Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers
Simple Summary
Abstract
1. Introduction
2. HPV Carcinogenesis
3. HPV Vaccines
3.1. HPV Preventive Vaccines
3.2. Therapeutic Vaccines
3.2.1. DNA Vaccines
3.2.2. RNA-Based Vaccines
4. The Role of Adjuvants in Cancer Vaccines
5. Plant Metabolites Targeting HPV Tumors
5.1. HPV-Related In Vitro and In Vivo Studies Based on Purified Phytochemicals
5.2. HPV-Related In Vitro and In Vivo Studies Based on Plant Extracts or Mixtures
5.3. Evaluation of Plant Compound Adjuvant Activity in Chemo- and Radio-Therapies for HPV-Associated Cancer
5.3.1. Phytochemicals with Chemosensitizing Effects on Cervical Cancer Cells in Vitro
5.3.2. Phytochemicals with Radiosensitizing Effects on HPV-Related Cancer Cells In Vitro and In Vivo
5.4. Clinical Evaluation of Plant Compounds
5.5. Cytotoxicity of Plant Compounds: Anti-HPV Cancer Efficacy Prediction and Concerns for Healthy Cells
6. Improving DNA Vaccine Effectiveness by Plant-Derived Solutions
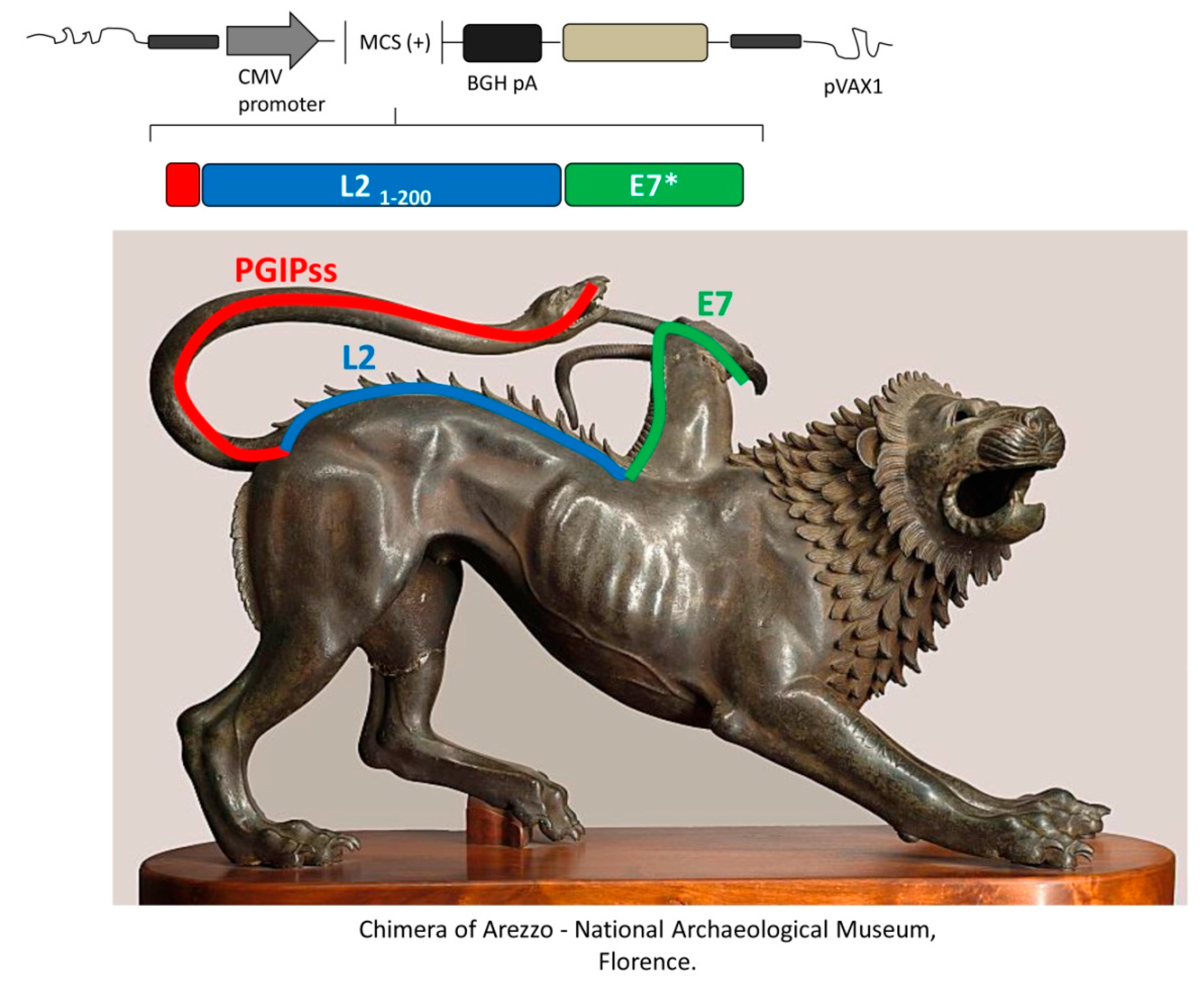
6.1. Improved HPV Genetic Vaccines Including Plant Immune-Modulating Sequences
6.2. Combinations of Plant Molecules with HPV DNA Vaccination
7. Future Perspectives and Clinical Translation: What Is Needed
8. Conclusions
9. Patents
Author Contributions
Funding
Conflicts of Interest
References
- McLaughlin-Drubin, M.E.; Munger, K. Viruses associated with human cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2008, 1782, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, G.R.; Leong, D.P.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Gupta, R.; Diaz, R.; Avezum, A.; Oliveira, G.B.F.; Wielgosz, A.; et al. Variations in Common Diseases, Hospital Admissions, and Deaths in Middle-Aged Adults in 21 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2020, 395, 785–794. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Cobos, C.; Figueroa, J.A.; Mirandola, L.; Colombo, M.; Summers, G.; Figueroa, A.; Aulakh, A.; Konala, V.M.; Verma, R.; Riaz, J.; et al. The Role of Human Papilloma Virus (HPV) Infection in Non-Anogenital Cancer and the Promise of Immunotherapy: A Review. Int. Rev. Immunol. 2014, 33, 383–401. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef]
- Lee, S.-J.; Yang, A.; Wu, T.; Hung, C.-F. Immunotherapy for human papillomavirus-associated disease and cervical cancer: Review of clinical and translational research. J. Gynecol. Oncol. 2016, 27, e51. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef]
- Vici, P.; Pizzuti, L.; Mariani, L.; Zampa, G.; Santini, D.; Di Lauro, L.; Gamucci, T.; Natoli, C.; Marchetti, P.; Barba, M.; et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: Hope or reality from clinical studies. Expert Rev. Vaccines 2016, 15, 1327–1336. [Google Scholar] [CrossRef]
- Cordeiro, M.N.; Lima, R.D.C.P.D.; Paolini, F.; Melo, A.R.D.S.; Campos, A.P.F.; Venuti, A.; Freitas, A.C. Current research into novel therapeutic vaccines against cervical cancer. Expert Rev. Anticancer. Ther. 2018, 18, 365–376. [Google Scholar] [CrossRef]
- Franconi, R.; Di Bonito, P.; Dibello, F.; Accardi, L.; Muller, A.; Cirilli, A.; Simeone, P.; Donà, M.G.; Venuti, A.; Giorgi, C. Plant-derived human papillomavirus 16 E7 oncoprotein induces immune response and specific tumor protection. Cancer Res. 2002, 62, 3654–3658. [Google Scholar]
- Franconi, R.; Massa, S.; Illiano, E.; Müller, A.; Cirilli, A.; Accardi, L.; Di Bonito, P.; Giorgi, C.; Venuti, A. Exploiting the Plant Secretory Pathway to Improve the Anticancer Activity of a Plant-Derived HPV16 E7 Vaccine. Int. J. Immunopathol. Pharmacol. 2006, 19, 187–19719. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Grasso, F.; Mangino, G.; Massa, S.; Illiano, E.; Franconi, R.; Fanales-Belasio, E.; Falchi, M.; Affabris, E.; Giorgi, C. Immunomodulatory Activity of a Plant Extract Containing Human Papillomavirus 16-E7 Protein in Human Monocyte-Derived Dendritic Cells. Int. J. Immunopathol. Pharmacol. 2009, 22, 967–978. [Google Scholar] [CrossRef]
- Demurtas, O.C.; Massa, S.; Ferrante, P.; Venuti, A.; Franconi, R.; Giuliano, G. A Chlamydomonas-Derived Human Papillomavirus 16 E7 Vaccine Induces Specific Tumor Protection. PLoS ONE 2013, 8, e61473. [Google Scholar] [CrossRef]
- Chuang, C.-M.; Monie, A.; Wu, A.A.; Hung, C.-F. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J. Biomed. Sci. 2009, 16, 49. [Google Scholar] [CrossRef]
- Kang, T.H.; Lee, J.H.; Kil Song, C.; Han, H.D.; Shin, B.C.; Pai, S.I.; Hung, C.-F.; Trimble, C.; Lim, J.-S.; Kim, T.W.; et al. Epigallocatechin-3-Gallate Enhances CD8+ T Cell-Mediated Antitumor Immunity Induced by DNA Vaccination. Cancer Res. 2007, 67, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Khavari, A.; Bolhassani, A.; Alizadeh, F.; Bathaie, S.Z.; Balaram, P.; Agi, E.; Vahabpour, R. Chemo-immunotherapy using saffron and its ingredients followed by E7-NT (gp96) DNA vaccine generates different anti-tumor effects against tumors expressing the E7 protein of human papillomavirus. Arch. Virol. 2014, 160, 499–508. [Google Scholar] [CrossRef]
- Massa, S.; Simeone, P.; Muller, A.; Benvenuto, E.; Venuti, A.; Franconi, R. Antitumor Activity of DNA Vaccines Based on the Human Papillomavirus-16 E7 Protein Genetically Fused to a Plant Virus Coat Protein. Hum. Gene Ther. 2008, 19, 354–364. [Google Scholar] [CrossRef]
- Massa, S.; Paolini, F.; Spanò, L.; Franconi, R.; Venuti, A. Mutants of plant genes for developing cancer vaccines. Hum. Vaccines 2011, 7, 147–155. [Google Scholar] [CrossRef]
- Massa, S.; Paolini, F.; Curzio, G.; Cordeiro, M.N.; Illiano, E.; Demurtas, O.C.; Franconi, R.; Venuti, A. A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines. Hum. Vaccines Immunother. 2017, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Venuti, A.; Franconi, R.; Massa, S.; Mariani, L.; Paolini, F. Therapeutic/Preventive Hpv Vaccines, an Old Idea with Something New: Dna Chimeric Vaccines with Plant-Derived Signal Sequences. In Proceedings of the 32nd International Papillomavirus Conference, Sydney, Australia, 2–6 October 2018. [Google Scholar]
- Cheng, M.A.; Farmer, E.; Huang, C.; Lin, J.; Hung, C.-F.; Wu, T.-C. Therapeutic DNA Vaccines for Human Papillomavirus and Associated Diseases. Hum. Gene Ther. 2018, 29, 971–996. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Gillison, M.L. Human Papillomavirus-Related Diseases: Oropharynx Cancers and Potential Implications for Adolescent HPV Vaccination. J. Adolesc. Heal. 2008, 43, S52–S60. [Google Scholar] [CrossRef]
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar] [PubMed]
- Pullos, A.; Castilho, R.; Squarize, C. HPV Infection of the Head and Neck Region and Its Stem Cells. J. Dent. Res. 2015, 94, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Paolini, F.; Frank, D.; Kamdar, D.; Curzio, G.; Pichi, B.; Pellini, R.; Spriano, G.; Bonagura, V.; Venuti, A.; et al. Latent human papillomavirus type 16 infection is widespread in patients with oropharyngeal cancers. Oral Oncol. 2018, 78, 222–224. [Google Scholar] [CrossRef]
- Forcier, M.; Musacchio, N. An overview of human papillomavirus infection for the dermatologist: Disease, diagnosis, management, and prevention. Dermatol. Ther. 2010, 23, 458–476. [Google Scholar] [CrossRef]
