9G TestTM Cancer/Lung: A Desirable Companion to LDCT for Lung Cancer Screening
Simple Summary
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Determination of Lung Cancer Index Using 9G Test™ Cancer/Lung Test
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Shen, Y.; Li, W.; Chen, Y.; Wang, H. Low-dose computed tomography (LDCT) versus other cancer screenings in early diagnosis of lung cancer: A meta-analysis. Medicine 2018, 97, e11233. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.G.; Motsch, E.; Trotter, A.; Kauczor, H.; Heussel, C.; Hermann, S.; Zeissig, S.R.; Delorme, S.; Kaaks, R. Overdiagnosis in lung cancer screening: Estimates from the German Lung Cancer Screening Intervention Trial. Int. J. Cancer 2020, 1–9. [Google Scholar] [CrossRef]
- Patz, E.F., Jr.; Pinsky, P.; Gatsonis, C.; Sicks, J.D.; Kramer, B.S.; Tammemägi, M.C.; Chiles, C.; Black, W.C.; Aberle, D.R.; NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer. JAMA Intern. Med. 2014, 174, 269–274. [Google Scholar] [CrossRef]
- Wu, F.-Z.; Kuo, P.-L.; Huang, Y.-L.; Tang, E.-K.; Chen, C.-S.; Wu, M.-T.; Lin, Y.-P. Differences in lung cancer characteristics and mortality rate between screened and non-screened cohorts. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Marcus, P.M.; Bergstralh, E.J.; Zweig, M.H.; Harris, A.; Offord, K.P.; Fontana, R.S. Extended lung cancer incidence follow-up in the Mayo Lung Project and over diagnosis. J. Natl. Cancer Inst. 2006, 98, 748–756. [Google Scholar] [CrossRef]
- Oken, M.M.; Hocking, W.G.; Kvale, P.A.; Andriole, G.L.; Buys, S.S.; Church, T.R.; Crawford, E.D.; Fouad, M.N.; Isaacs, C.; Reding, D.J.; et al. Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011, 306, 1865–1873. [Google Scholar] [CrossRef]
- Becker, N.; Motsch, E.; Trotter, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Kauczor, H.; Maldonado, S.G.; Miller, A.B.; Kaaks, R.; et al. Lung cancer mortality reduction by LDCT screening—Results from the randomized German LUSI trial. Int. J. Cancer 2020, 146, 1503–1513. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.; Javier, J.; Spira, A.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Ba, K.S.A.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef] [PubMed]
