Rare Germline Genetic Variants and the Risks of Epithelial Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Protein Truncating Variants in Confirmed Susceptibility Genes
2.1. BRCA1 and BRCA2
2.2. BRIP1
2.3. RAD51C and RAD51D
2.4. PALB2
2.5. Mismatch Repair (MMR) Genes
3. Missense Variants in Confirmed Susceptibility Genes
3.1. BRCA1/2 and MMR Genes
3.2. BRIP1, RAD51C/D and PALB2
4. Deleterious Variants in Other Proposed Susceptibility Genes
4.1. FANCM
4.2. ATM
4.3. BARD1 and NBN
4.4. CHECK2
4.5. Other Genes
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Thomas, D.M.; James, P.A.; Ballinger, M.L. Clinical implications of genomics for cancer risk genetics. Lancet Oncol. 2015, 16, e303–e308. [Google Scholar] [CrossRef]
- Jervis, S.; Song, H.; Lee, A.; Dicks, E.; Tyrer, J.; Harrington, P.; Easton, D.F.; Jacobs, I.J.; Pharoah, P.P.; Antoniou, A.C. Ovarian cancer familial relative risks by tumour subtypes and by known ovarian cancer genetic susceptibility variants. J. Med. Genet. 2014, 51, 108–113. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horváth, J.; Kast, K.; Niederacher, D.; Turnbull, C.; et al. Ovarian and Breast Cancer Risks Associated With Pathogenic Variants in RAD51C and RAD51D. J. Natl. Cancer Inst. 2020, 112. [Google Scholar] [CrossRef]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated with Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Gareth Evans, D.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef]
- Kar, S.P.; Berchuck, A.; Gayther, S.A.; Goode, E.L.; Moysich, K.B.; Pearce, C.L.; Ramus, S.J.; Schildkraut, J.M.; Sellers, T.A.; Pharoah, P.D.P. Common Genetic Variation and Susceptibility to Ovarian Cancer: Current Insights and Future Directions. Cancer Epidemiol. Biomark. Prev. 2018, 27, 395. [Google Scholar] [CrossRef]
- Phelan, C.M.; Kuchenbaecker, K.B.; Tyrer, J.P.; Kar, S.P.; Lawrenson, K.; Winham, S.J.; Dennis, J.; Pirie, A.; Riggan, M.J.; Chornokur, G.; et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017, 49, 680–691. [Google Scholar] [CrossRef]
- Ramus, S.J.; Harrington, P.A.; Pye, C.; DiCioccio, R.A.; Cox, M.J.; Garlinghouse-Jones, K.; Oakley-Girvan, I.; Jacobs, I.J.; Hardy, R.M.; Whittemore, A.S.; et al. Contribution of BRCA1 and BRCA2 mutations to inherited ovarian cancer. Hum. Mutat. 2007, 28, 1207–1215. [Google Scholar] [CrossRef]
- Ramus, S.J.; Gayther, S.A. The Contribution of BRCA1 and BRCA2 to Ovarian Cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef]
- Johannesdottir, G.; Gudmundsson, J.; Bergthorsson, J.T.; Arason, A.; Agnarsson, B.A.; Eiriksdottir, G.; Johannsson, O.T.; Borg, A.; Ingvarsson, S.; Easton, D.F.; et al. High Prevalence of the 999del5 Mutation in Icelandic Breast and Ovarian Cancer Patients. Cancer Res. 1996, 56, 3663–3665. [Google Scholar]
- Gayther, S.A.; Harrington, P.; Russell, P.; Kharkevich, G.; Garkavtseva, R.F.; Ponder, B.A. Frequently occurring germ-line mutations of the BRCA1 gene in ovarian cancer families from Russia. Am. J. Hum. Genet. 1997, 60, 1239–1242. [Google Scholar]
- Sokolenko, A.P.; Rozanov, M.E.; Mitiushkina, N.V.; Sherina, N.Y.; Iyevleva, A.G.; Chekmariova, E.V.; Buslov, K.G.; Shilov, E.S.; Togo, A.V.; Bit-Sava, E.M.; et al. Founder mutations in early-onset, familial and bilateral breast cancer patients from Russia. Fam. Cancer 2007, 6, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Menkiszak, J.; Gronwald, J.; Górski, B.; Jakubowska, A.; Huzarski, T.; Byrski, T.; Foszczyńska-Kłoda, M.; Haus, O.; Janiszewska, H.; Perkowska, M.; et al. Hereditary ovarian cancer in Poland. Int. J. Cancer 2003, 106, 942–945. [Google Scholar] [CrossRef]
- Heimdal, K.; Maehle, L.; Apold, J.; Pedersen, J.C.; Møller, P. The Norwegian founder mutations in BRCA1: High penetrance confirmed in an incident cancer series and differences observed in the risk of ovarian cancer. Eur. J. Cancer 2003, 39, 2205–2213. [Google Scholar] [CrossRef]