- Stillo, M.; Santisteve, P.C.; Lopalco, P.L. Safety of human papillomavirus vaccines: A review. Expert Opin. Drug Saf. 2015, 14, 697–712. [Google Scholar] [CrossRef]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, Â. Cervical cancer and HPV infection: Ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discov. Today 2019, 24, 2044–2057. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef]
- Villa, L.L. Prophylactic HPV vaccines: Reducing the burden of HPV-related diseases. Vaccine 2006, 24, S23–S28. [Google Scholar] [CrossRef]
- Christensen, N.D.; A Reed, C.; Cladel, N.M.; Han, R.; Kreider, J.W. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 1996, 70, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Roden, R.B.; Monie, A.; Wu, T.-C. Opportunities to improve the prevention and treatment of cervical cancer. Curr. Mol. Med. 2007, 7, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Frazer, I.H.; Lowry, D.R. Chapter Human papillomavirus vaccines. In Plotkin SA; Orenstein, W.A., Offit, P.A., Eds.; Vaccines; Saunders/Elsevier: Philadelphia, PA, USA, 2008; pp. 244–257. [Google Scholar]
- Schiller, J.T.; Müller, M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015, 16, e217–e225. [Google Scholar] [CrossRef]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Signorelli, C.; Odone, A.; Ciorba, V.; Cella, P.; Audisio, R.A.; Lombardi, A.; Mariani, L.; Mennini, F.S.; Pecorelli, S.; Rezza, G.; et al. Human papillomavirus 9-valent vaccine for cancer prevention: A systematic review of the available evidence. Epidemiol. Infect. 2017, 145, 1962–1982. [Google Scholar] [CrossRef]
- Ma, B.; Maraj, B.; Tran, N.P.; Knoff, J.; Chen, A.; Alvarez, R.D.; Hung, C.-F.; Wu, T.-C. Emerging human papillomavirus vaccines. Expert Opin. Emerg. Drugs 2012, 17, 469–492. [Google Scholar] [CrossRef]
- Yang, A.; Jeang, J.; Cheng, K.; Cheng, T.; Yang, B.; Wu, T.-C.; Hung, C.-F. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev. Vaccines 2016, 15, 989–1007. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, Élodie; Pérez, N.; Brisson, M.; Ali, H.; Boily, M.-C.; Baldo, V.; Brassard, P.; Brotherton, J.M.L.; Callander, D.; et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Borysiewicz, L.; Fiander, A.; Nimako, M.; Man, S.; Wilkinson, G.; Westmoreland, D.; Evans, A.; Adams, M.; Stacey, S.; Boursnell, M.; et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 1996, 347, 1523–1527. [Google Scholar] [CrossRef]
- Kenter, G.G.; Welters, M.J.P.; Valentijn, A.R.P.M.; Löwik, M.J.G.; Meer, D.M.A.B.-V.D.; Vloon, A.P.G.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 Oncoproteins for Vulvar Intraepithelial Neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef]
- Kenter, G.G.; Welters, M.J.; Valentijn, A.P.; Der Meer, D.M.B.-V.; Vloon, A.P.; Drijfhout, J.W.; Wafelman, A.R.; Oostendorp, J.; Fleuren, G.J.; Offringa, R.; et al. Phase I Immunotherapeutic Trial with Long Peptides Spanning the E6 and E7 Sequences of High-Risk Human Papillomavirus 16 in End-Stage Cervical Cancer Patients Shows Low Toxicity and Robust Immunogenicity. Clin. Cancer Res. 2008, 14, 169–177. [Google Scholar] [CrossRef] [PubMed]
- I E Van Poelgeest, M.; Welters, M.J.P.; Van Esch, E.M.G.; Stynenbosch, L.F.M.; Kerpershoek, G.; Meerten, E.L.V.P.V.; Hende, M.V.D.; Löwik, M.J.G.; Meer, D.M.A.B.-V.D.; Fathers, L.M.; et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Herrin, V.E.; Ibrahim, R.A.; Toubaji, A.; Bernstein, S.; Dakheel, O.; Steinberg, S.M.; Abu-Eid, R.; Mkrtichyan, M.; Berzofsky, J.A.; et al. Pre-immature dendritic cells (PIDC) pulsed with HPV16 E6 or E7 peptide are capable of eliciting specific immune response in patients with advanced cervical cancer. J. Transl. Med. 2014, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, L.; Xu, H.; Yang, Y.; Zhang, L.; Zhang, F. Effectiveness of immune therapy combined with chemotherapy on the immune function and recurrence rate of cervical cancer. Exp. Ther. Med. 2015, 9, 1063–1067. [Google Scholar] [CrossRef]
- Chang, E.Y.; Chen, C.-H.; Ji, H.; Wang, T.-L.; Hung, K.; Lee, B.P.; Huang, A.Y.C.; Kurman, R.J.; Pardoll, D.M.; Wu, T.-C. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. Int. J. Cancer 2000, 86, 725–730. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete Regression of Metastatic Cervical Cancer After Treatment With Human Papillomavirus–Targeted Tumor-Infiltrating T Cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef]
- Caballero, O.L.; Chen, Y.-T. Cancer/testis (CT) antigens: Potential targets for immunotherapy. Cancer Sci. 2009, 100, 2014–2021. [Google Scholar] [CrossRef]
- Parmiani, G.; De Filippo, A.; Novellino, L.; Castelli, C. Unique human tumor antigens: Immunobiology and use in clinical trials. J. Immunol. 2007, 178, 1975–1979. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 1–24. [Google Scholar] [CrossRef]
- Brun, J.; Rajaonarison, J.; Nocart, N.; Hoarau, L.; Brun, S.; Garrigue, I. Targeted immunotherapy of high-grade cervical intra-epithelial neoplasia: Expectations from clinical trials (Review). Mol. Clin. Oncol. 2017, 8, 227–235. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Kim, T.J.; Jin, H.-T.; Hur, S.-Y.; Yang, H.G.; Seon, J.M.; Hong, S.R.; Lee, C.-W.; Kim, S.; Chang-Woo, L.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hur, S.Y.; Kim, T.-J.; Hong, S.R.; Lee, J.-K.; Cho, C.-H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia. Clin. Cancer Res. 2019, 26, 1616–1623. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; A Welsh, F.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Hung, C.-F.; Juang, J.; He, L.; Hardwick, J.M.; Wu, T.-C. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene Ther. 2004, 11, 336–342. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nat. Cell Biol. 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Kübler, H.; Scheel, B.; Gnad-Vogt, U.; Miller, K.; Schultze-Seemann, W.; Dorp, F.V.; Parmiani, G.; Hampel, C.; Wedel, S.; Trojan, L.; et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: A first-in-man phase I/IIa study. J. Immunother. Cancer 2015, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Papachristofilou, A.; Hipp, M.M.; Klinkhardt, U.; Früh, M.; Sebastian, M.; Weiss, C.; Pless, M.; Cathomas, R.; Hilbe, W.; Pall, G.; et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Vaccine designers take first shots at COVID. Scienceence 2020, 368, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunology 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Pradere, J.-P.; Dapito, D.H.; Schwabe, R.F. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2013, 33, 3485–3495. [Google Scholar] [CrossRef]
