Medullary Thyroid Cancer in Patients Older than 45—Epidemiologic Trends and Predictors of Survival
Simple Summary
Abstract
1. Introduction
2. Results
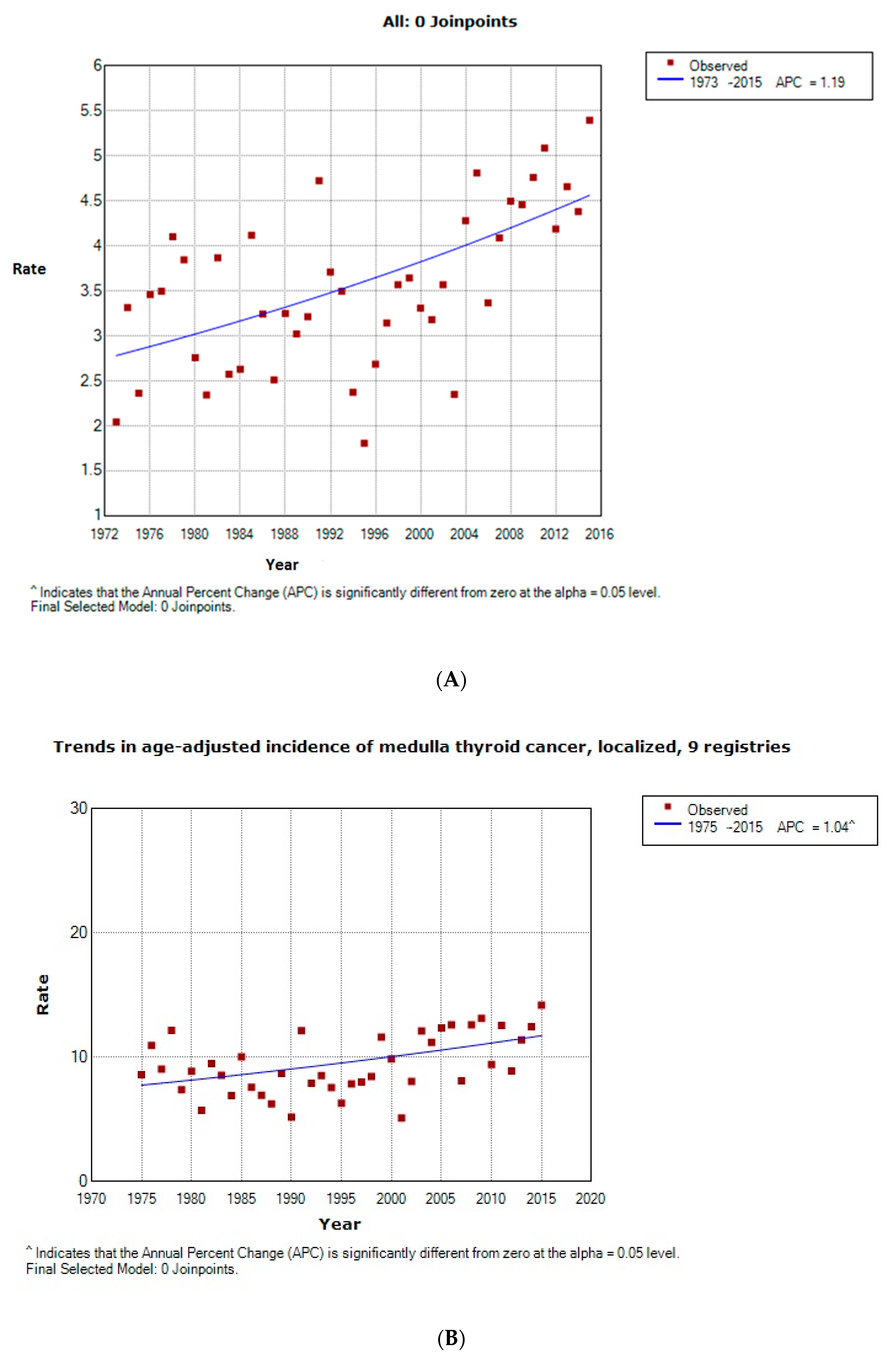
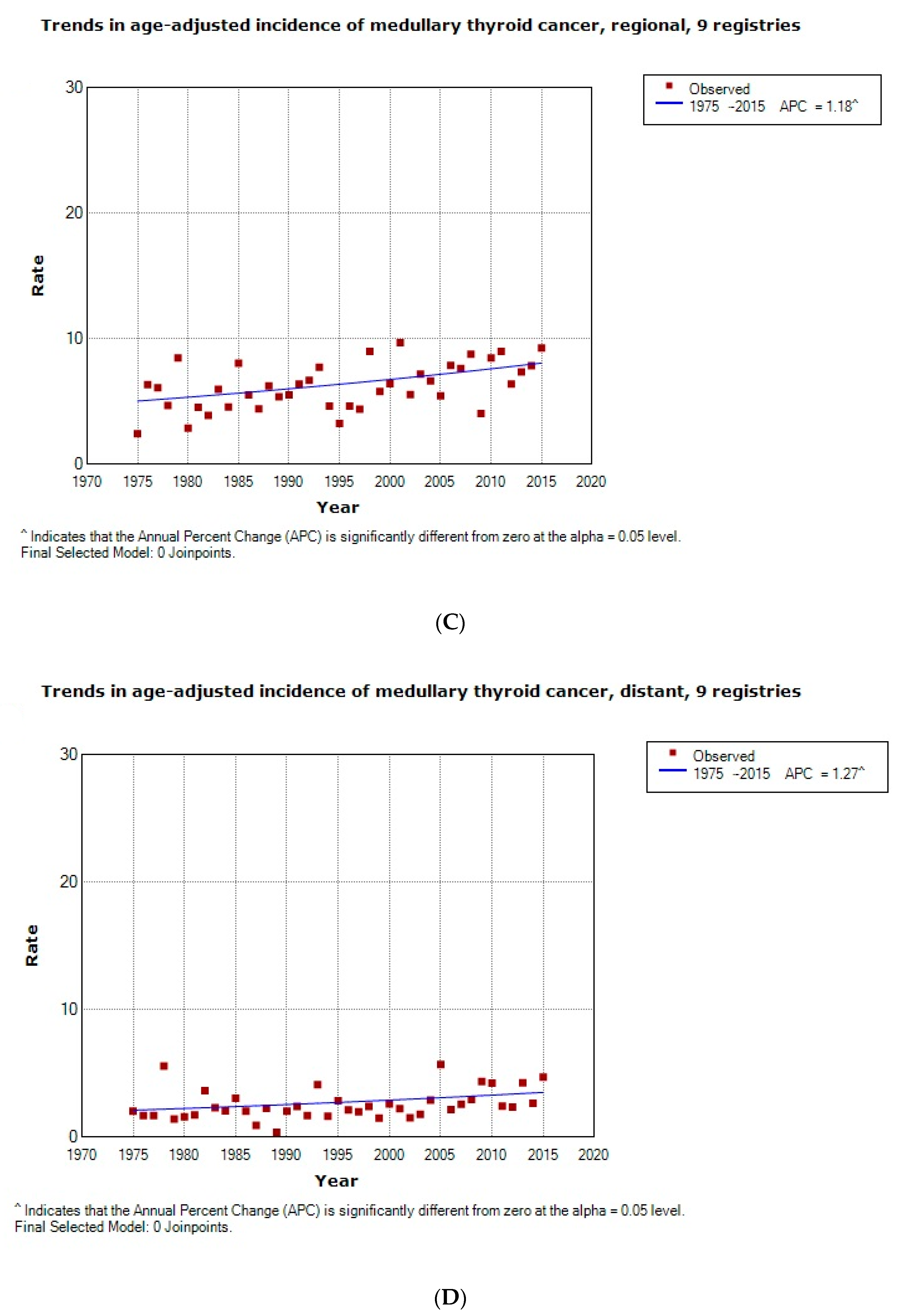
2.1. Epidemiology and Cancer-Free Survival Rate
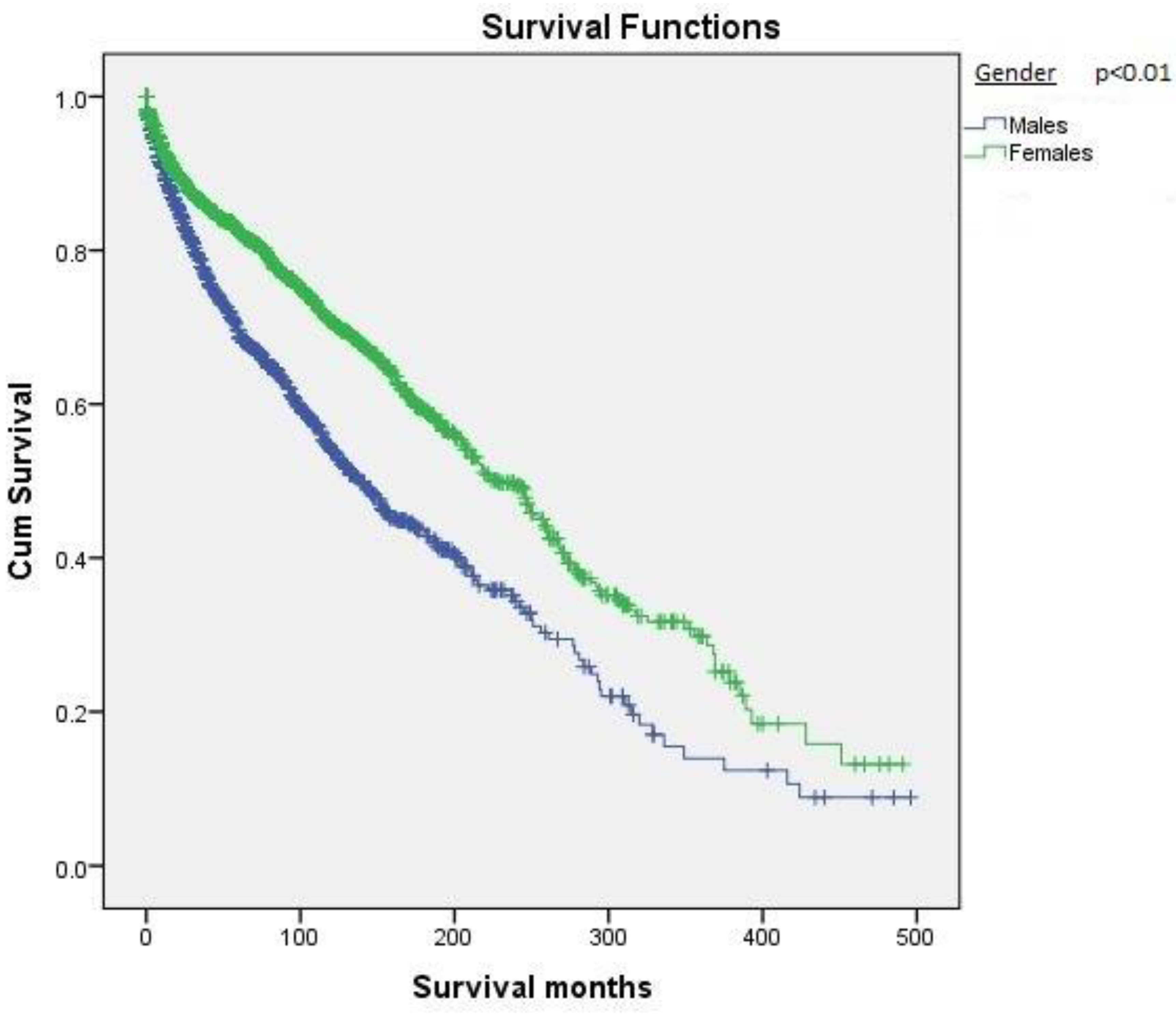
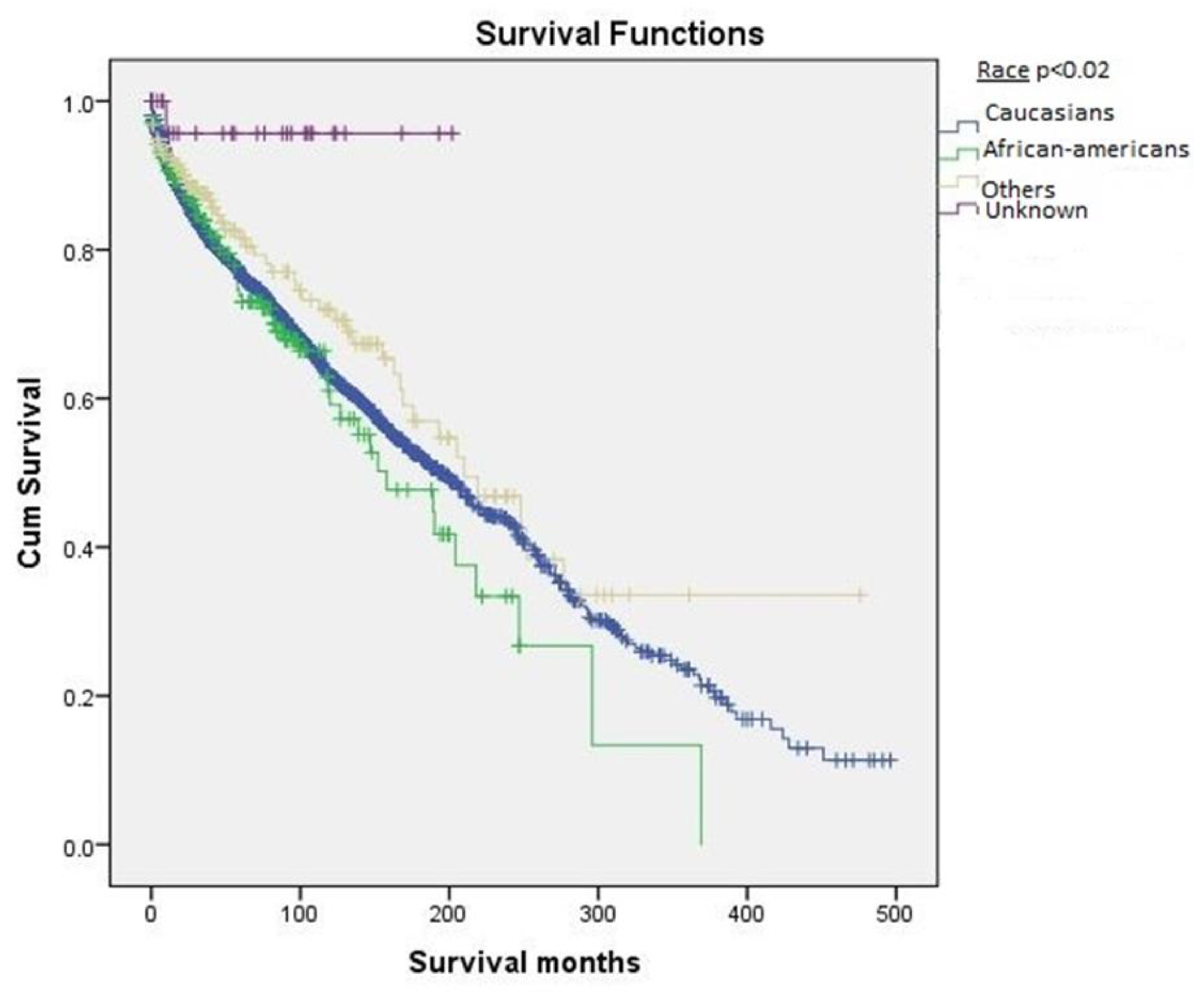
2.2. Survival Based on Demographics
2.3. Tumor Description and Survival Characteristics
2.4. Cox Proportional Hazard Model
3. Discussion
4. Materials and Methods
4.1. Inclusion Criteria
4.2. Exclusion Criteria
4.3. Patient-Related Variables
4.4. Outcome Measures
4.5. Survival Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ho, A.S.; Wang, L.; Palmer, F.L.; Yu, C.; Toset, A.; Patel, S.; Kattan, M.W.; Tuttle, R.M.; Ganly, I. Postoperative Nomogram for Predicting Cancer-Specific Mortality in Medullary Thyroid Cancer. Ann. Surg. Oncol. 2015, 22, 2700–2706. [Google Scholar] [CrossRef]
- Miyauchi, A.; Matsuzaka, F.; Hirai, K.; Yokozawa, T.; Kobayashi, K.; Ito, Y.; Nakano, K.; Kuma, K.; Futami, H.; Yamaguchi, K. Prospective trial of unilateral surgery for nonhereditary medullary thyroid carcinoma in patient without germline RET mutations. World J. Surg. 2002, 26, 1023–1028. [Google Scholar] [CrossRef]
- Larouche, V.; Akirov, A.; Thomas, C.M.; Krzyzanowska, M.K.; Ezzat, S. A primer on the genetics of medullary thyroid cancer. Curr. Oncol. 2019, 26, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017, 161, 137–146. [Google Scholar] [CrossRef]