- Sarantaus, L.; Huusko, P.; Eerola, H.; Launonen, V.; Vehmanen, P.; Rapakko, K.; Gillanders, E.; Syrjäkoski, K.; Kainu, T.; Vahteristo, P.; et al. Multiple founder effects and geographical clustering of BRCA1 and BRCA2 families in Finland. Eur. J. Hum. Genet. 2000, 8, 757–763. [Google Scholar] [CrossRef]
- Sekine, M.; Nagata, H.; Tsuji, S.; Hirai, Y.; Fujimoto, S.; Hatae, M.; Kobayashi, I.; Fujii, T.; Nagata, I.; Ushijima, K.; et al. Mutational analysis of BRCA1 and BRCA2 and clinicopathologic analysis of ovarian cancer in 82 ovarian cancer families: Two common founder mutations of BRCA1 in Japanese population. Clin. Cancer Res. 2001, 7, 3144–3150. [Google Scholar]
- Khoo, U.S.; Chan, K.Y.; Cheung, A.N.; Xue, W.C.; Shen, D.H.; Fung, K.Y.; Ngan, H.Y.; Choy, K.W.; Pang, C.P.; Poon, C.S.; et al. Recurrent BRCA1 and BRCA2 germline mutations in ovarian cancer: A founder mutation of BRCA1 identified in the Chinese population. Hum. Mutat. 2002, 19, 307–308. [Google Scholar] [CrossRef]
- Kurian, A.W.; Hughes, E.; Handorf, E.A.; Gutin, A.; Allen, B.; Hartman, A.-R.; Hall, M.J. Breast and Ovarian Cancer Penetrance Estimates Derived from Germline Multiple-Gene Sequencing Results in Women. JCO Precis. Oncol. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Lilyquist, J.; LaDuca, H.; Polley, E.; Davis, B.T.; Shimelis, H.; Hu, C.; Hart, S.N.; Dolinsky, J.S.; Couch, F.J.; Goldgar, D.E. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol. Oncol. 2017, 147, 375–380. [Google Scholar] [CrossRef]
- Castéra, L.; Harter, V.; Muller, E.; Krieger, S.; Goardon, N.; Ricou, A.; Rousselin, A.; Paimparay, G.; Legros, A.; Bruet, O.; et al. Landscape of pathogenic variations in a panel of 34 genes and cancer risk estimation from 5131 HBOC families. Genet. Med. 2018, 20, 1677–1686. [Google Scholar] [CrossRef]
- Suszynska, M.; Klonowska, K.; Jasinska, A.J.; Kozlowski, P. Large-scale meta-analysis of mutations identified in panels of breast/ovarian cancer-related genes—Providing evidence of cancer predisposition genes. Gynecol. Oncol. 2019, 153, 452–462. [Google Scholar] [CrossRef]
- Iqbal, J.; Ragone, A.; Lubinski, J.; Lynch, H.T.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Armel, S.; Eisen, A.; Neuhausen, S.L.; et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2012, 107, 2005–2009. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Song, H.; Cicek, M.S.; Dicks, E.; Harrington, P.; Ramus, S.J.; Cunningham, J.M.; Fridley, B.L.; Tyrer, J.P.; Alsop, J.; Jimenez-Linan, M.; et al. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum. Mol. Genet. 2014, 23, 4703–4709. [Google Scholar] [CrossRef]
- Walsh, T.; Casadei, S.; Lee, M.K.; Pennil, C.C.; Nord, A.S.; Thornton, A.M.; Roeb, W.; Agnew, K.J.; Stray, S.M.; Wickramanayake, A.; et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 18032–18037. [Google Scholar] [CrossRef]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women with Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 Mutations and Survival in Women with Invasive Epithelial Ovarian Cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Rosen, B.; Fan, I.; Moody, J.; McLaughlin, J.R.; Risch, H.; May, T.; Sun, P.; Narod, S.A. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol. Oncol. 2016, 140, 42–47. [Google Scholar] [CrossRef]
- McLaughlin, J.R.; Rosen, B.; Moody, J.; Pal, T.; Fan, I.; Shaw, P.A.; Risch, H.A.; Sellers, T.A.; Sun, P.; Narod, S.A. Long-Term Ovarian Cancer Survival Associated with Mutation in BRCA1 or BRCA2. J. Natl. Cancer Inst. 2013, 105, 141–148. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Genetics Screening (Version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf (accessed on 11 June 2020).
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef]
- Oza, A.M.; Tinker, A.V.; Oaknin, A.; Shapira-Frommer, R.; McNeish, I.A.; Swisher, E.M.; Ray-Coquard, I.; Bell-McGuinn, K.; Coleman, R.L.; O’Malley, D.M.; et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017, 147, 267–275. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsman, D.; Clemons, M.; Gilks, B.; et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]
