Prognostic and Monitoring Value of Circulating Tumor Cells in Adrenocortical Carcinoma: A Preliminary Monocentric Study
Simple Summary
Abstract
1. Introduction
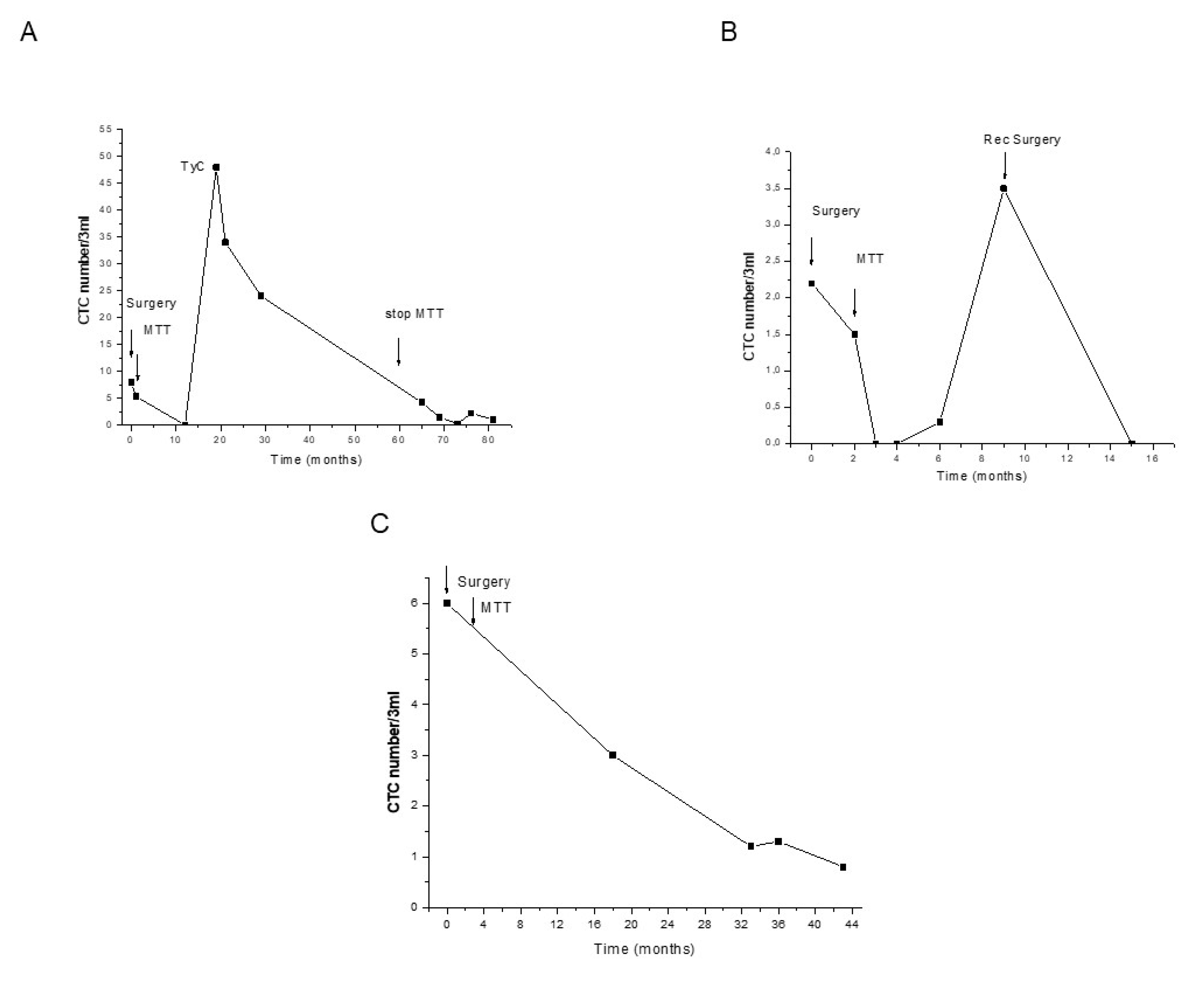
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Ethical Approval
4.2. Histologic Analysis and Immunohistochemistry of the Primary Tumour
4.3. Blood Sample Collection
4.4. Mitotane Measurement
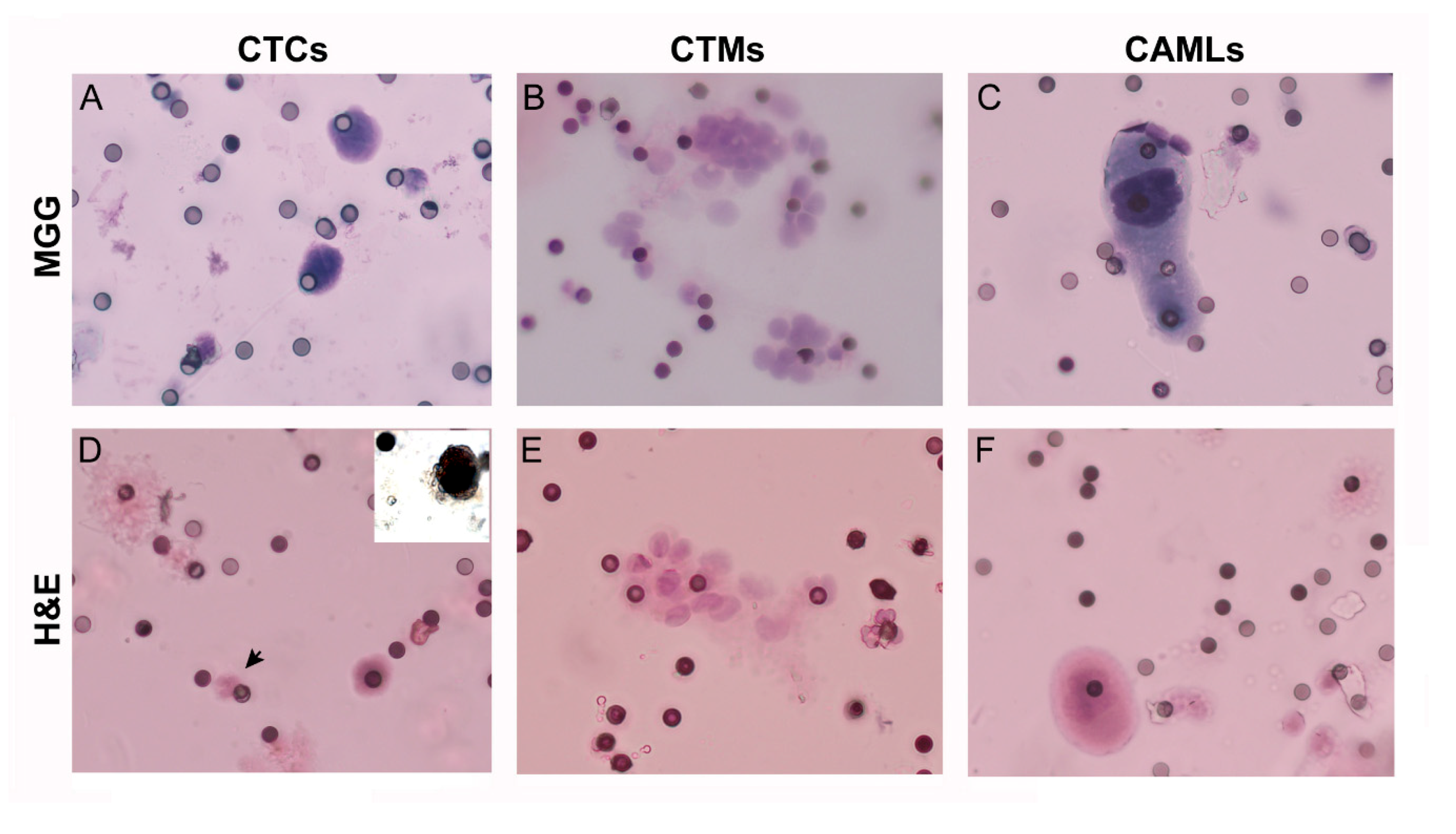
4.5. CTC Analysis
4.5.1. CTC Isolation
4.5.2. CTC Staining and Identification
4.5.3. Cytologic Characterization by Immunocytochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Laganà, M.; Grisanti, S.; Cosentini, D.; Ferrari, V.D.; Lazzari, B.; Ambrosini, R.; Sardini, C.; Volta, A.D.; Palumbo, C.; Poliani, P.L.; et al. Efficacy of the EDP-M Scheme Plus Adjunctive Surgery in the Management of Patients with Advanced Adrenocortical Carcinoma: The Brescia Experience. Cancers 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Gillis, A.; Alobuia, W.M.; Wild, H.; Kebebew, E. Surgery for adrenocortical carcinoma: When and how? Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101408. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Salvianti, F.; Pinzani, P. The diagnostic potential of mutation detection from single circulating tumor cells in cancer patients. Expert Rev. Mol. Diagn. 2017, 17, 975–981. [Google Scholar] [CrossRef]
- Lalli, E.; Luconi, M. The next step: Mechanisms driving adrenocortical carcinoma metastasis. Endocr. Relat. Cancer 2018, 25, R31–R48. [Google Scholar] [CrossRef]
- Pinzani, P.; Scatena, C.; Salvianti, F.; Corsini, E.; Canu, L.; Poli, G.; Paglierani, M.; Piccini, V.; Pazzagli, M.; Nesi, G.; et al. Detection of circulating tumor cells in patients with adrenocortical carcinoma: A monocentric preliminary study. J. Clin. Endocrinol. Metab. 2013, 98, 3731–3738. [Google Scholar] [CrossRef]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Hayes, D.F.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Reuben, J.M.; Doyle, G.V.; Matera, J.; Allard, W.J.; Miller, M.C.; et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 2005, 23, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Michiels, S.; Riethdorf, S.; Mueller, V.; Esserman, J.; Lucci, A.; Naume, B.; Horiguchi, J.; Gisbert-Criado, R.; Sleijfer, S.; et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 560–567. [Google Scholar] [CrossRef]
