Combination Therapy of High-Dose Rabeprazole Plus Metronomic Capecitabine in Advanced Gastro-Intestinal Cancer: A Randomized Phase II Trial
Simple Summary
Abstract
1. Introduction
2. Results
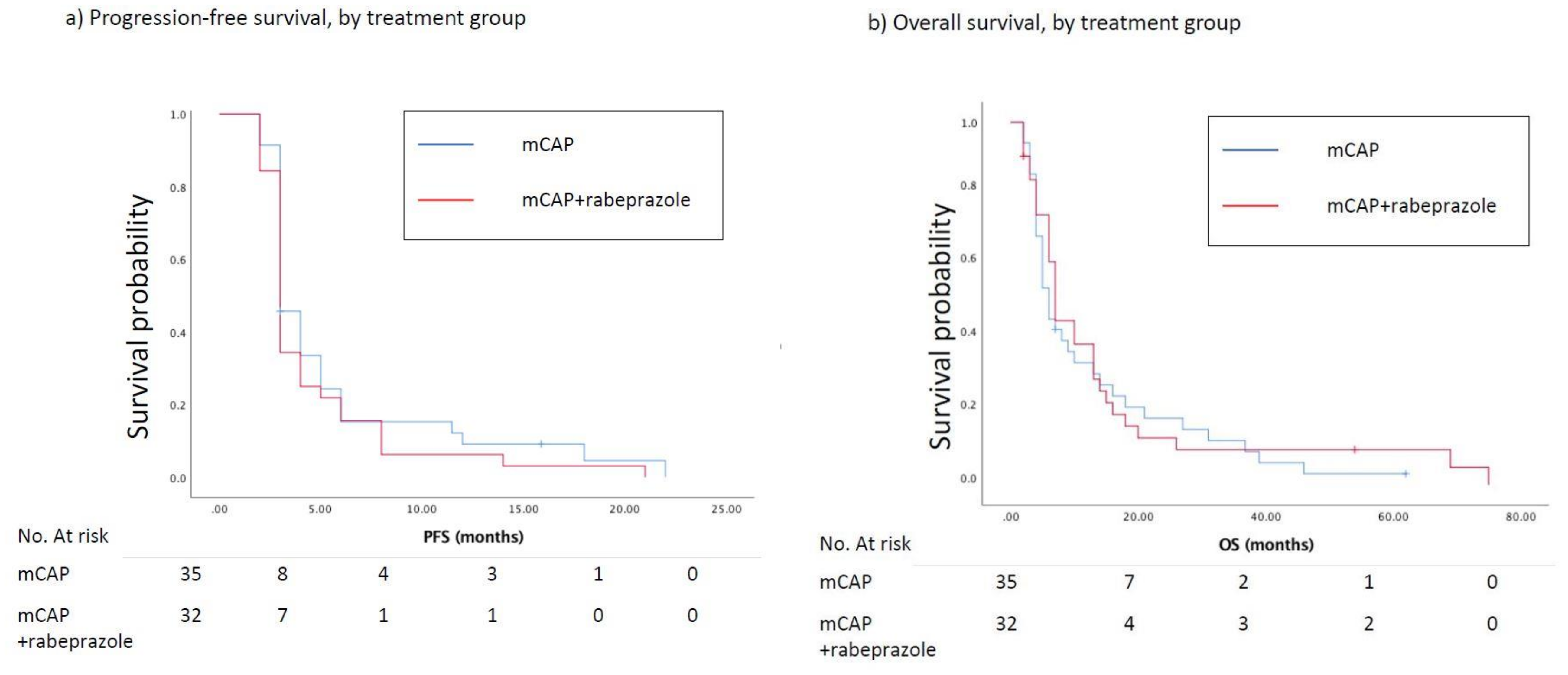
2.1. Efficacy
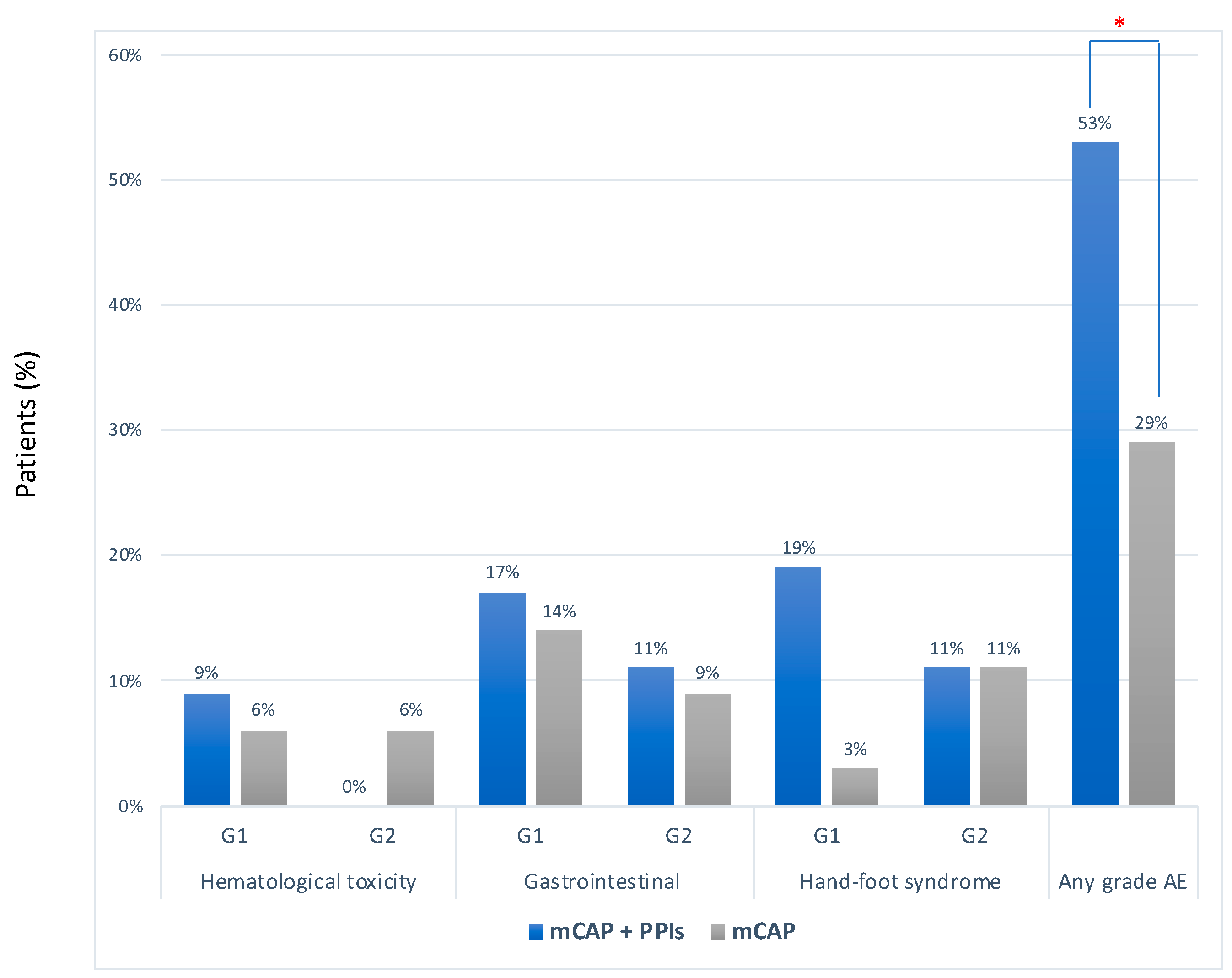
2.2. Safety
2.3. Plasma Concentration of Capecitabine and Its Metabolites
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Study Design and Protocol
4.3. Statistical Analysis, Sample Size
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Cmax | concentration at tmax |
| ECOG | Eastern Cooperative Oncology Group |
| GI | gastrointestinal |
| HR | hazard ratio |
| mCAP | metronomic capecitabine |
| OS | overall survival |
| PFS | progression free survival |
| PPIs | proton pump inhibitors |
| SD | standard deviation |
| RR | relative risk |
References
- Fais, S.; Venturi, G.; Gatenby, B. Microenvironmental acidosis in carcinogenesis and metastases: New strategies in prevention and therapy. Cancer Metastasis Rev. 2014, 33, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Fais, S. Proton pump inhibitor-induced tumour cell death by inhibition of a detoxification mechanism. J. Intern. Med. 2010, 267, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, O.; Tuccitto, A.; Citterio, D.; Huber, V.; Camisaschi, C.; Milione, M.; Vergani, B.; Villa, A.; Alison, M.R.; Carradori, S.; et al. pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Oncoimmunology 2018, 7, e1445452. [Google Scholar] [CrossRef] [PubMed]
- Luciani, F.; Spada, M.; De Milito, A.; Molinari, A.; Rivoltini, L.; Montinaro, A.; Marra, M.; Lugini, L.; Logozzi, M.; Lozupone, F.; et al. Effect of Proton Pump Inhibitor Pretreatment on Resistance of Solid Tumors to Cytotoxic Drugs. JNCI J. Natl. Cancer Inst. 2004, 96, 1702–1713. [Google Scholar] [CrossRef]
- De Milito, A.; Canese, R.; Marino, M.L.; Borghi, M.; Iero, M.; Villa, A.; Venturi, G.; Lozupone, F.; Iessi, E.; Logozzi, M.; et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 2010, 127, 207–219. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Baldi, A.; Buglioni, S.; Carocci, F.; de Bazzichini, G.M.; Betti, G.; Pantaleo, I.; Menicagli, F.; Citro, G.; Fais, S. Lansoprazole as a rescue agent in chemoresistant tumors: A phase I/II study in companion animals with spontaneously occurring tumors. J. Transl. Med. 2011, 9, 221. [Google Scholar] [CrossRef]