- Viola, D.; Elisei, R. Management of medullary thyroid cancer. Endocrinol. Metab. Clin. 2019, 48, 285–301. [Google Scholar] [CrossRef]
- Moo-Young, T.A.; Traugott, A.L.; Moley, J.F. Sporadic and familial medullary thyroid carcinoma, state of the art. Surg. Clin. 2009, 89, 1193–1204. [Google Scholar] [CrossRef]
- Fallah, M.; Sundquist, K.; Hemminki, K. Risk of thyroid cancer in relatives of patients with medullary thyroid carcinoma by age at diagnosis. Endocr.-Relat. Cancer 2013, 20, 717–724. [Google Scholar] [CrossRef]
- Qu, N.; Shi, R.L.; Luo, T.X.; Wang, Y.L.; Li, D.S.; Wang, Y.; Huang, C.P.; Ji, Q.H. Prognostic significance and optimal cutoff of age in medullary thyroid cancer. Oncotarget 2016, 7, 15937–15947. [Google Scholar] [CrossRef]
- Lennon, P.; Deady, S.; White, N.; Lambert, D.; Healy, M.L.; Green, A.; Kinsella, J.; Timon, C.; O’Neill, J.P.O. Aggressive medullary thyroid cancer, an analysis of the Irish National Cancer Registry. Ir. J. Med. Sci. 2017, 186, 89–95. [Google Scholar] [CrossRef]
- Gundara, J.S.; Zhao, J.; Gill, A.J.; Lee, J.C.; Delbridge, L.; Robinson, B.G.; McLean, C.; Serpell, J.; Sidhu, S.B. Noncoding RNA blockade of autophagy is therapeutic in medullary thyroid cancer. Cancer Med. 2015, 4, 174–182. [Google Scholar] [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J., III; et al. Executive summary of the American Association of Endocrine Surgeons Guidelines for the Definitive