- Parmiani, G.; Castelli, C.; Pilla, L.; Santinami, M.; Colombo, M.P.; Rivoltini, L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann. Oncol. 2007, 18, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological Properties and Clinical Application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.L.; A Westwood, J.; Darcy, P.K.; Sharkey, J.; Tsuji, M.; Franck, R.W.; Porcelli, S.A.; Besra, G.S.; Takeda, K.; Yagita, H.; et al. Combined Natural Killer T-Cell Based Immunotherapy Eradicates Established Tumors in Mice. Cancer Res. 2007, 67, 7495–7504. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Dai, Z.; Jaffarzad, N.; Ye, Y.; A Medina, M.; Huang, X.-F.; Dorta-Estremera, S.M.; Greeley, N.R.; Nitti, G.; Peng, W.; et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013, 19, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Landsberg, J.; Lopez-Ramos, D.; Renn, M.; Glodde, N.; Jansen, P.; Gaffal, E.; Steitz, J.; Tolba, R.H.; Kalinke, U.; et al. Immune Cell-Poor Melanomas Benefit from PD-1 Blockade after Targeted Type I IFN Activation. Cancer Discov. 2014, 4, 674–687. [Google Scholar] [CrossRef]
- Dubensky, J.T.W.; Kanne, D.B.; Leong, M.L. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines 2013, 1, 131–143. [Google Scholar] [CrossRef]
- Barber, G.N. Cytoplasmic DNA innate immune pathways. Immunol. Rev. 2011, 243, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Kanne, D.B.; Leong, M.; Glickman, L.H.; McWhirter, S.M.; Lemmens, E.; Mechette, K.; Leong, J.J.; Lauer, P.; Liu, W.; et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015, 7, 283ra52. [Google Scholar] [CrossRef]
- Pilla, L.; Rivoltini, L.; Patuzzo, R.I.; Marrari, A.; Valdagni, R.; Parmiani, G. Multipeptide vaccination in cancer patients. Expert Opin. Biol. Ther. 2009, 9, 1043–1055. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Ruby, C.; Hughes, T.; SlingluffJr, C.L. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.; Wang, F.; Kuniyoshi, J.; Rubio, V.; Stuge, T.B.; Groshen, S.; Gee, C.; Lau, R.; Jeffery, G.; Margolin, K.; et al. Effects of Interleukin-12 on the Immune Response to a Multipeptide Vaccine for Resected Metastatic Melanoma. J. Clin. Oncol. 2001, 19, 3836–3847. [Google Scholar] [CrossRef]
- Barrios, K.; Celis, E. TriVax-HPV: An improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol. Immunother. 2012, 61, 1307–1317. [Google Scholar] [CrossRef]
- Massa, S.; Franconi, R.; Brandi, R.; Muller, A.; Mett, V.; Yusibov, V.; Venuti, A. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine 2007, 25, 3018–3021. [Google Scholar] [CrossRef]
- Venuti, A.; Massa, S.; Mett, V.; Vedova, L.D.; Paolini, F.; Franconi, R.; Yusibov, V. An E7-based therapeutic vaccine protects mice against HPV16 associated cancer. Vaccine 2009, 27, 3395–3397. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Paolini, F.; Marino, C.; Franconi, R.; Venuti, A. Bioproduction of a Therapeutic Vaccine Against Human Papillomavirus in Tomato Hairy Root Cultures. Front. Plant Sci. 2019, 10, 452. [Google Scholar] [CrossRef]
- Whitehead, M.; Öhlschläger, P.; Almajhdi, F.N.; Alloza, L.; Marzábal, P.; E Meyers, A.; Hitzeroth, I.; Rybicki, E.P. Human papillomavirus (HPV) type 16 E7 protein bodies cause tumour regression in mice. BMC Cancer 2014, 14, 367. [Google Scholar] [CrossRef]
- Brower, V. Back to Nature: Extinction of Medicinal Plants Threatens Drug Discovery. J. Natl. Cancer Inst. 2008, 100, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Royston, K.J.; Tollefsbol, T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Azqueta, A.; Langie, S. Effects of micronutrients on DNA repair. Eur. J. Nutr. 2012, 51, 261–279. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid Redox Sign. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Sgambato, A.; Zannoni, G.F.; Faraglia, B.; Camerini, A.; Tarquini, E.; Spada, D.; Cittadini, A. Decreased expression of the CDK inhibitor p27Kip1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol. Oncol. 2004, 92, 776–783. [Google Scholar] [CrossRef]
- Looi, M.L.; Dali, A.Z.H.M.; Ali, S.A.M.; Ngah, W.Z.W.; Yusof, Y.A.M. Oxidative damage and antioxidant status in patients with cervical intraepithelial neoplasia and carcinoma of the cervix. Eur. J. Cancer Prev. 2008, 17, 555–560. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.W.; Ko, Y.S.; Koo, J.E.; Chung, H.Y.; Lee-Kim, Y.C. Changes in Lipid Peroxidation and Antioxidant Trace Elements in Serum of Women with Cervical Intraepithelial Neoplasia and Invasive Cancer. Nutr. Cancer 2003, 47, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Španinger, E.; Hrnčič, M.K.; Škerget, M.; Knez, Željko; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the Propagation in Vitro Of Poliomyelitis Viruses. J. Exp. Med. 1953, 97, 695–710. [Google Scholar] [CrossRef]
- Gey, G.O. Tissue Culture Studies of the Proliferative Capacity of Cervical Carcinoma and Normal Epithelium. Cancer Res. 1952, 12, 264–265. [Google Scholar]
- Wang, S.; Zheng, C.-J.; Peng, C.; Zhang, H.; Jiang, Y.-P.; Han, T.; Qin, L.-P. Plants and cervical cancer: An overview. Expert Opin. Investig. Drugs 2013, 22, 1133–1156. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, L.; Qiu, X.; Zhang, N.; Guo, Q.; Wang, Y.; Wang, M.; Gober, H.-J.; Li, D.; Wang, L. Traditional Chinese medicine for human papillomavirus (HPV) infections: A systematic review. Biosci. Trends 2017, 11, 267–273. [Google Scholar] [CrossRef]
- Song, Q.I.N.; Li-qin, J.I.N. The Studies of Cyanidin 3-Glucoside-Induced Apoptosis in Human Cervical Cancer Hela Cells and its Mechanism. Chin. J. Biochem. Pharm. 2008, 6, 369–373. [Google Scholar]
- Ahn, W.S.; Huh, S.W.; Bae, S.-M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.-I. A Major Constituent of Green Tea, EGCG, Inhibits the Growth of a Human Cervical Cancer Cell Line, CaSki Cells, through Apoptosis, G1 Arrest, and Regulation of Gene Expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef]
- Sharma, C.; Nusri, Q.E.-A.; Begum, S.; Javed, E.; Rizvi, T.A.; Hussain, A. (-)-Epigallocatechin-3-Gallate Induces Apoptosis and Inhibits Invasion and Migration of Human Cervical Cancer Cells. Asian Pac. J. Cancer Prev. 2012, 13, 4815–4822. [Google Scholar] [CrossRef]
- Qiao, Y.; Cao, J.; Xie, L.; Shi, X. Cell growth inhibition and gene expression regulation by (-)-epigallocatechin-3-gallate in human cervical cancer cells. Arch. Pharmacal Res. 2009, 32, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.-H.S.; Garcia, F. Green Tea Compound in Chemoprevention of Cervical Cancer. Int. J. Gynecol. Cancer 2010, 20, 617–624. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao, W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp. Ther. Med. 2018, 17, 1742–1748. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.-Y.; Zhang, Z.-F.; Rao, J.; Le, A.D. Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1α protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol. Cancer Ther. 2006, 5, 1227–1238. [Google Scholar] [CrossRef]
- Ramesh, E.; Alshatwi, A.A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem. Toxicol. 2013, 51, 97–105. [Google Scholar] [CrossRef]
- Kim, N.-I.; Lee, S.-J.; Lee, S.-B.; Park, K.; Kim, W.-J.; Moon, S.-K. Requirement for Ras/Raf/ERK pathway in naringin-induced G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis 2008, 29, 1701–1709. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Sulfikkarali, N.; Rajendraprasad, N.; Karthikeyan, S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Biomed. Prev. Nutr. 2011, 1, 223–231. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2012, 27, 581–592. [Google Scholar] [CrossRef]