- Mikulová, V.; Cabiňaková, M.; Janatková, I.; Mestek, O.; Zima, T.; Tesařová, P. Detection of circulating tumor cells during follow-up of patients with early breast cancer: Clinical utility for monitoring of therapy efficacy. Scand. J. Clin. Lab. Invest. 2014, 74, 132–142. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Salvianti, F.; Pazzagli, M.; Pinzani, P. Single circulating tumor cell sequencing as an advanced tool in cancer management. Expert Rev. Mol. Diagn. 2016, 16, 51–63. [Google Scholar] [CrossRef]
- Ye, Y.; Li, S.L.; Wang, J.J.; Liu, B. The diagnostic value of circulating tumor cells for lung cancer: A systematic review and meta-analysis. Medicine 2019, 98, e14936. [Google Scholar] [CrossRef]
- Riethdorf, S.; O’Flaherty, L.; Hille, C.; Pantel, K. Clinical applications of the CellSearch platform in cancer patients. Adv. Drug Deliv. Rev. 2018, 125, 102–121. [Google Scholar] [CrossRef]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Falchini, M.; Maddau, C.; Salvianti, F.; Nistri, M.; Bertelli, E.; Sali, L.; Zuccherelli, S.; Vella, A.; Matucci, M.; et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J. Cancer Res. Clin. Oncol. 2016, 142, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Yeh, E.S.; Soloff, A.C. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2016, 2, 15025. [Google Scholar] [CrossRef]
- Adams, D.L.; Adams, D.K.; Alpaugh, R.K.; Cristofanilli, M.; Martin, S.S.; Chumsri, S.; Tang, C.M.; Marks, J.R. Circulating cancer-associated macrophage-like cells differentiate malignant breast cancer and benign breast conditions. Cancer Epidemiol. Prev. Biomark. 2016, 25, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Benali-Furet, N.; Uzan, G.; Znaty, A.; Ye, Z.; Paolillo, C.; Wang, C.; Austin, L.; Rossi, G.; Fortina, P.; et al. Detection and Characterization of Circulating Tumor Associated Cells in Metastatic Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1665. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, C.; Ye, Z.; Rossi, G.; Sun, C.; Li, L.; Zhu, Z.; Yang, H.; Cristofanilli, M. Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer. Breast Cancer Res. Treat. 2017, 165, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Qin, Q. Does the elevation of CAMLs predict metastatic breast cancer patients’ survival? Breast Cancer Res. Treat. 2018, 167, 819. [Google Scholar] [CrossRef]
- Paterlini-Brechot, P.; Benali, N.L. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef]
- Mazzini, C.; Pinzani, P.; Salvianti, F.; Scatena, C.; Paglierani, M.; Ucci, F.; Pazzagli, M.; Massi, D. Circulating tumor cells detection and counting in uveal melanomas by a filtration-based method. Cancers 2014, 6, 323–332. [Google Scholar] [CrossRef]
- Lau, S.K.; Weiss, L.M. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum. Pathol. 2009, 40, 757–768. [Google Scholar] [CrossRef]
- Morimoto, R.; Satoh, F.; Murakami, O.; Suzuki, T.; Abe, T.; Tanemoto, M.; Abe, M.; Uruno, A.; Ishidoya, S.; Arai, Y.; et al. Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr. J. 2008, 55, 49–55. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a Revised TNM Classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Salvianti, F.; Gelmini, S.; Costanza, F.; Mancini, I.; Sonnati, G.; Simi, L.; Pazzagli, M.; Pinzani, P. The pre-analytical phase of the liquid biopsy. N. Biotechnol. 2020, 55, 19–29. [Google Scholar] [CrossRef] [PubMed]



| Age (Years) | Sex | Tumor Ø (cm) | Ki67 (%) | Weiss | Stage | Secretion | CTC PreS | CTC PostS | |
|---|---|---|---|---|---|---|---|---|---|
| Total cohort (n = 19) | 53.8 ± 12.5 | F:9 (47%) | 11.0 ± 4.5 | 21.4 ± 11.0 | 6.8 ± 1.2 | I: 2 (10%) | NO: 4 (21%) | 1.7 [1–9] | 0.6 [0.1–1.2] |
| II: 6 (32%) | G: 10 (53%) | ||||||||
| M:10 (53%) | III: 7 (37%) | A: 4 (21%) | (0–30) | (0–5.2) | |||||
| IV: 4 (21%) | M: 1 (5%) | ||||||||
| Low CTC number PreS (n = 12) | 50.8 ± 9.5 | F: 7 (58%) | 11.2 ± 4.6 | 20.4 ± 11.6 | 6.7 ± 1.2 | I: 1 (8%) | NO: 2 (17%) | 1.1 [1–2.0] | 0.6 [0–0.9] |
