Acid-Producing Diet and Depressive Symptoms among Breast Cancer Survivors: A Longitudinal Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Assessment
2.3. Assessment of Depression Symptoms
2.4. Other Assessments
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics by Disease Outcomes in the Whole Cohort
3.2. Baseline Characteristics by Quartile of PRAL
3.3. Longitudinal Associations between Dietary Acid Load and Depression (CES-D Score)
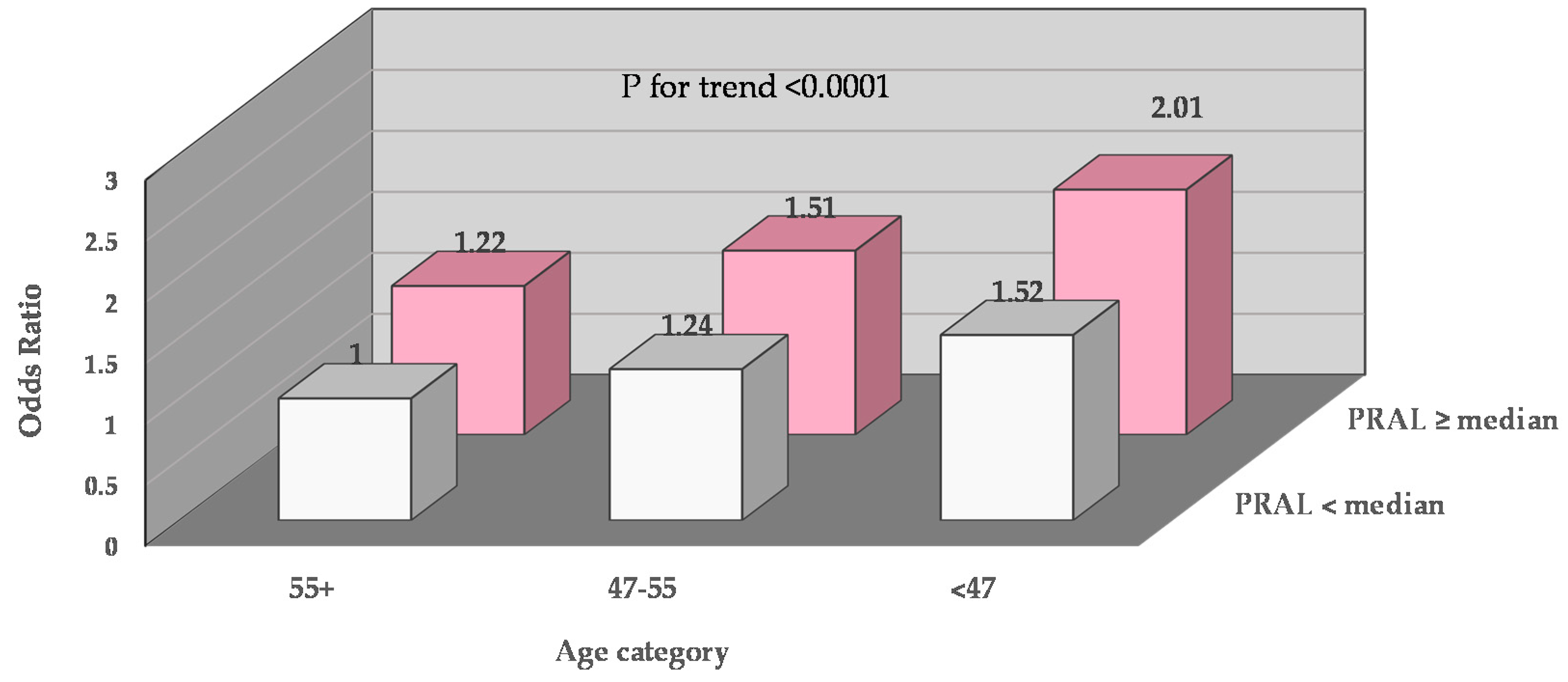
3.4. Joint Impacts of Dietary Acid Load and Age on Depression, and Joint Impacts of Dietary Acid Load and Physical Activity on Depression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Currier, M.B.; Nemeroff, C.B. Depression as a risk factor for cancer: From pathophysiological advances to treatment implications. Annu. Rev. Med. 2014, 65, 203–221. [Google Scholar] [CrossRef]
- Grassi, L.; Indelli, M.; Marzola, M.; Maestri, A.; Santini, A.; Piva, E.; Boccalon, M. Depressive symptoms and quality of life in home-care-assisted cancer patients. J. Pain Symptom Manag. 1996, 12, 300–307. [Google Scholar] [CrossRef]
- Wulsin, L.R.; Vaillant, G.E.; Wells, V.E. A systematic review of the mortality of depression. Psychosom. Med. 1999, 61, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.L.; Bibbins-Domingo, K.; Grossman, D.C.; Baumann, L.C.; Davidson, K.W.; Ebell, M.; García, F.A.R.; Gillman, M.; Herzstein, J.; Kemper, A.R.; et al. Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. J. Am. Med. Assoc. 2016, 315, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.Z.; Nik-Jaafar, N.R.; Baharudin, A.; Sabki, Z.A.; Ng, C.G. Prevalence of depression in breast cancer survivors: A systematic review of observational studies. Asian Pac. J. Cancer Prev. 2013, 14, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Burgess, C.; Cornelius, V.; Love, S.; Graham, J.; Richards, M.; Ramirez, A. Depression and anxiety in women with early breast cancer: Five-year observational cohort study. Br. Med. J. 2005, 330, 702. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [PubMed]