- Henschke, C.I.; McCauley, D.I.; Yankelevitz, D.F.; Naidich, D.P.; McGuinness, G.; Miettinen, O.S.; Libby, D.M.; Pasmantier, M.W.; Koizumi, J.; Altorki, N.K.; et al. Early Lung Cancer Action Project: Overall design and findings from baseline screening. Lancet 1999, 354, 99–105. [Google Scholar] [CrossRef]
- Infante, M.; Cavuto, S.; Lutman, F.R.; Brambilla, G.; Chiesa, G.; Ceresoli, G.; Passera, E.; Angeli, E.; Chiarenza, M.; Aranzulla, G.; et al. DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography (the DANTE Trial): Three-year results. Am. J. Respir. Crit. Care Med. 2009, 180, 445–453. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed]
- Sozzi, G.; Boeri, M. Potential biomarkers for lung cancer screening. Transl. Lung Cancer Res. 2014, 3, 139–148. [Google Scholar]
- Fahrmann, J.F.; Kim, K.; DeFelice, B.C.; Taylor, S.L.; Gandara, D.R.; Yoneda, K.Y.; Cooke, D.T.; Fiehn, O.; Kelly, K.; Miyamoto, S. Investigation of Metabolomic Blood Biomarkers for Detection of Adenocarcinoma Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1716–1723. [Google Scholar] [CrossRef]
- Carozzi, F.M.; Bisanzi, S.; Carrozzi, L.; Falaschi, F.; Pegna, A.L.; Mascalchi, M.; Picozzi, G.; Peluso, M.; Sani, C.; Greco, L.; et al. Multimodal lung cancer screening using the ITALUNG biomarker panel and low dose computed tomography. Results of the ITALUNG biomarker study. Int. J. Cancer 2017, 141, 94–101. [Google Scholar] [CrossRef]
- Bamji-Stocke, S.; Van Berkel, V.; Miller, D.M.; Frieboes, H.B. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics 2018, 14, 81. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.; Ahmed, R.; Huang, G.; Reid, J.; Mandal, R.; Maksymuik, A.; Sitar, D.S.; Tappia, P.S.; Ramjiawan, B.; et al. A High-Performing Plasma Metabolite Panel for Early-Stage Lung Cancer Detection. Cancers 2020, 12, 622. [Google Scholar] [CrossRef]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013, 5 (Suppl 5), S540–S550. [Google Scholar] [PubMed]
