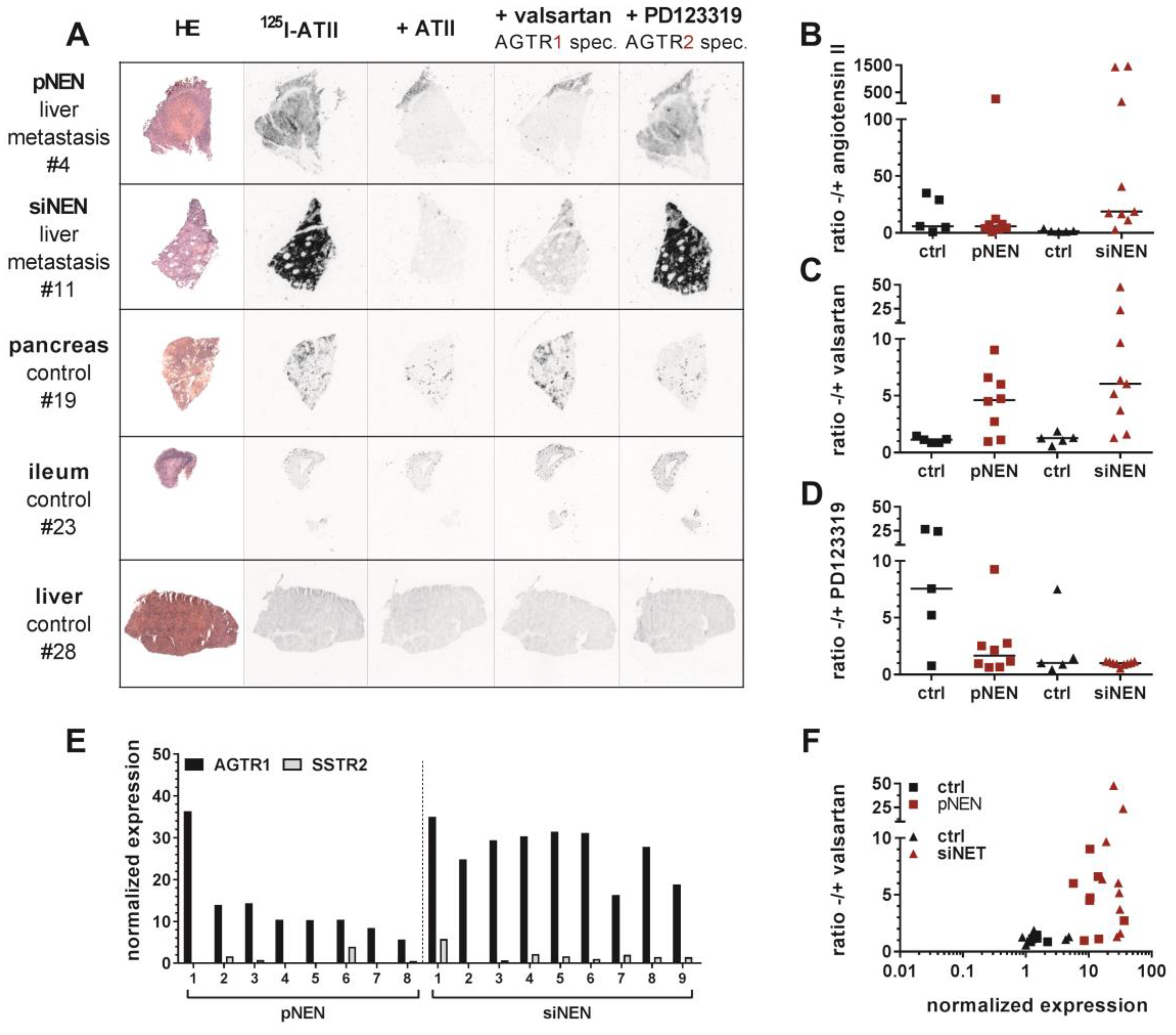
To verify AGTR1 gene expression employing an alternative methodology, ten normal control samples from pancreas and small intestine as well as eight pancreatic and nine small-intestinal NEN samples were selected for the determination of receptor binding sites by in vitro receptor autoradiography (patient characteristics:
Table S3). To this end, the natural receptor ligand ATII was radioactively labeled with iodine-125 (
125I–ATII) and purified by high-performance liquid chromatography (HPLC) (
Figure S2). Consecutive cryosections of each tissue were incubated with the radioligand in the absence or presence of an excess of non-labeled ATII. To distinguish between receptor subtypes, additional tissue sections were incubated with radioligand and AGTR1-specific antagonist valsartan or AGTR2-specific antagonist PD123319 (
Figure 3A,
Figures S4 and S5). Pancreatic NEN tissues showed a rather weak overall binding of
125I–ATII. Nevertheless, in tissues #4 (
Figure 3A), #1, #6 and #8 (
Figure S4), it could be clearly displaced by ATII and valsartan, but not by PD123319, indicating dominant AGTR1 expression. Small-intestinal NENs, on the other hand, demonstrated a strong binding in more than half of the samples (#9 to #13) (
Figure 3A,
Figure S4). This proved to be AGTR1-specific binding as it could only be displaced by valsartan. PD123319 was unable to compete with the radioligand. In addition, pancreatic and small-intestinal control tissues were included in the analysis (
Figure 3A,
Figure S5). Interestingly, a number of pancreatic samples exhibited strong radioligand binding. In comparison to pancreatic NEN samples, signals in normal pancreas were displaced solely by PD123319, indicating a specific expression of AGTR2. Small-intestinal control samples showed no binding at all (#23, #25) or non-specific binding (#24, #26, #27) (