surgical management of thyroid disease in adults. Ann. Surg. 2020, 271, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Rich, T.A.; Feng, L.; Busaidy, N.; Cote, G.J.; Gagel, R.F.; Hu, M.; Jimenez, C.; Lee, J.E.; Perrier, N.; Sherman, S.I.; et al. Prevalence by Age and Predictors of Medullary Thyroid Cancer in Patients with Lower Risk Germline RET Proto-Oncogene Mutations. Thyroid 2014, 24, 1096–1106. [Google Scholar] [CrossRef]
- Figlioli, G.; Landi, S.; Romei, C.; Elisei, R.; Gemignani, F. Medullary thyroid carcinoma (MTC) and RET proto-oncogene: Mutation spectrum in the familial cases and a meta-analysis of studies on the sporadic form. Mutat. Res. 2013, 752, 36–44. [Google Scholar] [CrossRef]
- Moura, M.M.; Cavaco, B.M.; Leite, V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr. Relat. Cancer 2015, 22, R235–R252. [Google Scholar] [CrossRef]
- Yamada, T.; Iwai, T.; Takahashi, G.; Kan, H.; Koizumi, M.; Matsuda, A.; Shinji, S.; Yamagishi, A.; Yokoyama, Y.; Tatsuguchi, A.; et al. Utility of KRAS mutation detection using circulating cell-free DNA from patients with colorectal cancer. Cancer Sci. 2016, 107, 936–943. [Google Scholar] [CrossRef]
- Fink, M.; Weinhausel, A.; Niederle, B.; Hass, O.A. Distinction between sporadic and hereditary medullary thyroid carcinoma (MTC) by mutation analysis of the RET proto-oncogene. Int. J. Cancer 1996, 69, 312–316. [Google Scholar] [CrossRef]