- Serra, M.C.; Goldberg, A.P.; Ryan, A.S. Increased depression and metabolic risk in postmenopausal breast cancer survivors. Diabetol. Metab. Syndr. 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.; Clarke, V. The psychological impact of a cancer diagnosis on families: The influence of family functioning and patients’ illness characteristics on depression and anxiety. Psycho-Oncology 2004, 13, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Linden, W.; Vodermaier, A.; MacKenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Tangney, C.C.; Young, J.; Murtaugh, M.A.; Cobleigh, M.; Oleske, D. Self-Reported Dietary Habits, Overall Dietary Quality and Symptomatology of Breast Cancer Survivors: A Cross-Sectional Examination. Breast Cancer Res. Treat. 2002, 71, 113–123. [Google Scholar] [CrossRef]
- Molendijk, M.; Molero, P.; Sánchez-Pedreño, F.O.; Van Der Does, W.; Martínez-González, M.A. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.-R.; Wei, Y.-J.; Sun, L.; Zhang, J.-X.; Zhang, H.-G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Rienks, J.; Dobson, A.J.; Mishra, G.D. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: Results from a large community-based prospective study. Eur. J. Clin. Nutr. 2012, 67, 75–82. [Google Scholar] [CrossRef]
- Chatzi, L.; Melaki, V.; Sarri, K.; Apostolaki, I.; Roumeliotaki, T.; Georgiou, V.; Vassilaki, M.; Koutis, A.; Bitsios, P.; Kogevinas, M. Dietary patterns during pregnancy and the risk of postpartum depression: The mother–child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr. 2011, 14, 1663–1670. [Google Scholar] [CrossRef]
- Saghafian, F.; Malmir, H.; Saneei, P.; Keshteli, A.; Hosseinzadeh-Attar, M.J.; Afshar, H.; Siassi, F.; Esmaillzadeh, A.; Adibi, P. Consumption of fruit and vegetables in relation with psychological disorders in Iranian adults. Eur. J. Nutr. 2018, 57, 2295–2306. [Google Scholar] [CrossRef]
- Saghafian, F.; Malmir, H.; Saneei, P.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Fruit and vegetable consumption and risk of depression: Accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br. J. Nutr. 2018, 119, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts and Figures 2019 from American Cancer Society. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (accessed on 20 April 2020).