- Ferrari, S.; Perut, F.; Fagioli, F.; Brach Del Prever, A.; Meazza, C.; Parafioriti, A.; Picci, P.; Gambarotti, M.; Avnet, S.; Baldini, N.; et al. Proton pump inhibitor chemosensitization in human osteosarcoma: From the bench to the patients’ bed. J. Transl. Med. 2013, 11, 268. [Google Scholar] [CrossRef]
- Brana, I.; Ocana, A.; Chen, E.X.; Razak, A.R.A.; Haines, C.; Lee, C.; Douglas, S.; Wang, L.; Siu, L.L.; Tannock, I.F.; et al. A phase I trial of pantoprazole in combination with doxorubicin in patients with advanced solid tumors: Evaluation of pharmacokinetics of both drugs and tissue penetration of doxorubicin. Invest. New Drugs 2014, 32, 1269–1277. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zhang, J.; Wang, J.-L.; Sun, S.; Wang, Z.-H.; Wang, L.-P.; Zhang, Q.-L.; Lv, F.-F.; Cao, E.-Y.; Shao, Z.-M.; et al. Erratum to: Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J. Exp. Clin. Cancer Res. 2015, 34, 109. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Fais, S. Drug repurposing for anticancer therapies. A lesson from proton pump inhibitors. Expert Opin. Ther. Pat. 2020, 30, 15–25. [Google Scholar] [CrossRef]
- Ikemura, K.; Hiramatsu, S.; Okuda, M. Drug Repositioning of Proton Pump Inhibitors for Enhanced Efficacy and Safety of Cancer Chemotherapy. Front. Pharmacol. 2017, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Romiti, A.; Cox, M.C.; Sarcina, I.; Di Rocco, R.; D’Antonio, C.; Barucca, V.; Marchetti, P. Metronomic chemotherapy for cancer treatment: A decade of clinical studies. Cancer Chemother. Pharmacol. 2013, 72, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Romiti, A.; Falcone, R.; Roberto, M.; Marchetti, P. Current achievements and future perspectives of metronomic chemotherapy. Invest. New Drugs 2017, 35, 359–374. [Google Scholar] [CrossRef]
- Hagman, H.; Frödin, J.-E.; Berglund, Å.; Sundberg, J.; Vestermark, L.W.; Albertsson, M.; Fernebro, E.; Johnsson, A. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: The Nordic ACT2 trial. Ann. Oncol. 2016, 27, 140–147. [Google Scholar] [CrossRef]
- Roberto, M.; Romiti, A.; Onesti, C.E.; D’Antonio, C.; Milano, A.; Falcone, R.; Barucca, V.; Palombi, L.; Righini, R.; Marchetti, P. A metronomic schedule as salvage chemotherapy for upper gastrointestinal tract cancer. Anticancer. Drugs 2015, 27, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Romiti, A.; Onesti, C.E.; Roberto, M.; Barucca, V.; Tomao, S.; D’Antonio, C.; Durante, V.; Milano, A.; Falcone, R.; Di Rocco, R.; et al. Continuous, low-dose capecitabine for patients with recurrent colorectal cancer. Med. Oncol. 2015, 32, 54. [Google Scholar] [CrossRef] [PubMed]