Figure S5).
Digitized autoradiograms were quantified by calculating the ratios of total binding to non-specific binding in the presence of excess ATII, valsartan or PD123319 (
Figure 3B–D). The higher a ratio, the higher the expression of the respective receptor subtype. Indeed, valsartan-related ratios were increased up to 5-fold in NEN tissues, especially in small-intestinal NENs, when compared to their respective control tissues. This confirmed a specific AGTR1 expression (
Figure 3C). To verify these findings using a different quantitative analysis, tissues were wiped off the slides after radioligand incubation and measured by a gamma counter. This alternative quantification yielded similar results (
Figure S3). As depicted in
Figure 3F, mRNA levels correlated well with binding levels for most tissues (Pearson
r = 0.43,
p ≤ 0.01,
r2 = 0.184). While controls showed low mRNA and low binding levels, NENs in general had relatively high mRNA and binding levels. A similar correlation using the alternative quantification confirmed this result (
Figure S3B). Furthermore, AGTR1 mRNA levels of the samples analyzed by autoradiography were individually compared to those of SSTR2 mRNA (
Figure 3E. All these NEN tissues showed higher AGTR1 than SSTR2 mRNA levels.
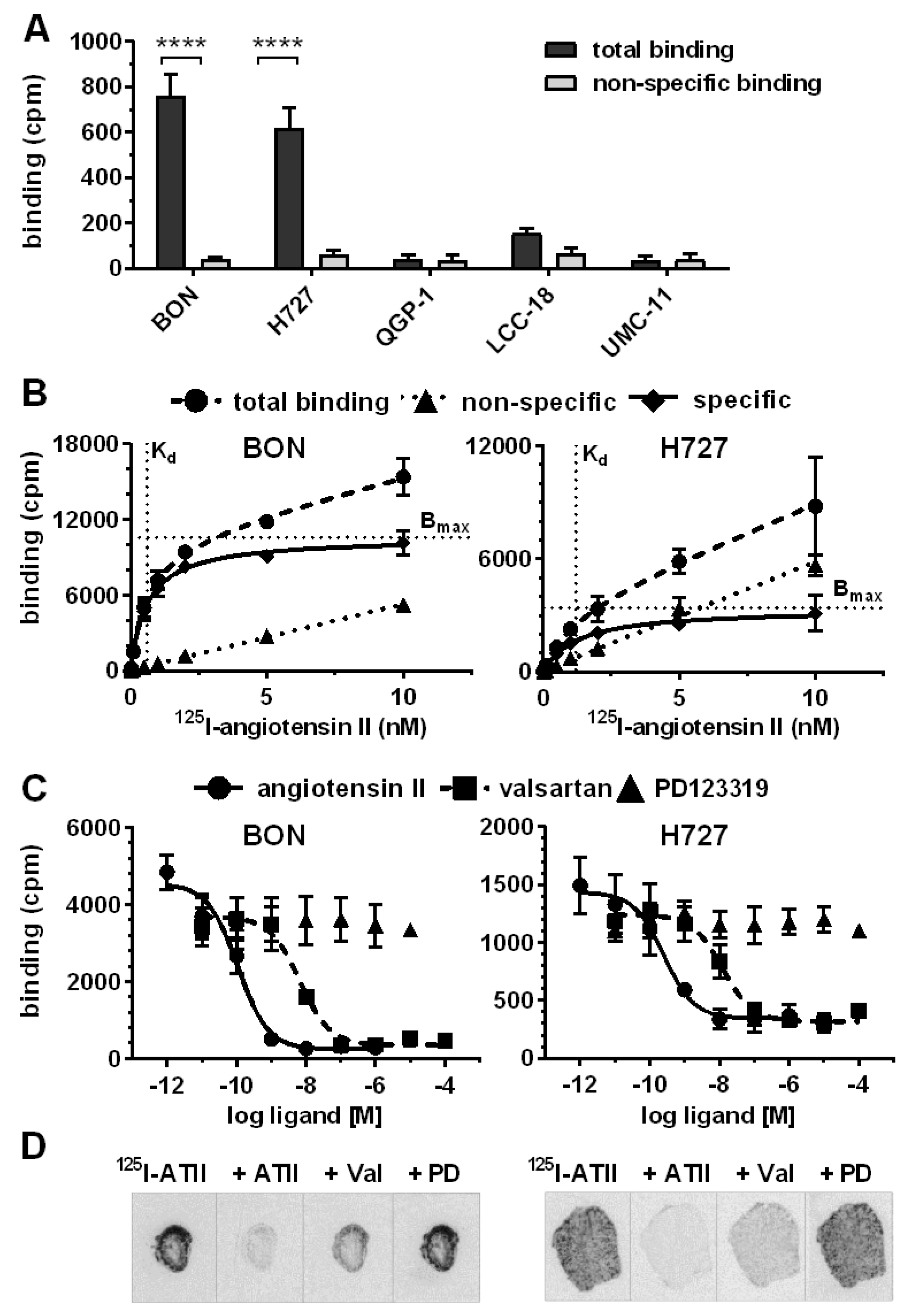
After target validation in patient tissues, receptor-positive cell lines BON and H727 were further evaluated as models for AGTR1 expression and function in NEN. Confirming gene expression data (
Figure 2A), both cell lines exhibited significant specific binding of
125I–ATII that could be displaced by unlabeled ATII, whereas the other NEN cell lines QGP-1, LCC-18 and UMC-1 demonstrated only background levels of radioligand binding (
Figure 4A). Saturation radioligand binding experiments determined K