- Zhang, J.; Dong, Y.; Zhu, W.; Xie, D.; Zhao, Y.; Yang, D.; Li, M. Ultrasensitive Detection of Circulating Tumor DNA of Lung Cancer via an Enzymatically Amplified SERS-Based Frequency Shift Assay. ACS Appl. Mater. Interfaces 2019, 11, 18145–18152. [Google Scholar] [CrossRef]
- Yu, H.; Guan, Z.; Cuk, K.; Brenner, H.; Zhang, Y. Circulating microRNA biomarkers for lung cancer detection in Western populations. Cancer Med. 2018, 7, 4849–4862. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.-H.; Boutros, J.; Benzaquen, J.; Ferreira, M.; Pastre, J.; Pison, C.; Padovani, B.; Bettayeb, F.; Fallet, V.; Guibert, N.; et al. Circulating tumour cells as a potential biomarker for lung cancer screening: A prospective cohort study. Lancet Respir. Med. 2020, 8, 709–716. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Ren, T.; Yin, Y. Autoantibodies as diagnostic biomarkers for lung cancer: A systematic review. Cell Death Discov. 2019, 5, 126. [Google Scholar] [CrossRef]
- Kammer, M.N.; Massion, P.P. Noninvasive biomarkers for lung cancer diagnosis, where do we stand? J. Thorac. Dis. 2020, 12, 3317–3330. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.; Chapman, C.J.; Holdenrieder, S.; Murray, A.; Robertson, C.; Wood, W.C.; Maddison, P.; Healey, G.; Fairley, G.H.; Barnes, A.C.; et al. Clinical validation of an autoantibody test for lung cancer. Ann. Oncol. 2011, 22, 383–389. [Google Scholar] [CrossRef]
- Sun, N.; Sun, S.; Gao, Y.; Li, Y.; Lu, Z.; Yuan, Z.; Che, Y.; Huang, J.; Mao, S.; Lei, Y.; et al. Utility of isocitrate dehydrogenase 1 as a serum protein biomarker for the early detection of non-small-cell lung cancer: A multicenter in vitro diagnostic clinical trial. Cancer Sci. 2020, 111, 1739–1749. [Google Scholar] [CrossRef]
- Faiz, K.W.; Einvik, G.; Brekke, P.H.; Omland, T. Cardiac Troponin T Increase in Patients with Acute Ischemic Stroke with and without Cancer. Clin. Chem. 2018, 64, 404–406. [Google Scholar] [CrossRef]