- Suszynska, M.; Ratajska, M.; Kozlowski, P. BRIP1, RAD51C, and RAD51D mutations are associated with high susceptibility to ovarian cancer: Mutation prevalence and precise risk estimates based on a pooled analysis of ~30,000 cases. J. Ovarian Res. 2020, 13, 50. [Google Scholar] [CrossRef]
- Meindl, A.; Hellebrand, H.; Wiek, C.; Erven, V.; Wappenschmidt, B.; Niederacher, D.; Freund, M.; Lichtner, P.; Hartmann, L.; Schaal, H.; et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 2010, 42, 410–414. [Google Scholar] [CrossRef]
- Loveday, C.; Turnbull, C.; Ramsay, E.; Hughes, D.; Ruark, E.; Frankum, J.R.; Bowden, G.; Kalmyrzaev, B.; Warren-Perry, M.; Snape, K.; et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat. Genet. 2011, 43, 879–882. [Google Scholar] [CrossRef]
- Song, H.; Dicks, E.; Ramus, S.J.; Tyrer, J.P.; Intermaggio, M.P.; Hayward, J.; Edlund, C.K.; Conti, D.; Harrington, P.; Fraser, L.; et al. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J. Clin. Oncol. 2015, 33, 2901–2907. [Google Scholar] [CrossRef]
- Manchanda, R.; Legood, R.; Antoniou, A.; Pearce, L.; Menon, U. Commentary on changing the risk threshold for surgical prevention of ovarian cancer. BJOG An. Int. J. Obstet. Gynaecol. 2018, 125, 541–544. [Google Scholar] [CrossRef]
- Manchanda, R.; Legood, R.; Antoniou, A.C.; Gordeev, V.S.; Menon, U. Specifying the ovarian cancer risk threshold of ’premenopausal risk-reducing salpingo-oophorectomy’ for ovarian cancer prevention: A cost-effectiveness analysis. J. Med. Genet. 2016, 53, 591–599. [Google Scholar] [CrossRef]
- Manchanda, R.; Legood, R.; Pearce, L.; Menon, U. Defining the risk threshold for risk reducing salpingo-oophorectomy for ovarian cancer prevention in low risk postmenopausal women. Gynecol. Oncol. 2015, 139, 487–494. [Google Scholar] [CrossRef]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkas, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-cancer risk in families with mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef]
- Ducy, M.; Sesma-Sanz, L.; Guitton-Sert, L.; Lashgari, A.; Gao, Y.; Brahiti, N.; Rodrigue, A.; Margaillan, G.; Caron, M.C.; Côté, J.; et al. The Tumor Suppressor PALB2: Inside out. Trends Biochem. Sci. 2019, 44, 226–240. [Google Scholar] [CrossRef]
- Song, H.; Ramus, S.; Dicks, E.; Tyrer, J.; Intermaggio, M.; Chenevix-Trench, G.; Bowtell, D.; Traficante, N.; Brenton, J.; Goranova, T.; et al. Population based targeted sequencing of 54 candidate genes identifies PALB2 as a susceptibility gene for high grade serous ovarian cancer. J Med Genet. 2020. [Google Scholar] [CrossRef]
- Osorio, A.; Endt, D.; Fernández, F.; Eirich, K.; de la Hoya, M.; Schmutzler, R.; Caldés, T.; Meindl, A.; Schindler, D.; Benitez, J. Predominance of pathogenic missense variants in the RAD51C gene occurring in breast and ovarian cancer families. Hum. Mol. Genet. 2012, 21, 2889–2898. [Google Scholar] [CrossRef]
- Ketabi, Z.; Bartuma, K.; Bernstein, I.; Malander, S.; Grönberg, H.; Björck, E.; Holck, S.; Nilbert, M. Ovarian cancer linked to Lynch syndrome typically presents as early-onset, non-serous epithelial tumors. Gynecol. Oncol. 2011, 121, 462–465. [Google Scholar] [CrossRef]
- Lu, H.M.; Li, S.; Black, M.H.; Lee, S.; Hoiness, R.; Wu, S.; Mu, W.; Huether, R.; Chen, J.; Sridhar, S.; et al. Association of Breast and Ovarian Cancers with Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol. 2019, 5, 51–57. [Google Scholar] [CrossRef]
- Spurdle, A.B.; Healey, S.; Devereau, A.; Hogervorst, F.B.; Monteiro, A.N.; Nathanson, K.L.; Radice, P.; Stoppa-Lyonnet, D.; Tavtigian, S.; Wappenschmidt, B.; et al. ENIGMA—Evidence-based network for the interpretation of germline mutant alleles: An international initiative to evaluate risk and clinical significance associated with sequence variation in BRCA1 and BRCA2 genes. Hum. Mutat 2012, 33, 2–7. [Google Scholar] [CrossRef]
- Li, H.; LaDuca, H.; Pesaran, T.; Chao, E.C.; Dolinsky, J.S.; Parsons, M.; Spurdle, A.B.; Polley, E.C.; Shimelis, H.; Hart, S.N.; et al. Classification of variants of uncertain significance in BRCA1 and BRCA2 using personal and family history of cancer from individuals in a large hereditary cancer multigene panel testing cohort. Genet. Med. 2020, 22, 701–708. [Google Scholar] [CrossRef]