d values of 0.6 nM for BON and 1.2 nM for H727 (
Figure 4B). In contrast, the cell lines differed in their receptor density as indicated by the B
max value, which was 3-fold higher for BON (~50,000 binding sites/cell) in comparison to H727 cells (~16,000 binding sites/cell). As depicted in
Figure 4C, binding of
125I–ATII to BON and H727 cells could be displaced in a dose-dependent fashion by ATII and the AGTR1-specific antagonist valsartan, but not by the AGTR2-specific antagonist PD123319. Calculated K
i values reflect the expected high affinity of ATII for AGTR1 (BON: 0.1 nM, H727: 0.2 nM). K
i values of valsartan were slightly higher, but still in the low nanomolar range (BON: 4.3 nM, H727: 9.4 nM). It was further shown that both cell lines retained AGTR1 expression and radioligand binding capacity during xenotransplantation and tumor growth in nude mice, as analyzed by receptor autoradiography (
Figure 4D).
Cellular signaling, secretion and cell viability of BON and H727 cells were evaluated upon receptor stimulation or inhibition. Receptor activation was studied by an intracellular calcium mobilization assay. While concentration-dependent response curves could be obtained for BON and H727, demonstrating functionally active receptor in these cells, QGP-1 and LCC-18 showed no calcium mobilization (
Figure 5A). Furthermore, valsartan was able to significantly diminish the induced signal, while PD123319 did not influence receptor activation, indicating selective AGTR1, not AGTR2 signaling (
Figure 5A). Valsartan inhibited ATII-mediated calcium mobilization in a concentration-dependent manner (
Figure 5B). Gα
q inhibition completely abolished the signal, whereas β-arrestin blockage did not modulate the effect. Inhibition of ERK or AKT also did not change the calcium response in BON cells (
Figure 5B).