- Oh, E.K.; Kim, H.J.; Bae, S.M.; Park, M.Y.; Kim, Y.W.; Kim, T.E.; Ahn, W.S. Apigenin-Induced Apoptosis in Cervical Cancer Cell Lines. Korean J. Obstet. Gynecol. 2008, 51, 874–881. [Google Scholar]
- Zheng, P.-W.; Chiang, L.-C.; Lin, C.-C. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005, 76, 1367–1379. [Google Scholar] [CrossRef]
- Czyż, J.; Madeja, Z.; Irmer, U.; Korohoda, W.; Hülser, D.F. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cellsin vitro. Int. J. Cancer 2004, 114, 12–18. [Google Scholar] [CrossRef]
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Liu, D.H.; Zhang, S. Effect of Daidzein on Cell Growth, Cell Cycle, and Telomerase Activity of Human Cervical Cancer in Vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar] [CrossRef]
- Lee, H.G.; Yu, K.A.; Oh, W.K.; Baeg, T.W.; Oh, H.C.; Ahn, J.S.; Jang, W.C.; Kim, J.W.; Lim, J.S.; Choe, Y.K.; et al. Inhibitory Effect of Jaceosidin Isolated from Artemisiaargyi on the Function of E6 and E7 Oncoproteins of HPV. J. Ethnopharmacol. 2005, 98, 339–343. [Google Scholar] [CrossRef]
- Cherry, J.J.; Rietz, A.; Malinkevich, A.; Liu, Y.; Xie, M.; Bartolowits, M.; Davisson, V.J.; Baleja, J.D.; Androphy, E.J. Structure Based Identification and Characterization of Flavonoids That Disrupt Human Papillomavirus-16 E6 Function. PLoS ONE 2013, 8, e84506. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Zheng, X.; Sun, H.; Zhou, Y.; Li, D.; Lin-Bo, G.; Wang, X. Luteolin enhances TNF-related apoptosis-inducing ligand’s anticancer activity in a lung cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 417, 842–846. [Google Scholar] [CrossRef]
- Kim, M.S.; Bak, Y.; Park, Y.S.; Lee, D.-H.; Kim, J.H.; Kang, J.W.; Song, H.-H.; Oh, S.-R.; Yoon, D.Y. Wogonin induces apoptosis by suppressing E6 and E7 expressions and activating intrinsic signaling pathways in HPV-16 cervical cancer cells. Cell Biol. Toxicol. 2013, 29, 259–272. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Li, C.; Wu, H.-Z.; Liu, Y.-W. Kaempferol-7-O-β-d-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008, 264, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.-H.; Yang, S.-F.; Tsai, S.-J.; Hsieh, S.-C.; Huang, Y.-C.; Bau, D.-T.; Hsieh, Y.-H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2011, 86, 263–273. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-F.; Wu, C.-L.; Huang, S.-C.; Wu, C.-M.; Hsiao, J.-R.; Yo, Y.-T.; Chen, Y.-H.; Shiau, A.-L.; Chou, C.-Y. Cathepsin L mediates resveratrol-induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy 2009, 5, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sull, J.W.; Sung, H.J. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol. Biol. Rep. 2012, 39, 8709–8716. [Google Scholar] [CrossRef]
- García-Zepeda, S.P.; García-Villa, E.; Díaz-Chávez, J.; Hernández-Pando, R.; Gariglio, P. Resveratrol induces cell death in cervical cancer cells through apoptosis and autophagy. Eur. J. Cancer Prev. 2013, 22, 577–584. [Google Scholar] [CrossRef]
- Xin, S.; Shulan, L.; Jing, Z.; Shengnan, L.; Jiyong, G.; Cheng’En, P. Effects of Res on proliferation and apoptosis of human cervical carcinoma cell lines C33A, SiHa and HeLa. J. Med Coll. PLA 2009, 24, 148–154. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.; Jernas, K.; Król, W. Dietary Flavonoids Sensitize HeLa Cells to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Int. J. Mol. Sci. 2008, 9, 56–64. [Google Scholar] [CrossRef]
- Maher, D.M.; Bell, M.C.; O’Donnell, E.A.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Curcumin suppresses human papillomavirus oncoproteins, restores p53, rb, and ptpn13 proteins and inhibits benzo[a]pyrene-induced upregulation of HPV. Mol. Carcinog. 2010, 50, 47–57. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H.; Ahn, K.S.; Sethi, G.; Sandur, S.K.; Natarajan, C.; Seeram, N.; Shishodia, S. Turmeric the Genus Curcuma; CRC: New York, NY, USA, 2007; pp. 297–368. [Google Scholar]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2006, 296, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Divya, C.S.; Pillai, M.R. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol. Carcinog. 2006, 45, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol. Cell. Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Hassan, S.S.; Naidu, R. The Curcumin Analogue 1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one Induces Apoptosis and Downregulates E6 and E7 Oncogene Expression in HPV16 and HPV18-Infected Cervical Cancer Cells. Molecules 2015, 20, 11830–11860. [Google Scholar] [CrossRef]
- Giridharan, P.; Somasundaram, S.T.; Perumal, K.; A Vishwakarma, R.; Karthikeyan, N.P.; Velmurugan, R.; Balakrishnan, A. Novel substituted methylenedioxy lignan suppresses proliferation of cancer cells by inhibiting telomerase and activation of c-myc and caspases leading to apoptosis. Br. J. Cancer 2002, 87, 98–105. [Google Scholar] [CrossRef]
- Gao, P.; Zhai, F.; Guan, L.; Zheng, J. Nordihydroguaiaretic acid inhibits growth of cervical cancer SiHa cells by up-regulating p21. Oncol. Lett. 2010, 2, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer. Cancer Lett. 2015, 356, 536–546. [Google Scholar] [CrossRef]
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; A Husain, S.; Das, B.C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer 2011, 10, 39. [Google Scholar] [CrossRef]
- Saha, S.K.; Khuda-Bukhsh, A.R. Berberine alters epigenetic modifications, disrupts microtubule network, and modulates HPV-18 E6–E7 oncoproteins by targeting p53 in cervical cancer cell HeLa: A mechanistic study including molecular docking. Eur. J. Pharmacol. 2014, 744, 132–146. [Google Scholar] [CrossRef]
- Munagala, R.; Kausar, H.; Munjal, C.; Gupta, R.C. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 2011, 32, 1697–1705. [Google Scholar] [CrossRef]
- Yim, N.-H.; Lee, J.H.; Cho, W.-K.; Yang, M.C.; Kwak, D.H.; Ma, J.Y. Decursin and decursinol angelate from Angelica gigas Nakai induce apoptosis via induction of TRAIL expression on cervical cancer cells. Eur. J. Integr. Med. 2011, 3, e299–e307. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.-W.; Gao, D.-W.; Han, Z.-S.; Lu, W.-Z. Antitumor and immunomodulating effects of polysaccharides isolated fromSolanum nigrumLinne. Phytother. Res. 2009, 23, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, Y.; Jiang, Z.; Sun, Y.; Zhu, L.; Ding, Z. Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus. Phytomedicine 2009, 16, 586–593. [Google Scholar] [CrossRef]
- Mancinelli, L.; De Angelis, P.M.; Annulli, L.; Padovini, V.; Elgjo, K.; Gianfranceschi, G.L. A class of DNA-binding peptides from wheat bud causes growth inhibition, G2 cell cycle arrest and apoptosis induction in HeLa cells. Mol. Cancer 2009, 8, 55. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.-C. Trichosanthin-induced specific changes of cytoskeleton configuration were associated with the decreased expression level of actin and tubulin genes in apoptotic Hela cells. Life Sci. 2007, 81, 1130–1140. [Google Scholar] [CrossRef]