- Thompson, B.A.; Spurdle, A.B.; Plazzer, J.P.; Greenblatt, M.S.; Akagi, K.; Al-Mulla, F.; Bapat, B.; Bernstein, I.; Capellá, G.; den Dunnen, J.T.; et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat. Genet. 2014, 46, 107–115. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7–20. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Tyrer, J.P.; Guo, Q.; Easton, D.F.; Pharoah, P.D. The admixture maximum likelihood test to test for association between rare variants and disease phenotypes. BMC Bioinform. 2013, 14, 177. [Google Scholar] [CrossRef]
- Bouwman, P.; van der Gulden, H.; van der Heijden, I.; Drost, R.; Klijn, C.N.; Prasetyanti, P.; Pieterse, M.; Wientjens, E.; Seibler, J.; Hogervorst, F.B.; et al. A high-throughput functional complementation assay for classification of BRCA1 missense variants. Cancer Discov. 2013, 3, 1142–1155. [Google Scholar] [CrossRef]
- Dicks, E.; Song, H.; Ramus, S.J.; Oudenhove, E.V.; Tyrer, J.P.; Intermaggio, M.P.; Kar, S.; Harrington, P.; Bowtell, D.D.; Group, A.S.; et al. Germline whole exome sequencing and large-scale replication identifies FANCM as a likely high grade serous ovarian cancer susceptibility gene. Oncotarget 2017, 8, 50930–50940. [Google Scholar] [CrossRef]
- Thompson, D.; Duedal, S.; Kirner, J.; McGuffog, L.; Last, J.; Reiman, A.; Byrd, P.; Taylor, M.; Easton, D.F. Cancer Risks and Mortality in Heterozygous ATM Mutation Carriers. JNCI J. Natl. Cancer Inst. 2005, 97, 813–822. [Google Scholar] [CrossRef]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.L.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Goldgar, D.E.; Healey, S.; Dowty, J.G.; Da Silva, L.; Chen, X.; Spurdle, A.B.; Terry, M.B.; Daly, M.J.; Buys, S.M.; Southey, M.C.; et al. Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res. 2011, 13, R73. [Google Scholar] [CrossRef]
- Weber-Lassalle, N.; Borde, J.; Weber-Lassalle, K.; Horváth, J.; Niederacher, D.; Arnold, N.; Kaulfuß, S.; Ernst, C.; Paul, V.G.; Honisch, E.; et al. Germline loss-of-function variants in the BARD1 gene are associated with early-onset familial breast cancer but not ovarian cancer. Breast Cancer Res. 2019, 21, 55. [Google Scholar] [CrossRef]
- Couch, F.J.; Shimelis, H.; Hu, C.; Hart, S.N.; Polley, E.C.; Na, J.; Hallberg, E.; Moore, R.; Thomas, A.; Lilyquist, J.; et al. Associations between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017, 3, 1190–1196. [Google Scholar] [CrossRef]
- Weischer, M.; Nordestgaard, B.G.; Pharoah, P.; Bolla, M.K.; Nevanlinna, H.; Van’t Veer, L.J.; Garcia-Closas, M.; Hopper, J.L.; Hall, P.; Andrulis, I.L.; et al. CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4308–4316. [Google Scholar] [CrossRef]
- Gylling, A.; Ridanpää, M.; Vierimaa, O.; Aittomäki, K.; Avela, K.; Kääriäinen, H.; Laivuori, H.; Pöyhönen, M.; Sallinen, S.-L.; Wallgren-Pettersson, C.; et al. Large genomic rearrangements and germline epimutations in Lynch syndrome. Int. J. Cancer 2009, 124, 2333–2340. [Google Scholar] [CrossRef]
- Easton, D.F.; Pharoah, P.D.; Antoniou, A.C.; Tischkowitz, M.; Tavtigian, S.V.; Nathanson, K.L.; Devilee, P.; Meindl, A.; Couch, F.J.; Southey, M.; et al. Gene-panel sequencing and the prediction of breast-cancer risk. N. Engl. J. Med. 2015, 372, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Prapa, M.; Solomons, J.; Tischkowitz, M. The use of panel testing in familial breast and ovarian cancer. Clin. Med. 2017, 17, 568–572. [Google Scholar] [CrossRef]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef]
- Domchek, S.M.; Robson, M.E. Update on Genetic Testing in Gynecologic Cancer. J. Clin. Oncol. 2019, 37, 2501–2509. [Google Scholar] [CrossRef]
- Manchanda, R.; Menon, U. Setting the Threshold for Surgical Prevention in Women at Increased Risk of Ovarian Cancer. Int. J. Gynecol. Cancer 2018, 28, 34–42. [Google Scholar] [CrossRef]