BON cell supernatants were tested for their chromogranin A (CgA) content after ATII treatment. Interestingly, CgA secretion in these cells was stimulated in a concentration-dependent manner. CgA secretion was diminished after preincubation with AGTR1 antagonist valsartan (
Figure 5C). Moreover, ATII treatment stimulated the release of serotonin (5HT) to the cell supernatant, while application of the receptor blockers valsartan and azilsartan did not modify basal 5HT secretion (
Figure 5D). When investigating metabolic activity as a readout for cell growth and viability, no change could be detected after 96 hours of treatment with different concentrations of agonist (ATII) or antagonist (valsartan) (
Figure 5E).
Finally, the capacity of AGTR1 to act as a target for in vivo molecular diagnostic imaging was assessed. To this end, the partial AGTR1/2 agonist saralasin was coupled via a 4,7,10-trioxatridecan-succinamic acid (TTDS) linker to the near-infrared fluorescent dye indotricarbocyanine (ITCC). Radioligand binding assays with
125I–ATII were conducted to determine the affinity of the fluorescent probe in vitro (
Figure 6A). In AGTR1-positive BON cells, the radioligand could be displaced by unlabeled ATII confirming its high affinity (K
i = 0.1 nM) as determined before (
Figure 4C). The binding curve of saralasin–ITCC was shifted to the right, indicating lower binding affinity at a K
i value of 246.5 nM. In contrast, receptor negative QGP-1 cells did not show binding at all. For in vivo imaging, immunodeficient nude mice were subcutaneously inoculated with BON cells on the right and QGP-1 cells on the left shoulder. Near-infrared fluorescent imaging of live animals was performed after sufficient tumor growth, and the ITCC-labeled probe was tested in four different animals. Biodistribution kinetics of saralasin–ITCC of one animal after intravenous application is shown in
Figure 6B. Visual evaluation of the images indicated a selective accumulation of saralasin–ITCC in AGTR1-positive BON tumors, which proved to be statistically significant at three, four, five and six hours post-injection (
Figure 6C). Tumor-to-background ratios were 2- to 3-fold higher when compared to the image acquired prior to injection. Low non-significant uptake was detected in receptor negative QGP-1 tumors. Ex vivo organ imaging 6 hours after injection confirmed the specificity of saralasin–ITCC for receptor-positive BON tumors as well as an accumulation in kidneys and liver (
Figure 6D). Similarly, AGTR1 small molecule antagonist valsartan was labeled with ITCC via a 1,3-diamino propane linker and used for the same type of imaging. Although the affinity of this fluorescent ligand conjugate (IC
50 = 18.6 nM) was higher than for saralasin–ITCC, the accumulation of valsartan–ITCC in AGTR1-positive NEN xenograft tumors proved to be less pronounced (
Figure S6).
3. Discussion
In search of alternative targets that may be used for diagnosis and treatment of NENs, this study employed cell-based screening in human NEN cells using a compound library of 998 peptides and small molecules, yielding 38 confirmed hits, including ATII and a number of other GPCR ligands. These results stress the validity of activity-based screening approaches (reverse genetics) to identify new targets in oncology. In a comprehensive analysis of AGTR1 expression in 123 NEN patient and 25 control samples, this study demonstrated an upregulation of AGTR1 in small-intestinal NENs. While absolute expression in cancer tissue may be the most important criterion for judging the usefulness of a theranostic target, comparison with expression in normal tissues can be indicative of potential background signals in the organs of origin (pancreas, small intestine). Correlation analyses revealed a positive correlation between AGTR1 and SSTR2 expression in these samples. However, a subset of patients with low SSTR2 and high AGTR1 expression could benefit from tumor targeting approaches based on AGTR1 ligands. Data for the other SSTR family members have not been obtained. However, as SSTR2 is dominant for targeting in terms of both tumor expression as well as somatostatin analogue affinity, the contribution of other SSTRs to the imaging success would probably be limited. While mRNA expression data provide important insights into differentially regulated transcript levels, the existence of sufficient receptor protein at the cell surface is a prerequisite for molecular targeting. In a previous study, AGTR1 protein expression in 44 pancreatic NENs had been evaluated by immunohistochemistry [
10]. This paper observed an expression in 80% of the patients. However, the antibody employed in this paper was demonstrated to be non-specific in two studies [
11,
12]. Due to the lack of validated AGTR1 antibodies, in the current study, protein expression was indirectly evaluated by receptor autoradiography as an alternative method. Quantification revealed an up to 5-fold higher binding of AGTR1 in particular in small-intestinal NENs. This was found to correlate well with mRNA expression for most samples.