| II: 4 (33%) | G: 6 (50%) | ||||||||
| M: 5 (42%) | III: 7 (59%) | A: 3 (25%) | (0–6.6) | (0.1–1.1) | |||||
| IV: 0 (0%) | M: 1 (8%) | ||||||||
| High CTC number PreS (n = 7) | 57.2 ± 11.8 | F: 4 (57%) | 9.6 ± 5.4 | 27.5 ± 9.6 | 7.8 ± 1.0 | I: 1 (14%) | NO: 2 (29%) | 17 [9–24] | 0.3 [0–0.3] |
| II: 2 (29%) | G: 4 (57%) | ||||||||
| M: 3 (43%) | III: 0 (0%) | A: 1 (14%) | (9–30) | (0–1) | |||||
| IV: 4 (57%) | M: 0 (0%) |
| HR (95%CI) Unadjusted | p | HR (95%CI) Adjusted | p | |
|---|---|---|---|---|
| Pre-S CTCs | 12.0 (1.3–110.0) | 0.026 | 22.0 (0.9–540.0) | 0.05 |
| Age | 1.05 (0.99–1.11) | 0.120 | 0.98 (0.86–1.10) | 0.675 |
| Stage | 4.37 (1.2–15.75) | 0.024 | 3.78 (0.7–19.7) | 0.114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantini, G.; Canu, L.; Armignacco, R.; Salvianti, F.; De Filpo, G.; Ercolino, T.; Nesi, G.; Maggi, M.; Mannelli, M.; Pinzani, P.; et al. Prognostic and Monitoring Value of Circulating Tumor Cells in Adrenocortical Carcinoma: A Preliminary Monocentric Study. Cancers 2020, 12, 3176. https://doi.org/10.3390/cancers12113176
Cantini G, Canu L, Armignacco R, Salvianti F, De Filpo G, Ercolino T, Nesi G, Maggi M, Mannelli M, Pinzani P, et al. Prognostic and Monitoring Value of Circulating Tumor Cells in Adrenocortical Carcinoma: A Preliminary Monocentric Study. Cancers. 2020; 12(11):3176. https://doi.org/10.3390/cancers12113176
Chicago/Turabian StyleCantini, Giulia, Letizia Canu, Roberta Armignacco, Francesca Salvianti, Giuseppina De Filpo, Tonino Ercolino, Gabriella Nesi, Mario Maggi, Massimo Mannelli, Pamela Pinzani, and et al. 2020. "Prognostic and Monitoring Value of Circulating Tumor Cells in Adrenocortical Carcinoma: A Preliminary Monocentric Study" Cancers 12, no. 11: 3176. https://doi.org/10.3390/cancers12113176
APA StyleCantini, G., Canu, L., Armignacco, R., Salvianti, F., De Filpo, G., Ercolino, T., Nesi, G., Maggi, M., Mannelli, M., Pinzani, P., & Luconi, M. (2020). Prognostic and Monitoring Value of Circulating Tumor Cells in Adrenocortical Carcinoma: A Preliminary Monocentric Study. Cancers, 12(11), 3176. https://doi.org/10.3390/cancers12113176