- André, N.; Carré, M.; Pasquier, E. Metronomics: Towards personalized chemotherapy? Nat. Rev. Clin. Oncol. 2014, 11, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Dadabhai, A.; Friedenberg, F.K. Rabeprazole: A pharmacologic and clinical review for acid-related disorders. Expert Opin. Drug Saf. 2009, 8, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; de Lemos, M.; Hunter, N.; Badry, N.; de Lemos, J. Concomitant use of capecitabine and proton pump inhibitors—Is it safe? J. Oncol. Pharm. Pract. 2019, 25, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.P.; Hecht, J.R.; Slamon, D.; Wainberg, Z.A.; Bang, Y.-J.; Hoff, P.M.; Sobrero, A.; Qin, S.; Afenjar, K.; Houe, V.; et al. Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 2017, 3, 767–773. [Google Scholar] [CrossRef]
- Sun, J.; Ilich, A.I.; Kim, C.A.; Chu, M.P.; Wong, G.G.; Ghosh, S.; Danilak, M.; Mulder, K.E.; Spratlin, J.L.; Chambers, C.R.; et al. Concomitant Administration of Proton Pump Inhibitors and Capecitabine is Associated With Increased Recurrence Risk in Early Stage Colorectal Cancer Patients. Clin. Colorectal Cancer 2016, 15, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.; Roberto, M.; D’Antonio, C.; Romiti, A.; Milano, A.; Onesti, C.E.; Marchetti, P.; Fais, S. High-doses of proton pump inhibitors in refractory gastro-intestinal cancer: A case series and the state of art. Dig. Liver Dis. 2016, 48, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Prager, G.W.; Quintela, A.; Stein, A.; Moreno Vera, S.; Mounedji, N.; Taieb, J. Beyond second-line therapy in patients with metastatic colorectal cancer: A systematic review. Ann. Oncol. 2018, 29, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.; Di Emidio, K.; Tarantino, V.; Cascinu, S. Second-line treatments: Moving towards an opportunity to improve survival in advanced gastric cancer? ESMO Open 2017, 2, e000206. [Google Scholar] [CrossRef]
- Hua, J.; Shi, S.; Liang, D.; Liang, C.; Meng, Q.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Current status and dilemma of second-line treatment in advanced pancreatic cancer: Is there a silver lining? Onco. Targets. Ther. 2018, 11, 4591–4608. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Federici, C.; Borghi, M.; Azzarito, T.; Marino, M.L.; Cesolini, A.; Spugnini, E.P.; Fais, S. Proton pump inhibitors while belonging to the same family of generic drugs show different anti-tumor effect. J. Enzyme Inhib. Med. Chem. 2016, 31, 538–545. [Google Scholar] [CrossRef]