- Mahata, S.; Maru, S.; Shukla, S.; Pandey, A.; Mugesh, G.; Das, B.C.; Bharti, A.C. Anticancer property of Bryophyllum pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC Complement. Altern. Med. 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.; Pandey, A.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C.; Bharti, A.C. Anticancer Activity of Phyllanthus emblicaLinn. (Indian Gooseberry): Inhibition of Transcription Factor AP-1 and HPV Gene Expression in Cervical Cancer Cells. Nutr. Cancer 2013, 65, 88–97. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, X.-J.; Pan, L.-L.; Zhang, S.-H.; Zheng, F.-Y. Effects of Brucea javanica oil emulsion on human papilloma virus type 16 infected cells and mechanisms research. Chin. J. Integr. Tradit. West. Med. 2013, 33, 1545–1551. [Google Scholar]
- Li, G.; Jiang, W.; Xia, Q.; Chen, S.-H.; Ge, X.-R.; Gui, S.-Q.; Xu, C.-J. HPV E6 down-regulation and apoptosis induction of human cervical cancer cells by a novel lipid-soluble extract (PE) from Pinellia pedatisecta Schott in vitro. J. Ethnopharmacol. 2010, 132, 56–64. [Google Scholar] [CrossRef]
- Talwar, G.P.; Sharma, R.; Singh, S.; Das, B.C.; Bharti, A.C.; Sharma, K.; Singh, P.; Atrey, N.; Gupta, J.C. BASANT, a Polyherbal Safe Microbicide Eliminates HPV-16 in Women with Early Cervical Intraepithelial Lesions. J. Cancer Ther. 2015, 6, 1163–1166. [Google Scholar] [CrossRef]
- Kwon, S.-B.; Kim, M.-J.; Yang, J.M.; Lee, H.-P.; Hong, J.T.; Jeong, H.-S.; Kim, E.S.; Yoon, D.-Y. Cudrania tricuspidata Stem Extract Induces Apoptosis via the Extrinsic Pathway in SiHa Cervical Cancer Cells. PLoS ONE 2016, 11, e0150235. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, A.; Le Gresley, A.; Naughton, D.; Kuhnert, N.; Sirbu, D.; Ashrafi, G.H. Biological activities of Ficus carica latex for potential therapeutics in Human Papillomavirus (HPV) related cervical cancers. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Sánchez-Carballido, M.A.; Suárez, C.D.; Gómez-Mora, J.A.; Bonneau, N. Effects of flavonoids on tongue squamous cell carcinoma. Cell Biol. Int. 2020, 44, 686–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-N.; Kuo, W.-H.; Chiang, C.-L.; Chiou, H.-L.; Hsieh, Y.-S.; Chu, S.-C. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem. Interact. 2006, 163, 218–229. [Google Scholar] [CrossRef]
- Hausen, H.Z. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (−)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M. Curcumin as an Anti-Cancer Agent: Review of the Gap Between Basic and Clinical Applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef]
- Talwar, G.P.; Dar, S.A.; Rai, M.; Reddy, K.; Mitra, D.; Kulkarni, S.V.; Doncel, G.F.; Buck, C.B.; Schiller, J.T.; Muralidhar, S.; et al. A novel polyherbal microbicide with inhibitory effect on bacterial, fungal and viral genital pathogens. Int. J. Antimicrob. Agents 2008, 32, 180–185. [Google Scholar] [CrossRef]
- Bava, S.V.; Sreekanth, C.N.; Thulasidasan, A.K.T.; Anto, N.P.; Cheriyan, V.T.; Puliyappadamba, V.T.; Menon, S.G.; Ravichandran, S.D.; Anto, R.J. Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. Int. J. Biochem. Cell Biol. 2011, 43, 331–341. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Paduch, R.; Piersiak, T.; Głowniak, K.; Gawron, A.; Kandefer-Szerszeń, M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem. Pharmacol. 2005, 69, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, X.-L.; Yang, L.; Shi, F.; Gao, L.-B.; Zhong, Y.-J.; Sun, H.; He, F.; Lin-Bo, G.; Wang, X. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 159. [Google Scholar] [CrossRef]
- He, F.; Wang, Q.; Zheng, X.-L.; Yan, J.-Q.; Yang, L.; Sun, H.; Hu, L.-N.; Lin, Y.; Wang, X. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol. Rep. 2012, 28, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic Effects of Apigenin and Paclitaxel on Apoptosis of Cancer Cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-L.; Wang, W. Formononetin potentiates epirubicin-induced apoptosis via ROS production in HeLa cells in vitro. Chem. Interact. 2013, 205, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Periasamy, V.S.; Athinarayanan, J.; Elango, R. Synergistic anticancer activity of dietary tea polyphenols and bleomycin hydrochloride in human cervical cancer cell: Caspase-dependent and independent apoptotic pathways. Chem. Interact. 2016, 247, 1–10. [Google Scholar] [CrossRef]
- Zoberi, I.; Bradbury, C.; Curry, H.A.; Bisht, K.S.; Goswami, P.C.; Roti, J.L.R.; Gius, D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 2002, 175, 165–173. [Google Scholar] [CrossRef]
- Yashar, C.M.; Spanos, W.J.; Taylor, U.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.-Y.; Pan, J.; Han, S.-P.; Yin, X.-X.; Wang, B.; Hu, G. Combined treatment of ionizing radiation with genistein on cervical cancer HeLa cells. J. Pharmacol. Sci. 2006, 102, 129–135. [Google Scholar] [CrossRef]
- Shin, J.-I.; Shim, J.-H.; Kim, K.-H.; Choi, H.-S.; Kim, J.-W.; Lee, H.G.; Kim, B.Y.; Park, S.-N.; Park, O.-J.; Yoon, D.-Y. Sensitization of the apoptotic effect of gamma-irradiation in genistein-pretreated CaSki cervical cancer cells. J. Microbiol. Biotechnol. 2008, 18, 523–5317183. [Google Scholar]
- Javvadi, P.; Hertan, L.; Kosoff, R.; Datta, T.; Kolev, J.; Mick, R.; Tuttle, S.W.; Koumenis, C. Thioredoxin Reductase-1 Mediates Curcumin-Induced Radiosensitization of Squamous Carcinoma Cells. Cancer Res. 2010, 70, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Kanimozhi, G.; Prasad, N.R.; Mahalakshmi, R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol. Vitr. 2011, 25, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yu, Y.; Zhao, H.-G.; Yang, A.; Yan, H.; Cui, Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012, 104, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2004, 113, 951–960. [Google Scholar] [CrossRef]
- Hillman, G.G.; Forman, J.D.; Kucuk, O.; Yudelev, M.; Maughan, R.L.; Rubio, J.; Layer, A.; Tekyi-Mensah, S.; Abrams, J.; Sarkar, F.H. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin. Cancer Res. 2001, 7, 382–390. [Google Scholar]
- Ahn, W.-S.; Yoo, J.; Huh, S.-W.; Kim, C.K.; Lee, J.-M.; Namkoong, S.-E.; Bae, S.-M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Shukla, S.; Bharti, A.C.; Hussain, S.; Mahata, S.; Hedau, S.; Kailash, U.; Kashyap, V.; Bhambhani, S.; Roy, M.; Batra, S.; et al. Elimination of high-risk human papillomavirus type HPV16 infection by ‘Praneem’ polyherbal tablet in women with early cervical intraepithelial lesions. J. Cancer Res. Clin. 2009, 135, 1701–1709. [Google Scholar] [CrossRef]
- Basu, P.; Dutta, S.; Begum, R.; Mittal, S.; Das Dutta, P.; Bharti, A.C.; Panda, C.K.; Biswas, J.; Dey, B.; Talwar, G.P.; et al. Clearance of cervical human papillomavirus infection by topical application of curcumin and curcumin containing polyherbal cream: A phase II randomized controlled study. Asian Pac. J. Cancer Prev. 2013, 14, 5753–5759. [Google Scholar] [CrossRef]