- Roy, M.; Chen, H.; Sippel, R.S. Current understanding and management of medullary thyroid cancer. Oncologist 2013, 18, 1093–1100. [Google Scholar] [CrossRef]
- Hazard, J.B.; HAWK, W.A.; CRILE, J.R.G. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J. Clin. Endocrinol. Metab. 1959, 19, 152–161. [Google Scholar] [CrossRef]
- Machens, A.; Dralle, H. Surgical cure rates of sporadic medullary thyroid cancer in the era of calcitonin screening. Eur. J. Endocrinol. 2016, 175, 219–228. [Google Scholar] [CrossRef]
- Grubbs, E.G.; Williams, M.D.; Scheet, P.; Vattathil, S.; Perrier, N.D.; Lee, J.E.; Gagel, R.F.; Hai, T.; Feng, L.; Cabanillas, M.E.; et al. Role of CDKN2C copy number in sporadic medullary thyroid carcinoma. Thyroid 2016, 26, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Takahasi, M.; Ritz, J.; Cooper, G.M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985, 42, 581–588. [Google Scholar] [CrossRef]
- Pasini, A.; Geneste, O.; Legrand, P.; Schlumberger, M.; Rossel, M.; Fournier, L.; Rudkin, B.B.; Schuffenecker, I.; Lenoir, G.M.; Billaud, M. Oncogenic activation of RET by two distinct FMTC mutations affecting the tyrosine kinase domain. Oncogene 1997, 15, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M.; Kwok, J.B.; Healey, C.S.; Elsdon, M.J.; Eng, C.; Gardner, E.; Love, D.R.; Mole, S.E.; Moore, K.K.; Papi, L.; et al. Germline mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993, 363, 458–460. [Google Scholar] [CrossRef]