- Shirali, A. Electrolyte and Acid–Base Disorders in Malignancy, in Onco-Nephrology Curriculum; American Society of Nephrology: Washington, DC, USA, 2016. [Google Scholar]
- Wu, T.; Seaver, P.; Lemus, H.; Hollenbach, K.; Wang, S.E.; Pierce, J.P. Associations between Dietary Acid Load and Biomarkers of Inflammation and Hyperglycemia in Breast Cancer Survivors. Nutrients 2019, 11, 1913. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The Prevalence of Comorbid Depression in Adults with Diabetes: A meta-analysis. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Merriam, R.L.; Morris, R.C. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am. J. Clin. Nutr. 2002, 76, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Faerber, S.; Wright, F.A.; Rock, C.L.; Newman, V.; Flatt, S.W.; Kealey, S.; Jones, V.E.; Caan, B.J.; Gold, E.B.; et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival. Control. Clin. Trials 2002, 23, 728–756. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a Diet Very High in Vegetables, Fruit, and Fiber and Low in Fat on Prognosis Following Treatment for Breast Cancer. J. Am. Med Assoc. 2007, 298, 289–298. [Google Scholar] [CrossRef]
- Frassetto, L.; Todd, K.M.; Morris, R.C.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential Renal Acid Load of Foods and its Influence on Urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef]
- Wassertheil-Smoller, S.; Shumaker, S.A.; Ockene, J.K.; Talavera, G.A.; Greenland, P.; Cochrane, B.B.; Robbins, J.; Aragaki, A.K.; Dunbar-Jacob, J. Depression and Cardiovascular Sequelae in Postmenopausal Women. Arch. Intern. Med. 2004, 164, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; Andrykowski, M.; Wilson, J.; Hall, L.; Rayens, M.K.; Sachs, B.; Cunningham, L. Psychometrics for two short forms of the center for epidemiologic studies-depression scale. Issues Ment. Health Nurs. 1998, 19, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Robins, L.N.; Helzer, J.E.; Croughan, J.; Ratcliff, K.S. National Institute of Mental Health Diagnostic Interview Schedule. Arch. Gen. Psychiatry 1981, 38, 381–389. [Google Scholar] [CrossRef]
- Tuunainen, A.; Langer, R.D.; Klauber, M.R.; Kripke, D.F. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001, 103, 261–270. [Google Scholar] [CrossRef]
- Johnson-Kozlow, M.; Rock, C.L.; Gilpin, E.A.; Hollenbach, K.A.; Pierce, J.P. Validation of the WHI Brief Physical Activity Questionnaire among Women Diagnosed with Breast Cancer. Am. J. Health Behav. 2007, 31, 193–202. [Google Scholar] [CrossRef]
- Hong, S.; Bardwell, W.A.; Natarajan, L.; Flatt, S.W.; Rock, C.L.; Newman, V.A.; Madlensky, L.; Mills, P.J.; Dimsdale, J.E.; Thomson, C.A.; et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res. Treat. 2006, 101, 225–232. [Google Scholar] [CrossRef]
- Daneshzad, E.; Keshavarz, S.-A.; Qorbani, M.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Association of dietary acid load and plant-based diet index with sleep, stress, anxiety and depression in diabetic women. Br. J. Nutr. 2019, 123, 901–912. [Google Scholar] [CrossRef]