- Lyon, A.R. Disparate worlds drawing closer together: Cardiovascular biomarkers predict cancer outcomes in treatment-naïve patients. Heart 2015, 101, 1853–1854. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Demers, M.; Blomgren, B.; Wong, S.L.; Von Arbin, M.; Von Heijne, A.; Laska, A.C.; Wallén, H.; Wagner, D.D.; Aspberg, S. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 2016, 139, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, M.; Johnson, N.; Cheadle, C.; Cho-Chung, Y. Autoantibody biomarker opens a new gateway for cancer diagnosis. Biochim. Biophys. Acta 2006, 1762, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Saini, S.; Parashar, D.; Verma, A.; Sinha, A.; Jagadish, N.; Batra, A.; Suri, S.; Gupta, A.; Ansari, A.S.; et al. The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. OncoImmunology 2013, 2, e24270. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.J.; Vanhoorelbeke, K.; Leypoldt, F.; Kaya, Z.; Bieber, K.; McLachlan, S.M.; Komorowski, L.; Luo, J.; Cabral-Marques, O.; Hammers, C.M.; et al. Mechanisms of Autoantibody-Induced Pathology. Front. Immunol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef]
- Song, K.-S.; Nimse, S.B.; Warkad, S.D.; Oh, A.-C.; Kim, T.; Hong, Y.J. Quantification of CYFRA 21-1 and a CYFRA 21-1-anti-CYFRA 21-1 autoantibody immune complex for detection of early stage lung cancer. Chem. Commun. 2019, 55, 10060–10063. [Google Scholar] [CrossRef]
- Baron, J.A. Screening for cancer with molecular markers: Progress comes with potential problems. Nat. Rev. Cancer 2012, 12, 368–371. [Google Scholar] [CrossRef]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef]
- Shitrit, D.; Zingerman, B.; Shitrit, A.B.-G.; Shlomi, D.; Kramer, M.R. Diagnostic Value of CYFRA 21-1, CEA, CA 19-9, CA 15-3, and CA 125 Assays in Pleural Effusions: Analysis of 116 Cases and Review of the Literature. Oncologist 2005, 10, 501–507. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhou, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci. Rep. 2018, 8, 2732. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Takayama, K.; Izumi, M.; Harada, T.; Furuyama, K.; Nakanishi, Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer 2013, 80, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zaenker, P.; Gray, E.; Ziman, M. Autoantibody Production in Cancer—The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun. Rev. 2016, 15, 477–483. [Google Scholar] [CrossRef]
- Zamay, T.N.; Zamay, G.S.; Kolovskaya, O.S.; Zukov, R.A.; Petrova, M.; Gargaun, A.; Berezovski, M.V.; Kichkailo, A.S. Current and Prospective Protein Biomarkers of Lung Cancer. Cancers 2017, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Meaney, C.L.; Zingone, A.; Brown, D.; Yu, Y.; Cao, L.; Ryan, B.M. Identification of serum inflammatory markers as classifiers of lung cancer mortality for stage I adenocarcinoma. Oncotarget 2017, 8, 40946–40957. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Luo, L.; Wampfler, J.A.; Wang, Y.; Liu, D.; Chen, Y.-M.; Adjei, A.A.; Midthun, D.E.; Yang, P. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol. 2019, 20, 1098–1108. [Google Scholar] [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Consortium for Early Detection of Lung Cancer; Guida, F.; Sun, N.; Bantis, L.E.; Muller, D.C.; Li, P.; Taguchi, A.; Dhillon, D.; Kundnani, D.L.; Patel, N.J.; et al. Assessment of Lung Cancer Risk on the Basis of a Biomarker Panel of Circulating Proteins. JAMA Oncol. 2018, 4, e182078. [Google Scholar] [CrossRef]
| Sources of Clinical Samples | Healthy Individuals | Lung Cancer Patients * | IRB No. |
|---|---|---|---|
| Nowon Eulji Medical Center, Eulji University, Seoul, South Korea | 175 a | - | 2020-EC-01-008 |
| Korea Cancer Central Hospital, Seoul, South Korea | 120 | 50 | KIRAMS 2018-10-006 |
| Ajou Human Bio-Resource Bank (AHBB) | 64 b | - | AJHB-2019-28 |
| Biobank of Gyeongsang National University Hospital, | - | 12 | 2019-021 |
| Asan Bio-Resource Center, Korea Biobank Network | - | 182 | 2019-14(193) |
| Total | 359 | 244 | - |