- Donis-Keller, H.; Dou, S.; Chi, D.; Carlson, K.M.; Toshima, K.; Lairmore, T.C.; Howe, J.R.; Moley, J.F.; Goodfellow, P.; Wells, S.A., Jr.; et al. Mutations of the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993, 2, 851–856. [Google Scholar] [CrossRef]
- Elisei, R.; Cosci, B.; Romei, C.; Bottici, V.; Renzini, G.; Molinaro, E.; Agate, L.; Vivaldi, A.; Faviana, P.; Basolo, F.; et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Accardo, G.; Conzo, G.; Esposito, D.; Gambardella, C.; Mazzella, M.; Castaldo, F.; Donna, C.D.; Polistena, A.; Avenia, N.; Colantuoni, V.; et al. Genetics of medullary thyroid cancer: An overview. Int. J. Surg. 2017, 41, S2–S6. [Google Scholar] [CrossRef]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef]
- Farndon, J.R.; Leightt, G.S.; Dilley, W.G.; Baylin, S.B.; Smallridge, R.C.; Harrison, T.S.; Wells, S.A., Jr. Familial medullary thyroid carcinoma without associated endocrinopathies: A distinct clinical entity. Br. J. Surg. 1986, 73, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Ituarte, P.G.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Medullary Thyroid Carcinoma: Clinical Characteristics, Treatment, Prognostic Factors, and a Comparison of Staging Systems. Cancer 2000, 88, 1139–1148. [Google Scholar] [CrossRef]
- Roman, S.; Lin, R.; Sosa, J.A. Prognosis of Medullary Thyroid Carcinoma: Demographic, Clinical, and Pathologic Predictors of Survival in 1252 Cases. Cancer 2006, 107, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, S.; Reagh, J.; Bullock, M.; Aniss, A.; Clifton-Bligh, R.; Learoyd, D.; Robinson, B.; Delbridge, L.; Sidhu, S.; Gill, A.J.; et al. Medullary thyroid carcinoma: Survival analysis and evaluation of mutation-specific immunohistochemistry in the detection of sporadic disease. World J. Surg. 2018, 42, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
| Variables | N (%) | Survival (Months) | p-Value |
|---|---|---|---|
| Age | |||
| 45–64 years | 1362 (53.7%) | 254.2 ± 7.3 | |
| 65–84 years | 772 (30.4%) | 124.5 ± 6.5 | <0.01 |
| ≥85 years | 399 (15.7%) | 43.6 ± 6.5 | |
| Gender | <0.01 | ||
| Males (N, %) | 1049 (41.4%) | 180.7 ± 8.4 | |
| Females (N, %) | 1484 (58.6%) | 239.0 ± 7.5 | |
| Race | 0.02 | ||
| Caucasians | 2172 (85.7%) | 215.1 ± 6.0 | |
| African-Americans | 177 (7.0%) | 173.0 ± 16.0 | |
| Others (American Indians, Asian Pacific islanders) | 156 (6.2%) | 251 ± 16.3 | |
| Unknown | 28 (1.1%) | 194 ± 8.1 |
| Variables | Mean | SD | p-Value |
|---|---|---|---|
| Tumor size (±SD) | 25.6 (mm) | 2.7 (mm) | |
| Number of cervical lymph nodes isolated (N ± SD) | 42.4 | 97.6 | |
| Number of cervical lymph nodes positive for metastasis (N ± SD) | 36.0 | 44.6 | |
| N (%) | Survival (months) | ||
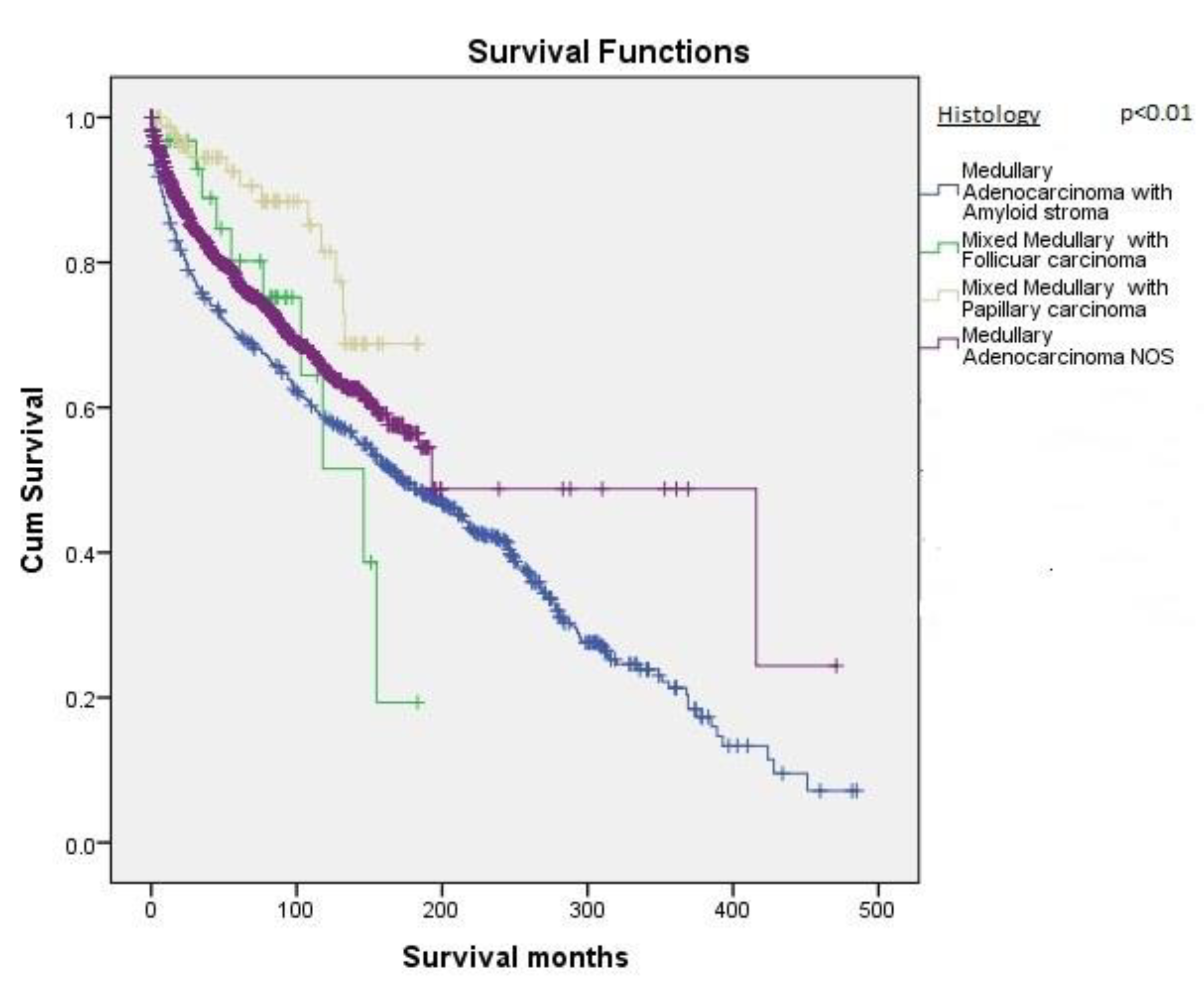
| Histological subtypes | <0.01 | ||
| Medullary adenocarcinoma NOS | 1704 (67.3%) | 250 ± 16 | |
| Medullary adenocarcinoma with an amyloid stoma | 702 (27.7%) | 202.6 ± 7.0 | |
| Medullary adenocarcinoma with papillary variant | 89 (3.5%) | 156.5 ± 6.7 | |