- Azzarito, T.; Venturi, G.; Cesolini, A.; Fais, S. Lansoprazole induces sensitivity to suboptimal doses of paclitaxel in human melanoma. Cancer Lett. 2015, 356, 697–703. [Google Scholar] [CrossRef]
- Lu, Z.N.; Tian, B.; Guo, X.L. Repositioning of proton pump inhibitors in cancer therapy. Cancer Chemother. Pharmacol. 2017, 80, 925–937. [Google Scholar] [CrossRef]
- Papagerakis, S.; Bellile, E.; Peterson, L.A.; Pliakas, M.; Balaskas, K.; Selman, S.; Hanauer, D.; Taylor, J.M.G.; Duffy, S.; Wolf, G. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Chest 2014, 146, 1258–1269. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, C.Z.; Lin, Y.C.; Kao, L.T.; Lin, H.C. Negative Association of Proton Pump Inhibitors With Subsequent Development of Breast Cancer: A Nationwide Population-Based Study. J. Clin. Pharmacol. 2019, 59, 350–355. [Google Scholar] [CrossRef]
- Ding, D.C.; Sung, F.C.; Chen, W.; Wang, J.H.; Lin, S.Z. Proton pump inhibitors reduce breast cancer risk in gastric ulcer patients: A population-based cohort study. Breast J. 2020, 26, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Milano, A.; D’Antonio, C.; Romiti, A.; Falcone, R.; Roberto, M.; Fais, S. Association Between Proton Pump Inhibitors and Metronomic Capecitabine as Salvage Treatment for Patients With Advanced Gastrointestinal Tumors: A Randomized Phase II Trial. Clin. Colorectal Cancer 2016, 15, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Reigner, B.; Blesch, K.; Weidekamm, E. Clinical pharmacokinetics of capecitabine. Clin. Pharmacokinet. 2001, 40, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Di Desidero, T.; Orlandi, P.; Fioravanti, A.; Cremolini, C.; Loupakis, F.; Marmorino, F.; Antoniotti, C.; Masi, G.; Lonardi, S.; Bergamo, F.; et al. Pharmacokinetic analysis of metronomic capecitabine in refractory metastatic colorectal cancer patients. Investig. New Drugs 2018, 36, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Le J Drug Absorption-Clinical Pharmacology-Merck Manuals Professional Edition. Available online: https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-absorption (accessed on 9 March 2020).