- Rosenthal, A.N.; Fraser, L.S.M.; Philpott, S.; Manchanda, R.; Burnell, M.; Badman, P.; Hadwin, R.; Rizzuto, I.; Benjamin, E.; Singh, N.; et al. Evidence of Stage Shift in Women Diagnosed with Ovarian Cancer during Phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J. Clin. Oncol. 2017, 35, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.W. Ethical, legal, and social implications of genomic medicine. N. Engl. J. Med. 2003, 349, 562–569. [Google Scholar] [CrossRef]
- Mersch, J.; Brown, N.; Pirzadeh-Miller, S.; Mundt, E.; Cox, H.C.; Brown, K.; Aston, M.; Esterling, L.; Manley, S.; Ross, T. Prevalence of Variant Reclassification Following Hereditary Cancer Genetic Testing. JAMA 2018, 320, 1266–1274. [Google Scholar] [CrossRef]
- LaDuca, H.; Stuenkel, A.J.; Dolinsky, J.S.; Keiles, S.; Tandy, S.; Pesaran, T.; Chen, E.; Gau, C.-L.; Palmaer, E.; Shoaepour, K.; et al. Utilization of multigene panels in hereditary cancer predisposition testing: Analysis of more than 2,000 patients. Genet. Med. Off. J. Am. Coll. Med Genet. 2014, 16, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Tandy-Connor, S.; Guiltinan, J.; Krempely, K.; LaDuca, H.; Reineke, P.; Gutierrez, S.; Gray, P.; Tippin Davis, B. False-positive results released by direct-to-consumer genetic tests highlight the importance of clinical confirmation testing for appropriate patient care. Genet. Med. 2018, 20, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.; Domchek, S.M.; Stadler, Z.; Nathanson, K.L.; Couch, F.; Garber, J.E.; Offit, K.; Robson, M.E. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat. Rev. Clin. Oncol. 2016, 13, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Boolbol, S.; Degnim, A.; Kuerer, H.; Leitch, A.M.; Morrow, M. Society of Surgical Oncology: Position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Ann. Surg Oncol. 2007, 14, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Gabai-Kapara, E.; Lahad, A.; Kaufman, B.; Friedman, E.; Segev, S.; Renbaum, P.; Beeri, R.; Gal, M.; Grinshpun-Cohen, J.; Djemal, K.; et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc. Natl. Acad. Sci. USA 2014, 111, 14205–14210. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Loggenberg, K.; Sanderson, S.; Burnell, M.; Wardle, J.; Gessler, S.; Side, L.; Balogun, N.; Desai, R.; Kumar, A.; et al. Population Testing for Cancer Predisposing BRCA1/BRCA2 Mutations in the Ashkenazi-Jewish Community: A Randomized Controlled Trial. JNCI J. Natl. Cancer Inst. 2014, 107. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, H.; Li, L.; Zang, R.; Wang, C.; Song, F.; Shi, T.; Yu, D.; Yang, M.; Xue, W.; et al. Genome-wide association study identifies new susceptibility loci for epithelial ovarian cancer in Han Chinese women. Nat. Commun. 2014, 5, 4682. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Longo, D.L. Personalized Medicine for Primary Treatment of Serous Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
| Gene | Study | Cases Carriers/Total (Frequency) | Controls Carriers/Total (Frequency) | OR (95% CI) | Cumulative Risk (95% CI) a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HGS | END | CCC | LGS | MUC | Mixed/ Unk b | All EOC | Matched | Publicly Available | ||||
| BRCA1 | Walsh et al., 2011 [34] | 31/242 ** | 2/23 | 1/17 | ** | - | 6/78 | 40/360 | - | - | NC | NC |
| (12.8%) | (8.7%) | (5.9%) | (7.7%) | (11.1%) | ||||||||
| Alsop et al., 2012 † [32] | 74/709 ** | 7/119 | 4/63 | ** | - | 3/110 | 88/1001 | - | - | NC | NC | |
| (10.4%) | (5.9%) | (6.3%) | (2.7%) | (8.79%) | ||||||||
| Song et al., 2014 ^ [33] | 58/1105 * | 3/322 | 3/192 | 6/172 | 1/157 | 13/274 | 84/2222 | 1/1528 | - | 60 # (10–2100) | 61% (15–99%) | |
| (5.2%) | (0.9%) | (1.6%) | (3.5%) | (0.6%) | (4.7%) | (3.78%) | (0.07%) | |||||
| Norquist et al., 2016 [35] | 155/1498 | 4/77 | 4/58 | 3/70 | 0/16 | 16/196 | 142/1915 | - | 114/36276 | 48.9 (24–100) | NC | |
| (10.3%) | (5.2%) | (6.9%) | (4.3%) | (0%) | (8.2%) | (7.41%) | (0.31%) | |||||
| Total | 278/3554 | 16/541 | 12/330 | 9/242 | 1/173 | 38/658 | 354/5498 | 1/1528 | - | - | - | |