- Talwar, G.; Raghuvanshi, P.; Mishra, R.; Banerjee, U.; Rattan, A.; Whaley, K.J.; Achilles, S.L.; Zeitlin, L.; Barré-Sinoussi, F.; David, A.; et al. Polyherbal Formulations with Wide Spectrum Antimicrobial Activity Against Reproductive Tract Infections and Sexually Transmitted Pathogens. Am. J. Reprod. Immunol. 2000, 43, 144–151. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Yao, S.; Li, G.; Xu, C.; Ye, Y.; Gui, S. A lipid-soluble extract of Pinellia pedatisecta Schott enhances antitumor T cell responses by restoring tumor-associated dendritic cell activation and maturation. J. Ethnopharmacol. 2019, 241, 111980. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing Delivery of Plant Based Anti-cancer Drugs. Front. Pharmacol. 2018, 8, 1002. [Google Scholar] [CrossRef] [PubMed]
- Gerloni, M.; Xiong, S.; Mukerjee, S.; Schoenberger, S.P.; Croft, M.; Zanetti, M. Functional cooperation between T helper cell determinants. Proc. Natl. Acad. Sci. USA 2000, 97, 13269–13274. [Google Scholar] [CrossRef] [PubMed]
- Savelyeva, N.; Zhu, D.; Stevenson, F.K. Engineering DNA vaccines that include plant virus coat proteins. Biotechnol. Genet. Eng. Rev. 2003, 20, 101–116. [Google Scholar] [CrossRef]
- Tan, B.K.H.; Vanitha, J. Immunomodulatory and antimicrobial effects of some traditional chinese medicinal herbs: A review. Curr. Med. Chem. 2004, 11, 1423–1430. [Google Scholar] [CrossRef]
- Ronald, P.C.; Beutler, B. Plant and Animal Sensors of Conserved Microbial Signatures. Science 2010, 330, 1061–1064. [Google Scholar] [CrossRef]
- Girbes, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, Distribution, Activity and Phylogenetic Relationship of Ribosome-Inactivating Proteins in Plants, Fungi and Bacteria. Mini-Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef]
- Fang, E.F.; Ng, T.B.; Shaw, P.C.; Wong, R.N.-S. Recent Progress in Medicinal Investigations on Trichosanthin and other Ribosome Inactivating Proteins from the Plant Genus Trichosanthes. Curr. Med. Chem. 2011, 18, 4410–4417. [Google Scholar] [CrossRef]
- Hajto, T.; Hostanska, K.; Weber, K.; Zinke, H.; Fischer, J.; Mengs, U.; Lentzen, H.; Saller, R. Effect of a recombinant lectin, Viscum album agglutinin on the secretion of interleukin-12 in cultured human peripheral blood mononuclear cells and on NK-cell-mediated cytotoxicity of rat splenocytes in vitro and in vivo. Nat. Immun. 1998, 16, 34–46. [Google Scholar] [CrossRef]
- Hajto, T.; Hostanska, K.; Frei, K.; Rordorf, C.; Gabius, H.J. Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res. 1990, 50, 3322–3326. [Google Scholar]
- Bhutia, S.K.; Mallick, S.K.; Maiti, T.K. In vitro immunostimulatory properties of Abrus lectins derived peptides in tumor bearing mice. Phytomedicine 2009, 16, 776–782. [Google Scholar] [CrossRef]
- Zhao, J.; Ben, L.-H.; Wu, Y.-L.; Hu, W.; Ling, K.; Xin, S.-M.; Nie, H.-L.; Ma, L.; Pei, G. Anti-HIV Agent Trichosanthin Enhances the Capabilities of Chemokines to Stimulate Chemotaxis and G Protein Activation, and This Is Mediated through Interaction of Trichosanthin and Chemokine Receptors. J. Exp. Med. 1999, 190, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sikriwal, D.; Ghosh, P.; Batra, J.K. Ribosome inactivating protein saporin induces apoptosis through mitochondrial cascade, independent of translation inhibition. Int. J. Biochem. Cell Biol. 2008, 40, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.; Lord, J. Cytotoxic ribosome-inactivating lectins from plants. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2004, 1701, 1–14. [Google Scholar] [CrossRef]
- Chandra, J.; Dutton, J.L.; Li, B.; Woo, W.-P.; Xu, Y.; Tolley, L.K.; Yong, M.; Wells, J.W.; Leggatt, G.R.; Finlayson, N.; et al. DNA Vaccine Encoding HPV16 Oncogenes E6 and E7 Induces Potent Cell-mediated and Humoral Immunity Which Protects in Tumor Challenge and Drives E7-expressing Skin Graft Rejection. J. Immunother. 2017, 40, 62–70. [Google Scholar] [CrossRef]
- Fonseca, J.A.; McCaffery, J.N.; Cáceres, J.; Kashentseva, E.; Singh, B.; Dmitriev, I.P.; Curiel, D.T.; Moreno, A. Inclusion of the murine IgGκ signal peptide increases the cellular immunogenicity of a simian adenoviral vectored Plasmodium vivax multistage vaccine. Vaccine 2018, 36, 2799–2808. [Google Scholar] [CrossRef]
- Yolitz, J.; Schwing, C.; Chang, J.; Van Ryk, D.; Nawaz, F.; Wei, D.; Cicala, C.; Arthos, J.; Fauci, A.S. Signal peptide of HIV envelope protein impacts glycosylation and antigenicity of gp. Proc. Natl. Acad. Sci. USA 2018, 115, 2443–2448. [Google Scholar] [CrossRef]
- E Broderick, K.; Humeau, L.M. Enhanced Delivery of DNA or RNA Vaccines by Electroporation. Recent Res. Cancer 2016, 1499, 193–200. [Google Scholar] [CrossRef]
- Paolini, F.; Massa, S.; Manni, I.; Franconi, R.; Venuti, A. Immunotherapy in new pre-clinical models of HPV-associated oral cancers. Hum. Vaccines Immunother. 2013, 9, 534–543. [Google Scholar] [CrossRef]
- Venuti, A.; Curzio, G.; Mariani, L.; Paolini, F. Immunotherapy of HPV-associated cancer: DNA/plant-derived vaccines and new orthotopic mouse models. Cancer Immunol. Immunother. 2015, 64, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.M.; Liwski, R.S.; Mansour, M. Immune Modulation by Chemotherapy or Immunotherapy to Enhance Cancer Vaccines. Cancers 2011, 3, 3114–3142. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Adila, A.; Alimu, A.; Chen, Q.; Li, Y.; Li, J.; Fisch, L. Glycyrrhiza Uralensis Crude Polysaccharides Enhance Mouse Immunity and Immune Responses Induced by HPV-DNA Vaccine. Chinese J. Microbiol. Immunol. 2018, 38, 774–781. [Google Scholar]
- Tseng, C.-W.; Hung, C.-F.; Alvarez, R.D.; Trimble, C.; Huh, W.K.; Kim, D.; Chuang, C.-M.; Lin, C.-T.; Tsai, Y.-C.; He, L.; et al. Pretreatment with Cisplatin Enhances E7-Specific CD8+ T-Cell-Mediated Antitumor Immunity Induced by DNA Vaccination. Clin. Cancer Res. 2008, 14, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Meir, H.; Kenter, G.; Burggraaf, J.; Kroep, J.R.; Welters, M.; Melief, C.; Burg, S.; Poelgeest, M. The Need for Improvement of the Treatment of Advanced and Metastatic Cervical Cancer, the Rationale for Combined Chemo-Immunotherapy. Anti-Cancer Agents Med. Chem. 2014, 14, 190–203. [Google Scholar] [CrossRef]
- Cordeiro, M.N.; Paolini, F.; Massa, S.; Curzio, G.; Illiano, E.; Silva, A.J.D.; Franconi, R.; Bissa, M.; Morghen, C.D.G.; De Freitas, A.C.; et al. Anti-tumor effects of genetic vaccines against HPV major oncogenes. Hum. Vaccines Immunother. 2014, 11, 45–52. [Google Scholar] [CrossRef]
- Kanerva, A.; Raki, M.; Ranki, T.; Särkioja, M.; Koponen, J.; Desmond, R.A.; Helin, A.; Stenman, U.-H.; Isoniemi, H.; Höckerstedt, K.; et al. Chlorpromazine and apigenin reduce adenovirus replication and decrease replication associated toxicity. J. Gene Med. 2007, 9, 3–9. [Google Scholar] [CrossRef]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
| Compound Type | Phytochemicals | Study Type | Cell Type | Activity | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Purified | Black rice anthocyanin and cyanidin 3-glucoside | In vitro | HeLa | Inhibition of proliferation and induction of apoptosis | Dose- and time-dependent by apoptosis induction through Bax/Bcl-2 | [94] |
| Polyphenols (Flavonoids, Anthocyanins) | ||||||