- Milajerdi, A.; Keshteli, A.H.; Haghighatdoost, F.; Azadbakht, L.; Esmaillzadeh, A.; Adibi, P. Dietary acid load in relation to depression and anxiety in adults. J. Hum. Nutr. Diet. 2019, 33, 48–55. [Google Scholar] [CrossRef]
- Bühlmeier, J.; Harris, C.; Koletzko, S.; Lehmann, I.; Bauer, C.-P.; Schikowski, T.; Von Berg, A.; Berdel, D.; Heinrich, J.; Hebebrand, J.; et al. Dietary Acid Load and Mental Health Outcomes in Children and Adolescents: Results from the GINIplus and LISA Birth Cohort Studies. Nutrients 2018, 10, 582. [Google Scholar] [CrossRef]
- Almeida, O.P.; Ford, A.H.; Flicker, L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. Int. Psychogeriatr. 2015, 27, 727–737. [Google Scholar] [CrossRef]
- Stahl, S.T.; Beach, S.R.; Musa, D.; Schulz, R. Living alone and depression: The modifying role of the perceived neighborhood environment. Aging Ment. Health 2016, 21, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, M.; Podratz, J.; Windebank, A.J.; Low, P.A. Acidity is involved in the development of neuropathy caused by oxidized cellulose. J. Neurol. Sci. 1997, 146, 97–102. [Google Scholar] [CrossRef]
- Esche, J.; Shi, L.; Sánchez-Guijo, A.; Hartmann, M.F.; Wudy, S.A.; Remer, T. Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int. 2016, 90, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Mora, F.; Segovia, G.; Del Arco, A.; De Blas, M.; Garrido, P. Stress, neurotransmitters, corticosterone and body–brain integration. Brain Res. 2012, 1476, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Landfield, P.W.; Eldridge, J. Evolving aspects of the glucocorticoid hypothesis of brain aging: Hormonal modulation of neuronal calcium homeostasis. Neurobiol. Aging 1994, 15, 579–588. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal Volume Reduction in Major Depression. Am. J. Psychiatry 2000, 157, 115–118. [Google Scholar] [CrossRef]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 2017, 23, 48–58. [Google Scholar] [CrossRef]
- Lee, C.-H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Avis, N.E.; Levine, B.; Naughton, M.J.; Case, D.L.; Naftalis, E.; Van Zee, K.J. Explaining age-related differences in depression following breast cancer diagnosis and treatment. Breast Cancer Res. Treat. 2012, 136, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Greendale, G.A.; Petersen, L.; Kahn, B.; Bower, J.E. Breast Cancer in Younger Women: Reproductive and Late Health Effects of Treatment. J. Clin. Oncol. 2003, 21, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Patsou, E.D.; Alexias, G.D.; Anagnostopoulos, F.G.; Karamouzis, M.V. Effects of physical activity on depressive symptoms during breast cancer survivorship: A meta-analysis of randomised control trials. ESMO Open 2017, 2, e000271. [Google Scholar] [CrossRef]
- Craft, L.L.; VanIterson, E.H.; Helenowski, I.B.; Rademaker, A.W.; Courneya, K.S. Exercise Effects on Depressive Symptoms in Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2011, 21, 3–19. [Google Scholar] [CrossRef]