| (7.82%) | (2.95%) | (3.63%) | (3.72%) | (0.58%) | (5.77%) | (6.43%) | (0.07%) | |||||
| BRCA2 | Walsh et al., 2011 [34] | 18/242 ** | 0/23 | 0/17 | ** | - | 5/78 | 23/360 | - | - | NC | NC |
| (7.4%) | (0%) | (0%) | (6.4%) | (6.38%) | ||||||||
| Alsop et al., 2012 † [32] | 44/709 ** | 3/119 | 0/63 | ** | - | 6/110 | 53/1001 | - | - | NC | NC | |
| (6.2%) | (2.5%) | (0%) | (5.4%) | (5.29%) | ||||||||
| Song et al., 2014 ^ [33] | 64/1105 * | 10/322 | 3/192 | 4/172 | 1/157 | 12/274 | 94/2222 | 4/1528 | - | 17 # (6.3–63) | 24% (10–62%) | |
| (5.8%) | (3.1%) | (1.6%) | (2.3%) | (0.6%) | (4.4%) | (4.23%) | (0.26%) | |||||
| Norquist et al., 2016 [35] | 85/1498 | 3/77 | 0/58 | 1/70 | 0/16 | 9/196 | 98/1915 | - | 149/36276 | 14 (8.2–23.8) | NC | |
| (5.7%) | (3.9%) | (0%) | (1.4%) | (0%) | (4.6%) | (5.11%) | (0.41%) | |||||
| Total | 211/3554 | 16/541 | 3/330 | 5/242 | 1/173 | 32/658 | 268/5498 | 4/1528 | - | - | - | |
| (5.93%) | (2.95%) | (0.91%) | (2.0%) | (0.57%) | (4.86%) | (4.87%) | (0.26%) | |||||
| Gene | Study | Cases Carriers/Total (Frequency) | Controls Carriers/Total (Frequency) | Risk (95% CI) | Cumulative Risk (95% CI) a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HGS | END | CCC | LGS | MUC | Mixed/ Unk b | All EOC | Matched | Publicly Available | ||||
| BRIP1 | Walsh et al., 2011 [34] | 2/242 ** | 1/23 | 0/17 | ** | - | 1/78 | 4/360 | - | - | NC | NC |
| (0.8%) | (4.3%) | (0%) | (1.3%) | (1.1%) | ||||||||
| Ramus et al., 2015 [11] | 26/2535 * | 0/65 | 0/25 | 4/416 | 0/26 | 0/160 | 30/ 3227 | 3/3444 | - | RR: 11.2 # (3.2–34.1) | 5.8% (3.6–9.1%) | |
| (1.0%) | (0%) | (0%) | (0.9%) | (0%) | (0%) | (0.92%) | (0.09) | |||||
| Norquist et al., 2016 [35] | 22/1498 | 1/77 | 0/58 | 0/70 | 0/16 | 3/196 | 26/1915 | - | 60/36276 c | OR: 6.4 (3.8–10.6) | NC | |
| (1.5%) | (1.3%) | (0%) | (0%) | (0%) | (1.5%) | (1.36%) | (0.17%) | |||||
| Total | 50/4275 | 2/165 | 0/100 | 4/486 | 0/42 | 4/434 | 60/5502 | 3/3444 | - | - | - | |
| (1.17%) | (1.21%) | (0%) | (0.82%) | (0%) | (0.9%) | (1.1%) | (0.09%) | |||||
| RAD51C | Song et al., 2015 † [48] | 10/1806 * | 1/383 | 1/225 | 1/405 | 0/166 | 1/444 | 14/3429 | 2/2772 | - | OR: 5.2 # (1.1–24) | 5.2% ¶ (1.1–22%) |
| (0.6%) | (0.3%) | (0.4%) | (0.2%) | (0%) | (0.2%) | (0.40%) | (0.07%) | |||||
| Norquist et al., 2016 † [35] | 7/1498 | 1/77 | 0/58 | 0/70 | 0/16 | 3/196 | 11/1915 | - | 39/36276 c | OR: 3.4 (1.5–7.6) | NC | |
| (0.5%) | (1.3%) | (0%) | (0%) | (0%) | (1.5%) | (0.57%) | (0.11%) | |||||
| Total | 17/3304 | 2/460 | 1/283 | 1/475 | 0/182 | 4/640 | 25/5344 | 2/2772 | - | - | - | |
| (0.51%) | (0.43%) | (0.35%) | (0.21%) | (0%) | (0.62%) | (0.47%) | (0.07%) | |||||
| RAD51D | Song et al., 2015 [48] | 9/1806 * | 3/383 | 0/225 | 0/405 | 0/166 | 0/444 | 12/3429 | 1/2772 | - | OR: 12 # (1.5–90) | 12%¶ (1.5–60%) |
| (0.5%) | (0.8%) | (0%) | (0%) | (0%) | (0%) | (0.35%) | (0.04%) | |||||
| Norquist et al., 2016 [35] | 7/1498 | 1/77 | 0/58 | 0/70 | 0/16 | 3/196 | 11/1915 | - | 14/36276 c | OR: 10.9 (4.6–26.0) | NC | |
| (0.5%) | (1.3%) | (0%) | (0%) | (0%) | (1.5%) | (0.57%) | (0.04%) | |||||
| Total | 16/3304 | 4/460 | 0/283 | 0/475 | 0/182 | 3/640 | 23/5344 | 1/2772 | - | - | - | |
| (0.48%) | (0.87%) | (0%) | (0%) | (0%) | (0.47%) | (0.43%) | (0.04%) | |||||
| PALB2 | Ramus et al., 2015 [11] | 6/2535 * | 0/65 | 1/25 | 1/416 | 0/26 | 1/160 | 9/3227 | 3/3444 | - | NC | NC |
| (0.24%) | (0%) | (4%) | (0.24%) | (0%) | (0.62%) | (0.28%) | (0.09%) | |||||
| Norquist et al., 2016 [35] | 9/1498 | 0/77 | 1/58 | 0/70 | 0/16 | 2/196 | 12/1915 | - | 39/36276 c | OR: 4.4 (2.1–9.1) | NC | |
| (0.60%) | (0%) | (1.7%) | (0%) | (0%) | (1.0%) | (0.62%) | (0.10%) | |||||
| Song et al., 2019 [54] | 18/5123 | - | - | - | - | - | 18/5123 | 6/5202 | - | OR: 3.01 # (1.6–5.7) | 3.2% (1.8–5.7%) | |
| (0.35%) | (0.35%) | (0.12%) | ||||||||||
| Total ^ | 15/4033 | 0/142 | 2/83 | 1/486 | 0/42 | 3/356 | 21/5142 | 3/3444 | - | - | - | |
| (0.37%) | (0%) | (2.4%) | (0.2%) | (0%) | (0.84%) | (0.41%) | (0.09%) | |||||
| Gene | Study | Type of Study | Cases Carriers/Total (Frequency) | Controls (Carriers/Total Frequency) | Risk (95% CI) | Cumulative Risk (95% CI) | |