| Purified Polyphenols (Flavonoids, Cathechins) | Epigallocatechin-3-gallate (EGCG) | In vitro and in vivo | CaSki | Inhibition of proliferation and induction of apoptosis | Dose-dependent apoptosis induction through arrest of cell cycle in the G1 phase. | [95] |
| Possible gene regulatory role | ||||||
| In vitro | HeLa | Inhibition of proliferation and induction of apoptosis | Inhibition of HPV E6/E7 expression and of estrogen receptor α and aromatase by a time-dependent manner mediated by apoptosis | [96,97] | ||
| In vitro | TCL-1 (HPV-immortalized cervical epithelial cells) | Inhibition of proliferation (adenocarcinoma less responsive to EGCG growth inhibition) and apoptosis induction | Dose-dependent increased expression of p53 and p21 | [98] | ||
| Me18 | ||||||
| HeLa | ||||||
| In vitro | CaSki | Suppression of growth | Time- and dose- dependent, possibly via regulating the expression of miRNAs | [99] | ||
| HeLa | ||||||
| C33A | ||||||
| In vitro | HeLa | Repression of hypoxia- and serum-induced HIF-1α and VEGF | Via MAPK and PI3K/AKT pathways | [100] | ||
| Purified Polyphenols (Flavonoids, Flavanones) | Naringenin | In vitro | SiHa | Inhibition of proliferation and induction of apoptosis | Cell cycle arrest at the G2/M phase and induction of apoptosis | [101] |
| In vitro | HeLa | Inhibition of proliferation and induction of apoptosis | Reduced expression of NF-κB p65 subunit, COX-2, and caspase-1 | [102] | ||
| Naringenin-loaded nanoparticles | In vitro | HeLa | Inhibition of proliferation and induction of apoptosis and cytotoxicity | Dose-dependent cytotoxicity, apoptosis, reduction of intracellular glutathione levels, alterations in mitochondrial membrane potential, increased intracellular ROS and lipid peroxidation. | [103] | |
| Hesperetin | In vitro | SiHa | Reduction in cell viability and induction of apoptosis | Increased expression of caspases, p53, Bax, and Fas death receptor and its adaptor protein | [104] | |
| Purified Polyphenols (Flavonoids, Flavones) | Apigenin | In vitro | CaSki HeLa C33A | Inhibition of proliferation and induction of apoptosis | G1 phase growth arrest through p53-dependent apoptosis and increased expression of p21/WAF1, Fas/APO-1 and caspase-3. | [105] |
| Decreased expression of Bcl-2 | ||||||
| In vitro | HeLa | Modifications in cell motility, inhibition of translocation | Interference with gap junctions | [106,107] | ||
| Daidzein | In vitro | HeLa | Inhibition of proliferation | Cell cycle, cell growth, and telomerase activity alterations | [108] | |
| Jaceosidin | In vitro | SiHa Caski | Inhibition of the function of E6 and E7 oncogenes | Impairment of binding to p53 and pRb | [109] | |
| Luteolin | In vitro | Caski E6/E7 immortalized human foreskin keratinocytes (HFK) primary HFKs | E6 inhibition | Binding at the interface between E6 and E6AP mimicking leucines in the conserved α-helical motif of E6AP | [110] | |
| In vivo | HeLa | Induction of apoptosis | TRAIL-induced apoptosis by both extrinsic and intrinsic apoptotic pathways | [111] | ||
| Wogonin | In vitro | SiHa CasKi | Induction of apoptosis | Suppression of E6 and E7 and increase in p53 and pRb | [112] | |
| Purified Polyphenols (Flavonoids, Flavonols) | Quercetin | In vitro | HeLa | Induction of apoptosis Induction of mitochondrial apoptosis | G2/M phase cell cycle arrest, apoptosis, inhibition of anti-apoptotic AKT and Bcl-2 expression | [113] |
| Kaempferol | In vitro | HeLa | Inhibition of proliferation | G2/M phase growth arrest, decrease of cyclin B1 and CDK1, inhibition of NF-kB nuclear translocation, upregulation of Bax and downregulation of Bcl-2 | [114] | |
| Fisetin | In vivo/in vitro | HeLa | Inhibition of proliferation and reduction of tumor growth by apoptosis | Apoptosis due to activation of the phosphorylation ERK1/2, inhibition of ERK1/2 by PD98059, activation of caspase-8/caspase-3 pathway | [115] | |
| Purified Polyphenols (Flavonoids, Phenolic Acids) | Gallic acid | In vitro | HeLa | Induction of apoptosis and/or necrosis | ROS increase and GSH depletion | [116] |
| Purified Polyphenols (Flavonoids, Stilbenes) | Resveratrol | In vitro | SiHa HeLa | Inhibition of proliferation, induction of autophagy and apoptosis | Cathepsin L-mediated mechanism | [117] |
| In vitro | HeLa | Suppression of invasion and migration | Generation of ROS | [118] | ||
| In vitro | SiHa HeLa CaSki C33A | Decrease in the angiogenic activity, induction of autophagy | Decreased expression of metalloproteinases. Inhibition of AKT and ERK1/2, destabilization of lysosomes, increased cytosol translocation | [119] | ||
| In vitro | SiHa HeLa C33A | Inhibition of proliferation | Induction of cell apoptosis | [120] | ||
| In vitro | HeLa | Inhibition of proliferation | Sensitization to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) | [121] | ||
| Purified Polyphenols (Curcuminoids) | Curcumin (diferuloylmethane) | In vitro | HeLa SiHa C33A | Inhibition of proliferation Induction of apoptosis | Down-regulation of HPV-18 transcription, inhibition of AP-1 binding activity and reversion of the expression of c-fos and fra-1 | [122] |
| Downregulation of viral oncogenes E6 and E7, NF-kB and AP-1 COX-2, iNOS and cyclin D1 | [123,124,125] | |||||
| Upregulation of Bax, AIF, release of cytochrome c and downregulation of Bcl-2, Bcl-XL, COX-2, iNOS and cyclin D1 | [126] | |||||
| MS17 curcumin analogue 1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one | In vitro | HeLa CaSki | Cytotoxic, anti-proliferative and apoptosis-inducing potential. | Apoptosis through activation of caspase-3 in CaSki cells. Down-regulation of HPV18 and HPV16 E6 and E7 oncogene expression. | [127] | |
| Purified Polyphenols (Lignans) | Methylenedioxy lignan | In vitro | HeLa | Inhibition of proliferation and apoptosis | Apoptosis and inhibition of telomerase activity | [128] |
| Nor-dihydro-guaiaretic acid | In vitro | SiHa | Promotion of apoptosis | Downregulation of HPV E6 and E7 transcription and expression | [129] | |
| Purified Diterpenoids | Tanshinone IIA | In vitro | HeLa SiHa CasKi C33A | Inhibition of growth promotion of apoptosis | Downregulation of HPV E6 and E7 expression, cell cycle arrest | [130] |
| Purified Alkaloids | Berberine | In vitro | HeLa | Inhibition of growth | Reduced expression of E6 and E7 with increase in p53 and pRb expression, loss of telomerase protein, hTERT | [131] |
| SiHa | Alter epigenetic modifications and disrupt microtubule network by targeting p53 | [132] | ||||
| Purified Steroid Lactones | Withaferin A | In vitro | CasKi | Promotion of apoptosis | E6 and E7 repression | [133] |
| Purified Pyranocoumarin compounds | Decursin Decursinol | In vitro | Hela | Promotion of apoptosis | Induction of TRAIL expression | [134] |
| Polysaccharides fractions | From Solanum nigrum | In vitro and in vivo | U14 | Promotion of apoptosis and inhibition of tumor growth | CD4+/CD8+ ratio modification | [135] |
| Lectins | From Astragalus mongholicus | In vitro | HeLa | Promotion of apoptosis and Antiproliferation | Upregulation of p21 and p27 and reduction of active complex cyclin E/CDK2 kinase | [136] |