| Lung Cancer Type | Subtypes | Lung Cancer Stages | |||
|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | ||
| Adenocarcinoma | Adenoid cystic carcinoma | 1 | 2 | ||
| Bronchioloalveolar adenocarcinoma | 36 | 3 | 5 | ||
| Mucinous adenocarcinoma | 4 | ||||
| Adenocarcinoma | 43 | 16 | 9 | 2 | |
| Papillary adenocarcinoma | 40 | 2 | 8 | ||
| Squamous cell carcinoma | Adenosquamous carcinoma | 2 | 2 | 3 | |
| Squamous cell carcinoma | 18 | 3 | 8 | ||
| Squamous cell carcinoma, keratinizing | 4 | 4 | |||
| Squamous cell carcinoma, large cell, non-keratinizing | 4 | 2 | 1 | ||
| Mucoepidermoid carcinoma | 1 | ||||
| Small Cell | Combined small cell carcinoma | 1 | 1 | 1 | 1 |
| Small cell carcinoma | 2 | ||||
| Large cell carcinoma | Large cell carcinoma | 2 | 2 | 4 | |
| Large cell carcinoma with neuroendocrine feature | 2 | ||||
| Non-small cell carcinoma | Bronchogenic non-small cell carcinoma | 1 | 1 | 1 | |
| Sarcomatoid carcinoma | 1 | 1 | |||
| Characteristic | Healthy Population (n = 359) | Cancer Patients (n = 244) | p-Value (Healthy vs. Cancer) |
|---|---|---|---|
| Age, years (SD) | 57.3 (±12.0) | 62.7 (±9.30) | - |
| Male gender, n (%) | 140 (38.9) | 161 (65.9) | - |
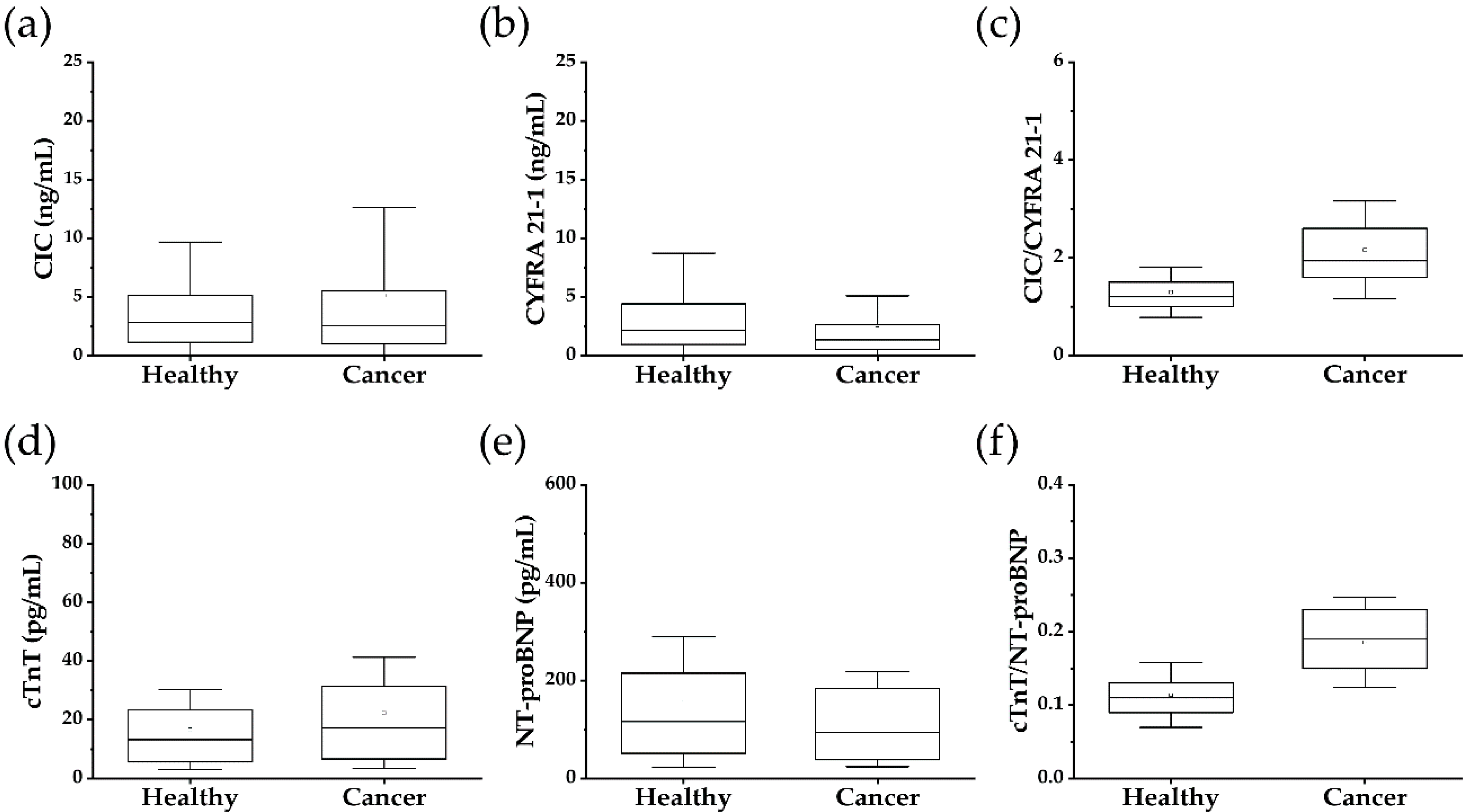
| CIC (pg/mL; SD) | 4.47 (±5.22) | 5.16 (±7.46) | 0.7056 |
| CYFRA 21-1 (pg/mL; SD) | 3.89 (±4.84) | 2.33 (±2.78) | 0.0001 |
| cTnT (pg/mL; SD) | 16.7 (±13.6) | 22.4 (±18.8) | 0.0015 |
| NT-proBNP (pg/mL; SD) | 155.6 (±133.6) | 121.7 (±97.5) | 0.005 |
| CIC/CYFRA 21-1 (SD) | 1.290 (±0.52) | 2.16 (±1.00) | 0.0001 |
| cTnT/NT-proBNP (SD) | 0.11 (±0.04) | 0.19 (±0.06) | 0.0001 |
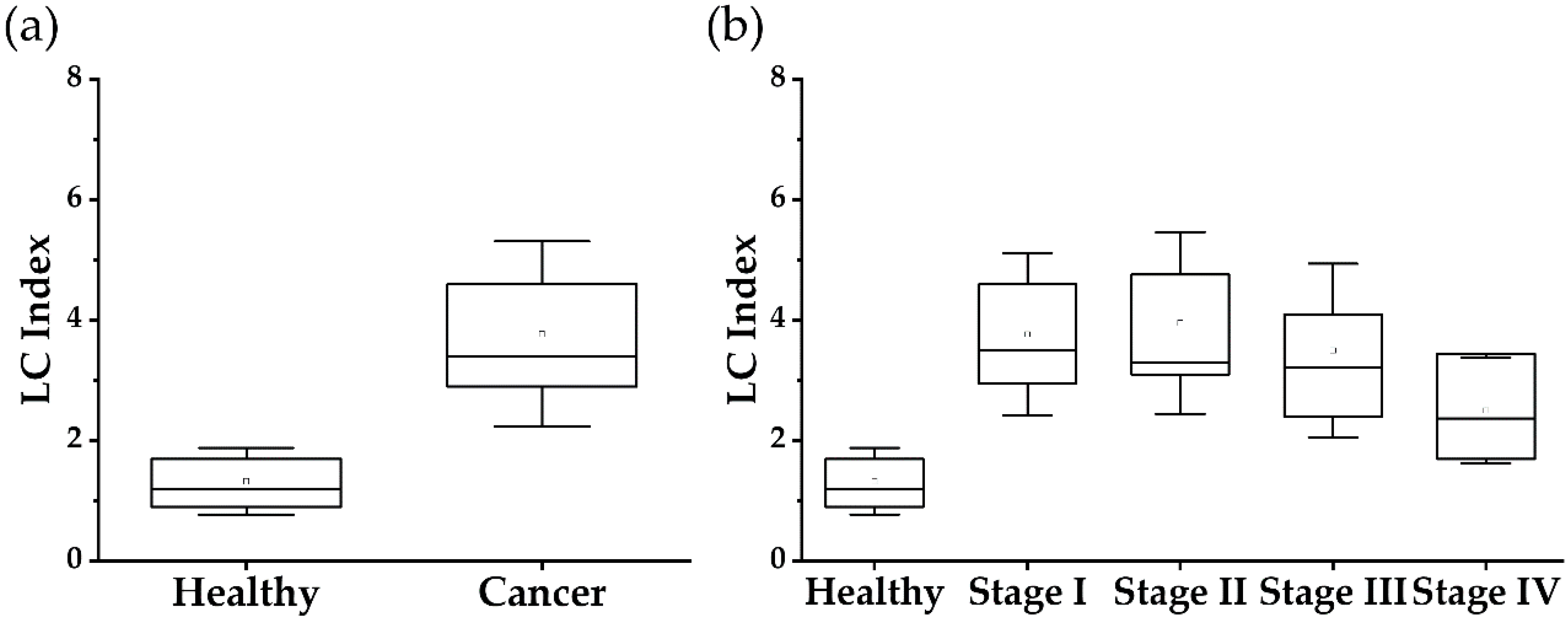
| LC Index (SD) | 1.45 (±0.73) | 3.90 (±1.87) | 0.0001 |
| Variable | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| CIC | 6.60 (3.80~10.4) | 97.3 (95.0~98.8) | 64.0 (44.4~79.8) | 59.0 (58.0~59.9) |
| CYFRA 21-1 | 0.00 (0.00~1.50) | 97.3 (95.0 ~98.8) | 0.00 | 57.3 (57.0~57.8) |
| cTnT | 0.00 (0.00~1.50) | 97.3 (95.0~98.8) | 0.00 | 57.3 (57.0~57.8) |