- Anders, C.K.; Fan, C.; Parker, J.S.; Carey, L.A.; Blackwell, K.L.; Klauber-Demore, N.; Perou, C.M. Breast Carcinomas Arising at a Young Age: Unique Biology or a Surrogate for Aggressive Intrinsic Subtypes? J. Clin. Oncol. 2011, 29, e18–e20. [Google Scholar] [CrossRef] [PubMed]
- Compas, B.E.; Stoll, M.F.; Thomsen, A.H.; Oppedisano, G.; Epping-Jordan, J.E.; Krag, D.N. Adjustment to breast cancer: Age-related differences in coping and emotional distress. Breast Cancer Res. Treat. 1999, 54, 195–203. [Google Scholar] [CrossRef]
- Azad, A.; Gharakhanlou, R.; Niknam, A.; Ghanbari, A. Effects of Aerobic Exercise on Lung Function in Overweight and Obese Students. Tanaffos 2011, 10, 24–31. [Google Scholar]
- Seifter, J.L.; Chang, H.-Y. Disorders of Acid-Base Balance: New Perspectives. Kidney Dis. 2016, 2, 170–186. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef]
| CES-D Score < 5 | CES-Score ≥ 5 | ||
|---|---|---|---|
| n = 2361 | n = 614 | p-Value | |
| PRAL (mEq/d) a | −4.49 (−14.21 to 4.15) | −1.55 (−10.47 to 7.23) | <0.0001 |
| NEAL (mEq/d) | 39.28 (32.16 to 47.82) | 42.30 (34.23 to 50.57) | <0.0001 |
| CES-D score | 1 (1 to 3) | 7 (5–8) | <0.001 |
| Basic | |||
| Age at diagnosis (years) | 51.0 (45.0 to 58.0) | 49.0 (44.0 to 55.0) | <0.0001 |
| White (%) | 85.4 | 82.4 | 0.06 |
| Body mass index | |||
| Normal weight (%) | 44.4 | 36.0 | <0.0001 |
| Overweight and obese (%) | 55.6 | 64.0 | |
| Education, at or above college (%) | 54.9 | 50.3 | 0.05 |
| Postmenopausal women (%) | 80.5 | 75.5 | 0.002 |
| Smoking status | |||
| Past smoker (%) | 41.0 | 42.5 | 0.1 |
| Never smoker (%) | 54.0 | 51.3 | |
| Pack-year status | |||
| Pack-years = 0 (%) | 54.5 | 51.5 | 0.1 |
| Pack-years > 0 to 15 (%) | 24.7 | 31.2 | |
| Pack-years > 15 (%) | 17.3 | 16.2 | |
| Alcohol abstainer (%) | 30.4 | 34.5 | 0.05 |
| Physical activity (MET/week) | 675 (225 to 1350) | 420 (105 to 1000) | <0.0001 |
| Intervention group (%) | 49.7 | 49.0 | 0.8 |
| Chemotherapy (%) | 69.1 | 71.6 | 0.4 |
| Radiation (%) | 61.2 | 62.8 | 0.3 |
| Hormone receptor status | |||
| ER+/PR+ (%) | 62.3 | 61.0 | 0.2 |
| ER−/PR− (%) | 21.2 | 24.5 | |
| Cancer stage at diagnosis (%) | |||
| I | 38.0 | 41.2 | 0.3 |
| II | 57.2 | 53.4 | |
| IIIa | 4.9 | 5.4 | |
| Tamoxifen use (%) | 67.7 | 61.0 | 0.007 |
| PRAL Score Quartiles (mEq/d) | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Value | |
| <−13.7 (n = 771) | −13.7 to <−3.7 (n = 769) | −3.7 to <4.7 (n = 771) | ≥4.7 (n = 770) | ||
| NEAP (mEq/d) a | 27.4 (23.9–30.7) | 36.4 (33.7–38.5) | 43.7 (41.1–46.3) | 55.4 (50.9–61.3) | <0.001 |
| CES-D score | 2 (1–3) | 2 (1–4) | 2 (1–4) | 2 (1–4) | <0.001 |
| Vegetable intakes (serving/d) | 4.13 (2.65–5.74) | 3.00 (2.05–4.00) | 2.37 (1.63–3.25) | 2.05 (1.40–2.83) | <0.001 |
| Vitamin B12 (μg/d) | 3.11 (1.98–4.76) | 3.13 (2.12–4.55) | 3.33 (2.26–4.71) | 3.71 (2.58–5.20) | <0.001 |
| General characteristics | |||||