| Medullary adenocarcinoma with follicular variant | 38 (1.5%) | 119.3 ± 12.4 | |
| Tumor extension | <0.01 | ||
| In-situ: Noninvasive | 464 (18.3%) | 172.6 ± 9.4 | |
| Single invasive tumor confined to the thyroid | 955 (37.7%) | 243 ± 6.2 | |
| Multiple foci confined to the thyroid | 275 (10.9%) | 229.6 ± 12.4 | |
| Localized, NOS | 165 (6.5%) | 171.2 ± 11.2 | |
| Into thyroid capsule, but not beyond | 317 (12.5%) | 179 ± 11.2 | |
| Pericapsular soft/connective tissue, Parathyroid Strap muscle(s), Nerves: Recurrent laryngeal, vagus | 97 (3.8%) | 136.1 ± 12 | |
| Extension to: Major blood vessel(s), SCM muscle Esophagus, Larynx (The tumor is described as fixed to adjacent tissues) | 23 (0.9%) | 80.1 ± 13 | |
| Trachea, Skeletal muscle, other than strap or SCM muscle, Bone | 29 (1.1%) | 46.3 ± 9.1 | |
| Extension into Mediastinal tissues | 41 (1.6%) | 55.3 ± 9.0 | |
| Metastasis | 76 (3.0%) | 37.0 ± 7.0 | |
| Unknown if extension present | 91 (3.4%) | 62.0 ± 7.7 | |
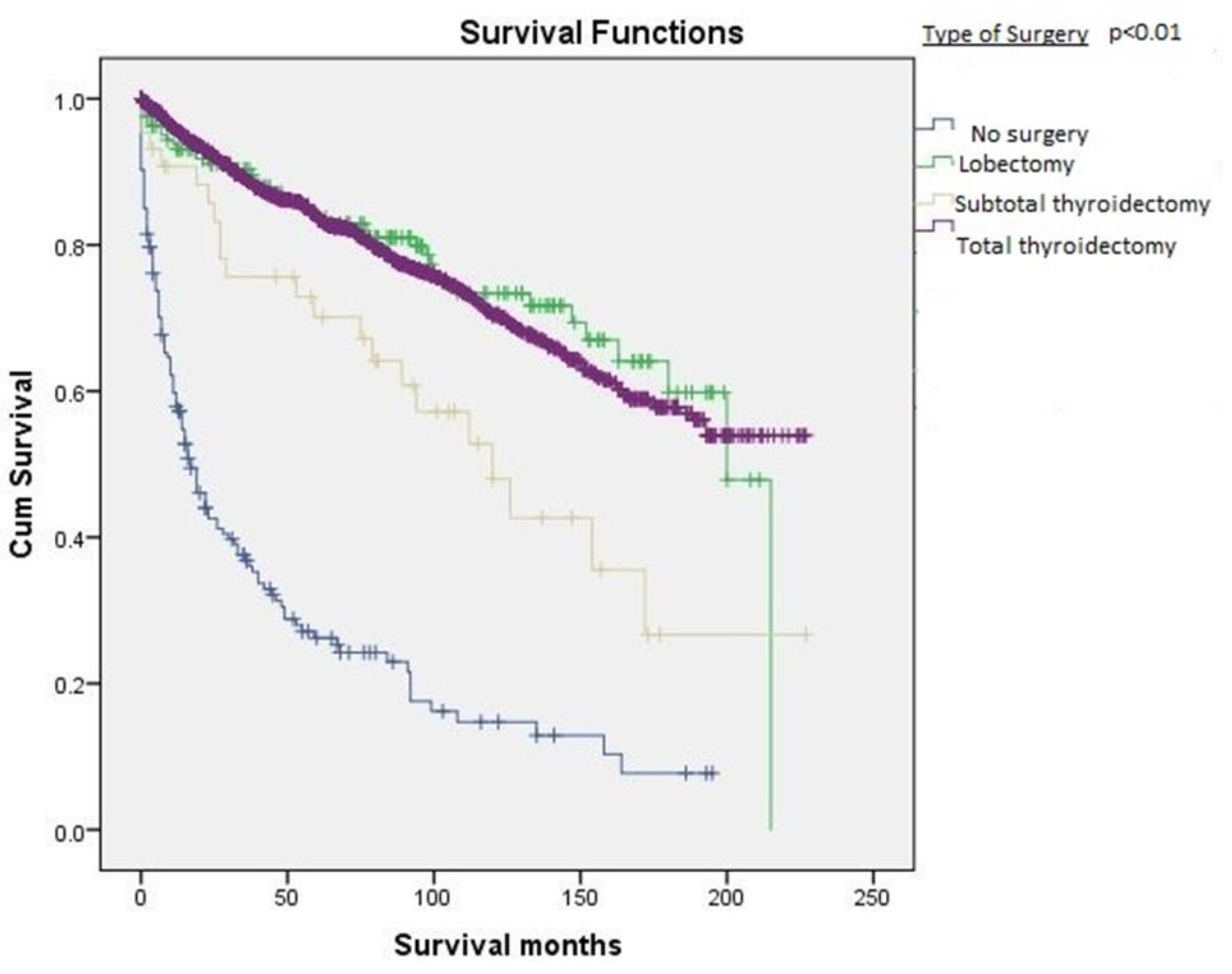
| Type of surgery performed | <0.01 | ||
| Total thyroidectomy | 1655 (65.3%) | 165.3 ± 2.7 | |
| Subtotal thyroidectomy | 344 (13%) | 122.2 ± 14.4 | |
| Thyroid Lobectomy | 262 (10%) | 162.1 ± 7.1 | |
| No surgery | 272 (10%) | 47.3 ± 5.3 |
| Factors | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.058 | 1.049–1.069 | <0.01 |
| Gender | |||
| Females (Reference) | 1 | ||
| Males | 1.204 | 1.009–1.437 | 0.04 |
| Nodal Status | |||