| NT-proBNP | 0.00 (0.00~1.50) | 97.3 (95.0~98.8) | 0.00 | 57.3 (57.0~57.8) |
| CIC/CYFRA 21-1 | 29.5 (23.9~35.7) | 97.3 (95.0~98.8) | 89.0 (80.3~94.0) | 65.6 (63.7~67.4) |
| cTnT/NT-proBNP | 34.0 (28.0~40.3) | 97.3 (95.0~98.8) | 90.2 (82.6~94.8) | 67.0 (65.0~69.0) |
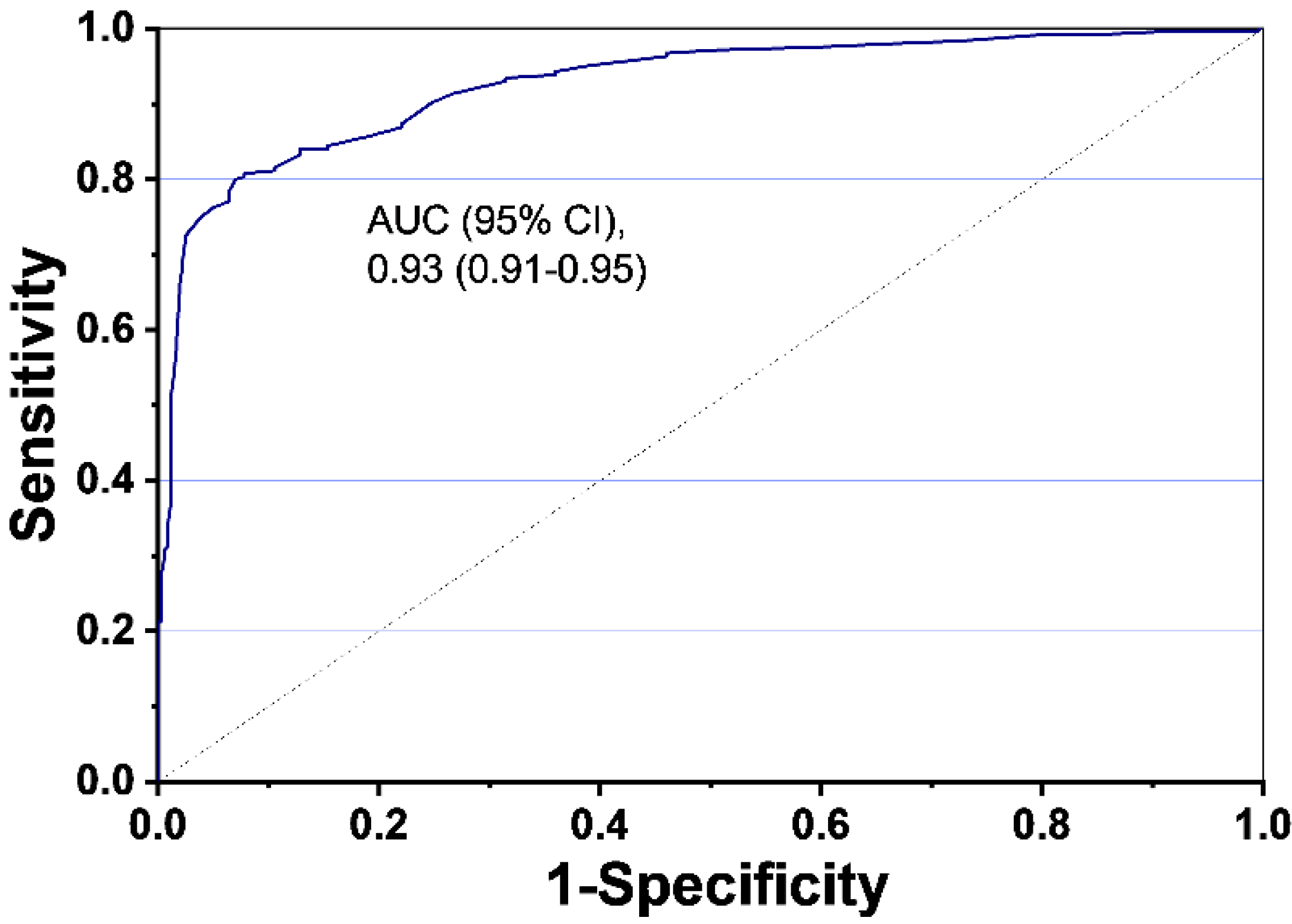
| LC Index | 75.0 (69.1~80.3) | 97.3 (95.0~98.8) | 95.4(91.4~97.5) | 84.3 (81.2~87.0) |
| Cancer Stages (n) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| Stage I (n = 160) | 77.5 (70.2~83.8) | 97.3 (95.0~98.8) | 93.2 (87.8~96.4) | 90.1 (87.2~92.4) |
| Stage II (n = 32) | 78.1 (60.0~90.0) | 97.3 (95.0~98.8) | 73.5 (58.7~84.5) | 97.9 (96.0~99.0) |
| Stage III (n = 49) | 67.4 (52.5~80.0) | 97.3 (95.0~98.8) | 78.6 (65.2~87.8) | 95.4 (93.2~96.8) |
| Stage IV (n = 3) * | 33.3 (0.80~90.6) | 97.3 (95.0~98.8) | 10.0 (2.00~38.4) | 99.4 (98.7~99.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, W.; Chae, J.D.; Lee, B.-H.; Kim, S.-H.; Park, S.Y.; Nimse, S.B.; Kim, J.; Warkad, S.D.; Song, K.-S.; Oh, A.-C.; et al. 9G TestTM Cancer/Lung: A Desirable Companion to LDCT for Lung Cancer Screening. Cancers 2020, 12, 3192. https://doi.org/10.3390/cancers12113192
Choe W, Chae JD, Lee B-H, Kim S-H, Park SY, Nimse SB, Kim J, Warkad SD, Song K-S, Oh A-C, et al. 9G TestTM Cancer/Lung: A Desirable Companion to LDCT for Lung Cancer Screening. Cancers. 2020; 12(11):3192. https://doi.org/10.3390/cancers12113192
Chicago/Turabian StyleChoe, Wonho, Jeong Don Chae, Byoung-Hoon Lee, Sang-Hoon Kim, So Young Park, Satish Balasaheb Nimse, Junghoon Kim, Shrikant Dashrath Warkad, Keum-Soo Song, Ae-Chin Oh, and et al. 2020. "9G TestTM Cancer/Lung: A Desirable Companion to LDCT for Lung Cancer Screening" Cancers 12, no. 11: 3192. https://doi.org/10.3390/cancers12113192
APA StyleChoe, W., Chae, J. D., Lee, B.-H., Kim, S.-H., Park, S. Y., Nimse, S. B., Kim, J., Warkad, S. D., Song, K.-S., Oh, A.-C., Hong, Y. J., & Kim, T. (2020). 9G TestTM Cancer/Lung: A Desirable Companion to LDCT for Lung Cancer Screening. Cancers, 12(11), 3192. https://doi.org/10.3390/cancers12113192