|---|---|---|---|---|---|---|---|
| All EOC | Matched | Publicly Available | |||||
| BRIP1 | Lilyquist et al., 2017 ¶ [27] | Clinical testing lab | 58/6294 (0.92%) | - | ExAC | SRR: 4.99 (3.8–6.4) | NC |
| Kurian et al., 2017 ¶ [26] | Clinical testing lab | 36/5020 (0.71%) | 161/64,649 (0.24%) | - | OR: 2.62 (1.7–3.9) | NC | |
| Castera et al., 2018 ¶ [28] | Genetic counselling | 21/4408 (0.48%) | - | 72/36,276 a (0.20%) | OR: 3.77 (0.7–9.4) | NC | |
| Suszynska et al., 2020 [45] | Meta-analysis ^ | 200/22,494 (0.89%) | - | 209/115,375 b (0.18%) | OR: 4.94 (4.0–6.0) | NC | |
| RAD51C | Meindl et al., 2010 [46] | Family study | 6/480 families | - | - | NC | NC |
| Lilyquist et al., 2017 ¶ [27] | Clinical testing lab | 44/6294 (0.7%) | - | ExAC | SRR: 5.12 (3.7–6.9) | NC | |
| Kurian et al., 2017 ¶ [26] | Clinical testing lab | 32/5020 (0.6%) | 72/64,649 | - | OR: 4.98 (3.0–8.0) | NC | |
| Castera et al., 2018 ¶ [28] | Genetic counselling | 23/4309 (0.5%) | - | 43/36,276 a (0.12%) | OR: 14.6 (5.3–29.5) | NC | |
| Suszynska et al., 2019 [29] | Meta-analysis ^^ | 21/3791 (0.6%) | - | - | OR: 4.3 (2.5–7.5) | NC | |
| Yang et al., 2020 [10] | RAD51C families # | 125 families | - | - | RR: 7.55 (5.6–10.2) | 11% (15–29%) | |
| Suszynska et al., 2020 [45] | Meta-analysis ^ | 149/23,802 (0.62%) | - | 130/115,475 b (0.11%) | OR: 5.59 (4.4–7.0) | NC | |
| RAD51D | Loveday et al., 2011 [47] | Family study | 8/911 families | 1/1060 (0.09%) | - | RR: 6.30 (2.8–13.8) | NC |
| Lilyquist et al., 2017 ¶ [27] | Clinical testing lab | 11/5743 (0.2%) | - | ExAC | SRR: 6.34 (3.1–11.3) | NC | |
| Kurian et al., 2017 ¶ [26] | Clinical testing lab | 9/5020 (0.2%) | 40/64,649 (0.06%) | - | OR: 4.78 (2.1–10.7) | NC | |
| Castera et al., 2018 ¶ [28] | Genetic counselling | 9/4011 (0.2%) | - | 18/36,276 a (0.05%) | OR: 11.8 (1.1–40) | NC | |
| Suszynska et al., 2019 [29] | Meta-analysis ^^ | 19/3258 (0.6%) | - | - | OR: 11.6 (5.9–23) | NC | |
| Yang et al., 2020 [10] | RAD51D families # | 60 families | - | - | RR: 7.6 (5.6–10.3) | 13% (7–23%) | |
| Suszynska et al., 2020 [45] | Meta-analysis ^ | 94/22,787 (0.45%) | - | 72/120,688 b (0.06%) | OR: 6.9 (5.1–9.4) | NC | |
| PALB2 | Yang et al., 2020 [12] | PALB2 families # | 524 families | - | - | RR: 2.91 (1.4–6.0) | 5% (2–10%) |
| Gene | Study | Cases Carriers/Total (Frequency) | Controls Carriers/Total (Frequency) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HGS | END | CCC | LGS | MUC | Mixed/ Unk a | All EOC | Matched | ||
| MLH1 | Song et al., 2014 [33] | 0/1105 * | 0/322 | 1/192 | 0/172 | 0/157 | 0/274 | 1/2222 | 2/1528 |
| (0%) | (0%) | (0.52%) | (0%) | (0%) | (0%) | (0.04%) | (0.13%) | ||
| Norquist et al., 2016 [35] | 0/1498 | 0/77 | 0/58 | 0/70 | 0/16 | 1/196 b | 1/1915 | - | |
| (0%) | (0%) | (0%) | (0%) | (0%) | (0.51%) | (0.05%) | |||
| Total | 0/2603 | 0/399 | 1/250 | 0/242 | 0/173 | 1/470 | 2/4137 | 2/1528 | |
| (0%) | (0%) | (0.40%) | (0%) | (0%) | (0.21%) | (0.04%) | (0.13%) | ||
| MSH2 | Song et al., 2014 [33] | 1/1105 * | 0/322 | 1/192 | 0/172 | 0/157 | 0/274 | 2/2222 | 0/1528 |
| (0.09%) | (0%) | (0.52%) | (0%) | (0%) | (0%) | (0.09%) | (0%) | ||
| Total | 1/1105 | 0/322 | 1/192 | 0/172 | 0/157 | 0/274 | 2/2222 | 0/1528 | |
| (0.09%) | (0%) | (0.52%) | (0%) | (0%) | (0%) | (0.09%) | (0%) | ||
| MSH6 | Walsh et al., 2011 [34] | 0/242 ** | 2/23 | 0/17 | ** | - | 0/78 | 2/360 | - |
| (0%) | (8.7%) | (0%) | (0%) | (0.55%) | |||||
| Song et al., 2014 [33] | 4/1105 * | 2/322 | 3/192 | 0/172 | 0/157 | 0/274 | 9/2222 | 1/1528 | |
| (0.36%) | (0.62%) | (1.56%) | (0%) | (0%) | (0%) | (0.40%) | (0.06%) | ||
| Norquist et al., 2016 [35] | 1/1498 | 2/77 | 0/58 | 0/70 | 0/16 | 0/196 | 3/1915 | - | |
| (0.06%) | (2.59%) | (0%) | (0%) | (0%) | (0%) | (0.15%) | |||
| Total | 5/2845 | 6/422 | 3/267 | 0/242 | 0/173 | 0/548 | 14/4497 | 1/1528 | |
| (0.17%) | (1.42%) | (1.12%) | (0%) | (0%) | (0%) | (0.31%) | (0.06%) | ||
| PMS2 | Song et al., 2014 [33] | 0/1105 * | 1/322 | 0/192 | 0/172 | 0/157 | 0/274 | 1/2222 | 0/1528 |