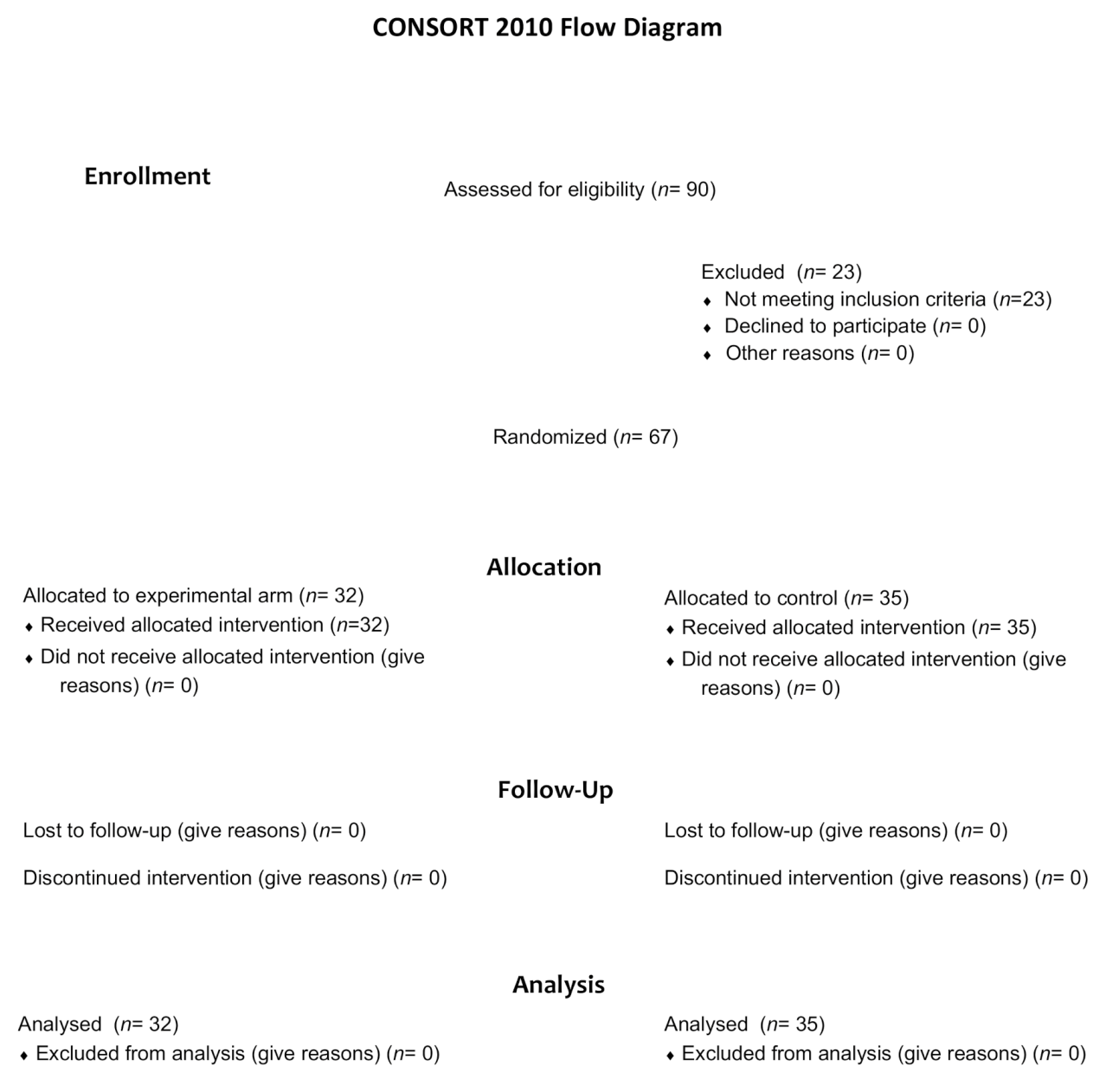
| Characteristics No. (%) | mCAP-Rabeprazole (n = 32) | mCAP (n = 35) | p | |
|---|---|---|---|---|
| Age—years | ||||
| Median (range) | 69 (39–85) | 69 (38–87) | 0.358 | |
| Sex | ||||
| Male | 22 (69) | 20 (57) | 0.449 | |
| Female | 10 (31) | 15 (43) | ||
| ECOG PS | ||||
| 0 | 14 (44) | 9 (26) | 0.197 | |
| 1 | 10 (31) | 18 (51) | ||
| 2 | 8 (25) | 8 (23) | ||
| Primary tumor site | ||||
| Colon or rectum | 28 (87) | 28 (80) | 0.134 | |
| Stomach | 0 (0) | 4 (11) | ||
| Pancreas | 4 (13) | 3 (9) | ||
| Site of metastasis | ||||
| Liver | 16 (50) | 15 (43) | 0.561 | |
| Multiorgan | 8 (25) | 13 (37) | ||
| Peritoneum | 8 (25) | 7 (20) | ||
| n. of prior regimens | ||||
| 1 | 6 (19) | 5 (14) | 0.349 | |
| 2 | 16 (50) | 15 (43) | ||
| ≥3 | 10 (31) | 15 (43) | ||
| Prior anticancer therapy | ||||
| Fluoropyrimidine-based | 14 (44) | 16 (46) | 0.244 | |
| Anti-VEGF | 16 (50) | 10 (28) | ||
| Anti-EGFR | 4 (12) | 6 (17) | ||
| Ctmax (μg/mL) | T1 (4 Weeks) | T2 (8 Weeks) | ||||
|---|---|---|---|---|---|---|
| mCAP+rabe (22) | mCAP (20) | p Value | mCAP+rabe (22) | mCAP (20) | p Value | |
| (Mean ± Std.dev.) | (Mean ± Std.dev.) | |||||
| Capecitabine | 0.58 ± 0.67 | 0.38 ± 0.46 | 0.315 | 0.38 ± 0.64 | 0.41 ± 0.32 | 0.160 |
| 5-DFUR | 1.85 ± 1.52 | 1.43 ± 2.01 | 0.168 | 1.41 ± 1.25 | 1.26 ± 0.65 | 0.018 |