| Negative cervical nodes | |||
| Positive cervical lymph nodes | 1.003 | 1.001–1.006 | <0.01 |
| Type of Surgical resection | |||
| Total thyroidectomy (Reference) | |||
| Subtotal thyroidectomy | 0.289 | 0.166–0.317 | <0.01 |
| Thyroid Lobectomy | 0.832 | 0.392–1.117 | 0.30 |
| No surgery | 2.180 | 1.390–3.419 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogna, S.; Goldberg, M.; Samson, D.; Gachabayov, M.; Felsenreich, D.M.; Azim, A.; Dong, X.D. Medullary Thyroid Cancer in Patients Older than 45—Epidemiologic Trends and Predictors of Survival. Cancers 2020, 12, 3124. https://doi.org/10.3390/cancers12113124
Gogna S, Goldberg M, Samson D, Gachabayov M, Felsenreich DM, Azim A, Dong XD. Medullary Thyroid Cancer in Patients Older than 45—Epidemiologic Trends and Predictors of Survival. Cancers. 2020; 12(11):3124. https://doi.org/10.3390/cancers12113124
Chicago/Turabian StyleGogna, Shekhar, Michael Goldberg, David Samson, Mahir Gachabayov, Daniel M. Felsenreich, Asad Azim, and Xiang D (Eric) Dong. 2020. "Medullary Thyroid Cancer in Patients Older than 45—Epidemiologic Trends and Predictors of Survival" Cancers 12, no. 11: 3124. https://doi.org/10.3390/cancers12113124
APA StyleGogna, S., Goldberg, M., Samson, D., Gachabayov, M., Felsenreich, D. M., Azim, A., & Dong, X. D. (2020). Medullary Thyroid Cancer in Patients Older than 45—Epidemiologic Trends and Predictors of Survival. Cancers, 12(11), 3124. https://doi.org/10.3390/cancers12113124