| (0%) | (0.31%) | (0%) | (0%) | (0.6%) | (0%) | (0.04%) | (0%) | ||
| Norquist et al., 2016 [35] | 4/1498 | 0/77 | 0/58 | 0/70 | 0/16 | 0/196 | 4/1915 | - | |
| (0.3%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0.20%) | |||
| Total | 4/2603 | 1/399 | 0/250 | 0/242 | 0/173 | 0/470 | 5/4137 | 0/1528 | |
| (0.15%) | (0.25%) | (0%) | (0%) | (0%) | (0%) | (0.12%) | (0%) | ||
| All 4 genes | Total $ | 10/2845 | 7/422 | 5/267 | 0/242 | 0/173 | 1/548 | 23/4497 | 3/1528 |
| (0.35%) | (1.65%) | (1.87%) | (0%) | (0%) | (0.18%) | (0.51%) | (0.19%) | ||
| Genes | Study | Cases Del Missense/Total (Frequency) | Predicted Deleterious | Evidence of Risk from RAML |
|---|---|---|---|---|
| BRIP1 | Ramus et al., 2015 [11] | 35/3227 a (1.1%) | SIFT, PolyPhen-2 and Provean | Yes (All EOC, but stronger in HGS) |
| Suszynska et al., 2020 [45] | 2 */22494 | Described in ClinVar | - | |
| RAD51C | Song et al., 2015 [11] | 12/3429 b (0.32%) | SIFT, PolyPhen-2 and Provean | Yes (All EOC, but stronger in HGS) |
| Lilyquist et al., 2017 [27] | 2 $/6294 | Described in ClinVar | - | |
| Norquist et al., 2016 [35] | 1 #/1915 c | Functional assay [65] | - | |
| Suszynska et al., 2020 [45] | 3 ^/22,494 | Described in ClinVar | - | |
| RAD51D | Song et al., 2015 [11] | 16/3429 (0.46%) | SIFT, PolyPhen-2 and Provean | Yes (Only in HGS) |
| Suszynska et al., 2020 [45] | 1 ¶/22,494 | Described in ClinVar | - | |
| PALB2 | Ramus et al., 2015 [11] | 26/3227 (0.80%) | SIFT, PolyPhen-2 and Provean | No |
| Song et al., 2019 [54] | 40/5123 a (0.78%) | SIFT, PolyPhen-2 and Provean | No |
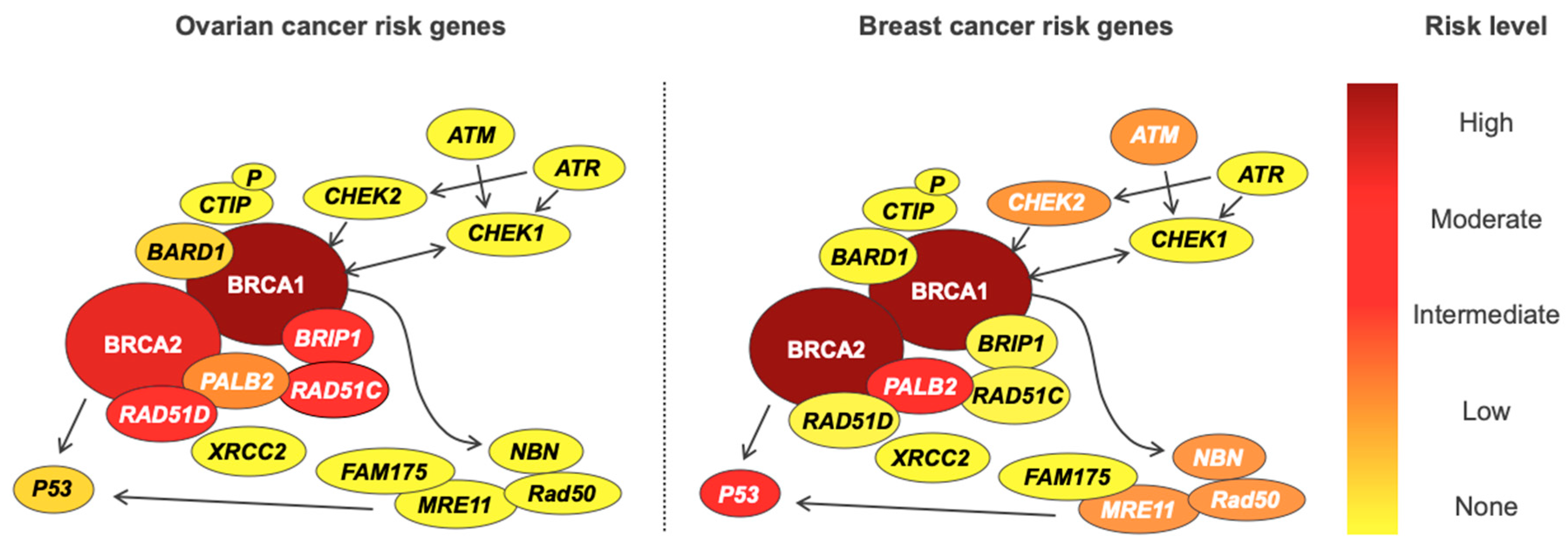
| Genes | Frequency (%) | Risk Est. ^ | Risk Level | Clinical Management $ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HGS | END | CCC | LGS | MUC | EOC | BC | EOC | BC | EOC | BC | |
| BRCA1 | 7.8 | 2.9 | 3.6 | 3.7 | <1 | 60 | 72% * | Very high | Very high | RRSO age 35 to 45 PARPi | RRM age 25 to 40 |
| BRCA2 | 5.9 | 2.9 | <1 | 2 | <1 | 17 | 69% * | High | Very high | ||
| MMR | <1 | 1.6 | 1.9 | 0 | 0 | 2.3 | - | Mod | None | RRSO with hysterectomy for LS | No increased risk |
| BRIP1 | 1.2 | 1.2 | 0 | <1 | 0 | 11.2 | - | Mod | None | RRSO age 45 to 50 no consensus | Insufficient evidence |
| RAD51C | <1 | <1 | <1 | <1 | 0 | 5.2 | 1.9 | Mod | None | ||
| RAD51D | <1 | <1 | 0 | 0 | 0 | 12 | 1.8 | Mod | None | ||
| PALB2 | <1 | 0 | 2.4 | <1 | 0 | 3.0 | 7.2 | Low | Mod | Insufficient evidence | Annual mammography/breast MRI age 30 no consensus |
| TP53 | Insufficient data | Insuf | Insuf | Low | Mod | Insufficient evidence | Insufficient evidence | ||||
| CHEK2 | No increased risk | - | 3.0 | None | Low | No increased risk | Annual mammography/breast MRI age 40 no consensus | ||||
| ATM | No increased risk | - | 2.8 | None | Low | ||||||
| NBN | No increased risk | - | 2.7 | None | Low | ||||||
| RAD50 | No increased risk | - | Insuf | None | Low | No increased risk | Insufficient evidence | ||||
| MRE11A | No increased risk | - | Insuf | None | Low | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavanello, M.; Chan, I.H.; Ariff, A.; Pharoah, P.D.; Gayther, S.A.; Ramus, S.J. Rare Germline Genetic Variants and the Risks of Epithelial Ovarian Cancer. Cancers 2020, 12, 3046. https://doi.org/10.3390/cancers12103046
Pavanello M, Chan IH, Ariff A, Pharoah PD, Gayther SA, Ramus SJ. Rare Germline Genetic Variants and the Risks of Epithelial Ovarian Cancer. Cancers. 2020; 12(10):3046. https://doi.org/10.3390/cancers12103046
Chicago/Turabian StylePavanello, Marina, Isaac HY Chan, Amir Ariff, Paul DP Pharoah, Simon A. Gayther, and Susan J. Ramus. 2020. "Rare Germline Genetic Variants and the Risks of Epithelial Ovarian Cancer" Cancers 12, no. 10: 3046. https://doi.org/10.3390/cancers12103046
APA StylePavanello, M., Chan, I. H., Ariff, A., Pharoah, P. D., Gayther, S. A., & Ramus, S. J. (2020). Rare Germline Genetic Variants and the Risks of Epithelial Ovarian Cancer. Cancers, 12(10), 3046. https://doi.org/10.3390/cancers12103046