| 5-FU | 0.29 ± 0.32 | 0.33 ± 0.62 | 0.270 | 0.31 ± 0.30 | 0.26 ± 0.34 | 0.968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberto, M.; Romiti, A.; Mazzuca, F.; Milano, A.; D’Antonio, C.; Lionetto, L.; Falcone, R.; Strigari, L.; Simmaco, M.; Fais, S.; et al. Combination Therapy of High-Dose Rabeprazole Plus Metronomic Capecitabine in Advanced Gastro-Intestinal Cancer: A Randomized Phase II Trial. Cancers 2020, 12, 3084. https://doi.org/10.3390/cancers12113084
Roberto M, Romiti A, Mazzuca F, Milano A, D’Antonio C, Lionetto L, Falcone R, Strigari L, Simmaco M, Fais S, et al. Combination Therapy of High-Dose Rabeprazole Plus Metronomic Capecitabine in Advanced Gastro-Intestinal Cancer: A Randomized Phase II Trial. Cancers. 2020; 12(11):3084. https://doi.org/10.3390/cancers12113084
Chicago/Turabian StyleRoberto, Michela, Adriana Romiti, Federica Mazzuca, Annalisa Milano, Chiara D’Antonio, Luana Lionetto, Rosa Falcone, Lidia Strigari, Maurizio Simmaco, Stefano Fais, and et al. 2020. "Combination Therapy of High-Dose Rabeprazole Plus Metronomic Capecitabine in Advanced Gastro-Intestinal Cancer: A Randomized Phase II Trial" Cancers 12, no. 11: 3084. https://doi.org/10.3390/cancers12113084
APA StyleRoberto, M., Romiti, A., Mazzuca, F., Milano, A., D’Antonio, C., Lionetto, L., Falcone, R., Strigari, L., Simmaco, M., Fais, S., & Marchetti, P. (2020). Combination Therapy of High-Dose Rabeprazole Plus Metronomic Capecitabine in Advanced Gastro-Intestinal Cancer: A Randomized Phase II Trial. Cancers, 12(11), 3084. https://doi.org/10.3390/cancers12113084