| Age at diagnosis (years) | 52.0 (47.0–58.0) | 51.0 (46.0–58.0) | 50.0 (45.0–57.0) | 48.0 (42.0–55.0) | <0.001 |
| Overweight and obese (%) | 43.4 | 53.3 | 63.9 | 67.2 | <0.001 |
| Education, at or above college (%) | 64.8 | 57.4 | 52.7 | 46.3 | <0.001 |
| Postmenopausal women (%) | 84.5 | 80.1 | 80.0 | 73.2 | 0.001 |
| Smoking status | |||||
| Past smoker (%) | 44.6 | 43.0 | 44.1 | 43.1 | 0.9 |
| Never smoker (%) | 55.4 | 56.9 | 55.9 | 56.9 | |
| Alcohol abstainer (%) | 32.1 | 30.5 | 33.7 | 30.8 | 0.3 |
| Physical activity (MET/week) | 825 (330–1500) | 630 (225–1335) | 480 (150–1080) | 405 (60–1080) | <0.001 |
| Chemotherapy (%) | 63.6 | 61.4 | 59.5 | 62.5 | 0.3 |
| Radiation (%) | 63.6 | 61.0 | 59.1 | 62.2 | 0.6 |
| Hormone receptor status | |||||
| ER+/PR+ (%) | 63.2 | 63.1 | 62.3 | 58.1 | 0.003 |
| Cancer stage at diagnosis (%) | |||||
| I | 38.8 | 36.7 | 38.7 | 38.9 | 0.4 |
| II | 55.4 | 59.6 | 56.7 | 55.0 | |
| IIIa | 5.7 | 3.7 | 4.6 | 6.2 | |
| Tamoxifen use (%) | 72.0 | 66.9 | 63.6 | 62.2 | 0.001 |
| CES-D Score (≥5 vs. <5) | ||||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Dietary acid load | Age-adjusted | Multi-model 1 | Multi-model 2 | Multi-model 3 | ||
| PRAL(mEq/d) | Range | |||||
| Quartile 1 | <−19.50 | Ref | Ref | Ref | Ref | |
| Quartile 2 | −19.50 to <−6.94 | 1.09 (0.91–1.29) | 1.01 (0.84–1.20) | 1.02 (0.86–1.23) | 1.01 (0.84–1.18) | |
| Quartile 3 | −6.94 to <3.22 | 1.35 (1.14–1.60) | 1.21 (1.02–1.45) | 1.24 (1.04–1.48) | 1.17 (0.96–1.43) | |
| Quartile 4 | ≥3.22 | 1.51 (1.27–1.80) | 1.29 (1.08–1.56) | 1.34 (1.11–1.62) | 1.26 (1.02–1.53) | |
| p for trend | <0.0001 | 0.002 | 0.0008 | 0.01 | ||
| NEAP(mEq/d) | Range | |||||
| Quartile 1 | <28.44 | Ref | Ref | Ref | Ref | |
| Quartile 2 | 28.44 to <37.25 | 0.91 (0.78–1.07) | 0.86 (0.73–1.02) | 0.88 (0.74–1.04) | 0.84 (0.71–1.00) | |
| Quartile 3 | 37.25 to <46.90 | 1.27 (1.08–1.49) | 1.16 (1.98–1.37) | 1.20 (1.02–1.43) | 1.13 (0.94–1.35) | |
| Quartile 4 | ≥46.90 | 1.29 (1.09–1.53) | 1.12 (0.93–1.34) | 1.17 (0.98–1.41) | 1.06 (0.87–1.30) | |
| p for trend | <0.0001 | 0.02 | 0.009 | 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Hsu, F.-C.; Pierce, J.P. Acid-Producing Diet and Depressive Symptoms among Breast Cancer Survivors: A Longitudinal Study. Cancers 2020, 12, 3183. https://doi.org/10.3390/cancers12113183
Wu T, Hsu F-C, Pierce JP. Acid-Producing Diet and Depressive Symptoms among Breast Cancer Survivors: A Longitudinal Study. Cancers. 2020; 12(11):3183. https://doi.org/10.3390/cancers12113183
Chicago/Turabian StyleWu, Tianying, Fang-Chi Hsu, and John P. Pierce. 2020. "Acid-Producing Diet and Depressive Symptoms among Breast Cancer Survivors: A Longitudinal Study" Cancers 12, no. 11: 3183. https://doi.org/10.3390/cancers12113183
APA StyleWu, T., Hsu, F.-C., & Pierce, J. P. (2020). Acid-Producing Diet and Depressive Symptoms among Breast Cancer Survivors: A Longitudinal Study. Cancers, 12(11), 3183. https://doi.org/10.3390/cancers12113183