| Peptide fractions | From Triticum aestivum | In vitro | HeLa | Antiproliferation | Induction of DNA damage and G2 arrest, inactivation of the CDK1-cyclin B1 complex and increase of active chk1 kinase expression | [137] |
| From Abrus precatorius | Induction of apoptosis; generation of ROS, decrease of Bcl-2/Bax ratio, induction of mitochondrial permeability transition | [138] | ||||
| Extracts | Fractionated extract of Bryophyllum pinnata (Bryophyllin A) Crude extract of Phyllanthus emblica fruits and Brucea javanica oil emulsion | In vitro | Hela SiHa Casky | Anti-HPV activities | Inhibitory action on AP-1 and STAT3 and specific downregulation of expression of viral oncogenes E6 and E7 | [139,140,141] |
| Lipid-soluble Rhizome extract of Pinellia pedatisecta | In vitro | Caski HeLa HBL-100 | Promotion of apoptosis | Increased expression of Caspase-8, Caspase-3, Bax, p53, p21 | [142] | |
| Basant (curcumin, purified saponins, extracts of Emblica officinalis, Mentha citrata oil, and gel extracts of Aloe vera) | In vitro | HeLa | Anti-HPV activities | Inhibition of transduction of HPV16 pseudovirus | [143] | |
| Cudrania tricuspidata stem extract | In vitro | SiHa HaCaT human normal keratinocytes | Apoptosis induction and cytotoxic effects in cervical cancer cells with no cytotoxic effect on HaCaT keratinocytes at concentrations of 0.125–0.5 mg/mL. | Dose-dependent mechanism by down-regulation of the E6 and E7 viral oncogenes. Apoptosis induction exclusively based on the increase of mRNA expression of extrinsic factors (i.e., Fas, death receptor 5 and TRAIL) and on activation of caspase-3/caspase-8 and cleavage of poly (ADP-ribose) polymerase (PARP) | [144] | |
| Ficus carica fruit latex | In vitro | CaSki HeLa | Inhibition of growth and invasion | Downregulation of the expression of p16 and HPV onco-proteins E6, E7 | [145] |
| Compound Type | Phytochemicals | Study Type | Cell Type | Activity | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Purified phytochemical with adjuvant activity in HPV chemo-therapies | Curcumin (diferuloylmethane) | In vitro | HeLa | Sensitization to cisplatin, paclitaxel | Induction of apoptosis by down-regulation of NF-kB. | [153] |
| Tetrahydrocurcuminoids | In vitro | Drug-resistant human cervical carcinoma cell line KB-31 and KB-V-1 | Sensitization to vinblastine, mitoxantrone, and etoposide | Down-regulation of HPV-18 transcription, inhibition of AP-1 binding activity, and reversion of the expression of c-fos and fra-1 | [124] | |
| Quercetin | In vitro | HeLa | Sensitization to cisplatin | Enhancement of cancer cells death levels. | [154] | |
| Saikasaponins | In vitro | HeLa SiHa | Sensitization to cisplatin | Reactive oxygen species generation | [155] | |
| Wogonin (5,7-dihydroxy-8-methoxyflavone) | In vitro | A549 HeLa | Sensitization to cisplatin | Reactive oxygen species generation | [156] | |
| Apigenin | In vitro | HeLa SiHa | Sensitization to paclitaxel | Apoptosis through intracellular ROS accumulation | [157] | |
| Formonetin | In vitro | HeLa | Sensitization to epirubicin | Potentiates epirubicin-induced apoptosis via ROS production. | [158] | |
| Tea polyphenols with EGCG | In vitro | SiHa | Sensitization to bleomycin | Activation of caspase-3, -8, -9, and up-regulation of the expression of P53 and Bcl-2 | [159] | |
| Purified phytochemical with adjuvant activity in HPV radio-therapies | Resveratrol | In vitro | HeLa SiHa | Increased radiosensitivity and potentiation of apoptosis | Dose-dependent alteration of cell cycle progression and cytotoxic response | [160] |
| Genistein | In vitro | CaSki Me180 Human esophageal cancer cell lines | Increased radiosensitivity and potentiation of apoptosis | Inhibition of Mcl-1; G(2)M arrest, and activation of the AKT gene | [161,162,163] | |
| Curcumin | In vitro | HeLa SiHa | Increased radiosensitivity and potentiation of apoptosis | ROS-dependent mechanism | [164] | |
| Ferulic acid | In vitro | HeLa Me180 | Increased radiosensitivity and potentiation of apoptosis | ROS-dependent mechanism | [165] | |
| Quercetin | In vitro and in vivo | DLD1 (human colorectal cancer xenografts) HeLa MCF-7 | Increased radiosensitivity and potentiation of apoptosis | Time- and dose-dependent mechanism and through ROS modulation and downregulation of E6 and E7 expression | [166] |
| Compound Type | Phytochemicals | Disease Stage | Route | Activity | References |
|---|---|---|---|---|---|
| Green tea compounds | 200 mg EGCG+/− “Poly E” (37 mg epigallocatechin + 31 mg epicatechin) | 51 patients with Chronic cervicitis mild dysplasia moderate dysplasia severe dysplasia | Orally (capsule) ± vaginally (ointment) | 20/27 patients (74%) under poly E ointment therapy showed a response. 6/8 patients (75%) under poly E ointment + poly E capsule therapy showed a response, 3/6 patients (50%) under poly E capsule therapy showed a response. 6/10 patients (60%) under EGCG capsule therapy showed a response. Overall, a 69% response rate (35/51) was noted for treatment with green tea extracts, as compared with a 10% response rate (4/39) in untreated controls (p < 0.05). | [169] |
| Curcumin-based | Curcumin | Phase I clinical testing 4 cervical intraepithelial neoplasia (CIN) cases | Oral administration of 0.5–12 mg for 3 months | Clinical safety (up to 8 mg/day Histological improvements in 1/4 patients. | [170] |
| Basant | Phase I/II double-blind clinical trial in women infected with HPV but without high grade CIN | Intra-vaginal application of curcumin-containing capsules or Basant cream | Higher clearance of cervical HPV infection (87.7%) in case of Basant cream 81.3% rate in the case of curcumin capsules compared to the controls (73.3%) with no serious adverse events. | [152] | |
| 11 women infected with HPV and low grade cervical abnormalities | Intra-vaginal application of Basant capsules | Clearance of HPV16 infection in all the patients (11/11) | [143] | ||
| Neem-based | Praneem | 20 HPV-infected women +/− early cervical intraepithelial lesions (placebo controlled) | Thirty days intra-vaginal application of Praneem tablets | Clearance of HPV16 infection in 60% of the patients (6/10). Clearance in another 50% after another administration, total 80% HPV clearance rate. | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franconi, R.; Massa, S.; Paolini, F.; Vici, P.; Venuti, A. Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers. Cancers 2020, 12, 3101. https://doi.org/10.3390/cancers12113101
Franconi R, Massa S, Paolini F, Vici P, Venuti A. Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers. Cancers. 2020; 12(11):3101. https://doi.org/10.3390/cancers12113101
Chicago/Turabian StyleFranconi, Rosella, Silvia Massa, Francesca Paolini, Patrizia Vici, and Aldo Venuti. 2020. "Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers" Cancers 12, no. 11: 3101. https://doi.org/10.3390/cancers12113101
APA StyleFranconi, R., Massa, S., Paolini, F., Vici, P., & Venuti, A. (2020). Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers. Cancers, 12(11), 3101. https://doi.org/10.3390/cancers12113101