In a panel of 28 cell lines tested for AGTR1 expression, the two cell lines with the highest AGTR1 mRNA expression were of NEN origin, whereas only one colon and one lung cancer cell line reached similarly high values. This may indicate that AGTR1 overexpression is not a general phenomenon in tumors, but may be of particular relevance to NENs. Specific AGTR1 expression in these cell lines was independently confirmed by radioactive binding assays and autoradiography.
Several studies showed that ATII facilitates cell proliferation in various human cancer cell lines, including breast [
13], prostate [
14] and pancreatic cells [
15]. The proposed proliferative effect, however, could not be confirmed in this study, as metabolic activity was not affected. Likewise, treatment with the AGTR1 antagonist valsartan revealed no inhibition of cell growth. BON and H727 might release ATII, leading to autocrine signaling and enhanced cell proliferation, making externally added treatment negligible. This would be in line with a previous study in which treatment with the ACE inhibitor enalapril, and thereby inhibition of ATII production, resulted in decreased BON cell growth in vitro and in vivo [
10]. Similarly, the effect of applied antagonists might be impaired as they have to compete with autocrine ATII binding for the receptors [
16]. Furthermore, ATII mediates its proliferative signaling not only by direct stimulation of tumor cells, but also indirectly affects stromal and vascular cells [
17], which are not present in most monolayer cell cultures.
Finally, BON cells harbor a number of untypical mutations (e.g., in TP53, TSC2) [
18] that may drive non-responsiveness of proliferation to regulation by AGTR1 agonists and antagonists. Similarly, QGP-1 cells are known for a number of mutations that are not typical for well-differentiated neuroendocrine tumors. This clearly constitutes a limitation of the experimental approach, as does the fact that it is a pancreatic NEN cell line (BON) that is strongly AGTR1-positive, while, overall, pancreatic NEN show no significant overexpression. Unfortunately, an AGTR1-overexpressing siNEN cell line that could be employed in the in vivo imaging experiments was not available. In contrast to other tumor entities like breast cancer or colorectal cancer with hundreds of cell lines, in the field of the heterogeneous neuroendocrine neoplasms, comparatively few cell lines have been developed, and almost none is available in public repositories. These cell lines do not at all recapitulate the landscape of these tumors. The lack of data for lung NEN is another limitation of this study, which was focused on NEN in the gastroenteropancreatic system. The scarcity of cell model systems in the field is currently improving step by step [
19], and more novel cell lines may become available with a more typical tumor biology. Primary cell cultures in 2D or 3D as well as patient-derived xenografts may pave the way for more appropriate and authentic NEN in vitro and in vivo models.
Radiolabeled AGTR1-targeted peptides and small molecules have been primarily utilized for cardiac and cardiovascular imaging to date, to select patients for distinct treatment options and to better predict therapy response [
20]. To the best of our knowledge, this is the first study investigating the applicability of AGTR1-based tumor targeting. Biodistribution of the two different ITCC-labeled targeted ligands was evaluated in a xenograft mouse model, revealing a significant accumulation of saralasin–ITCC in receptor-positive BON tumors. The superior performance of saralasin–ITCC was unexpected, as the introduction of the dye molecule ITCC decreased its affinity 250-fold when compared to the published K
i value of 1 nM [
21]. Although a slow dissociation rate from the receptor is mostly associated with high affinity binding, both parameters do not necessarily have to correlate [
22]. For a translation of this targeting principle into a clinically relevant theranostics approach, the affinity of a tracer will have to be improved, e.g., by coupling the chelator to another site in the molecule or by the introduction of another spacer.