Commonly Applied Selection Criteria for Lung Cancer Screening May Have Strongly Varying Diagnostic Performance in Different Countries
Simple Summary
Abstract
1. Introduction
2. Results
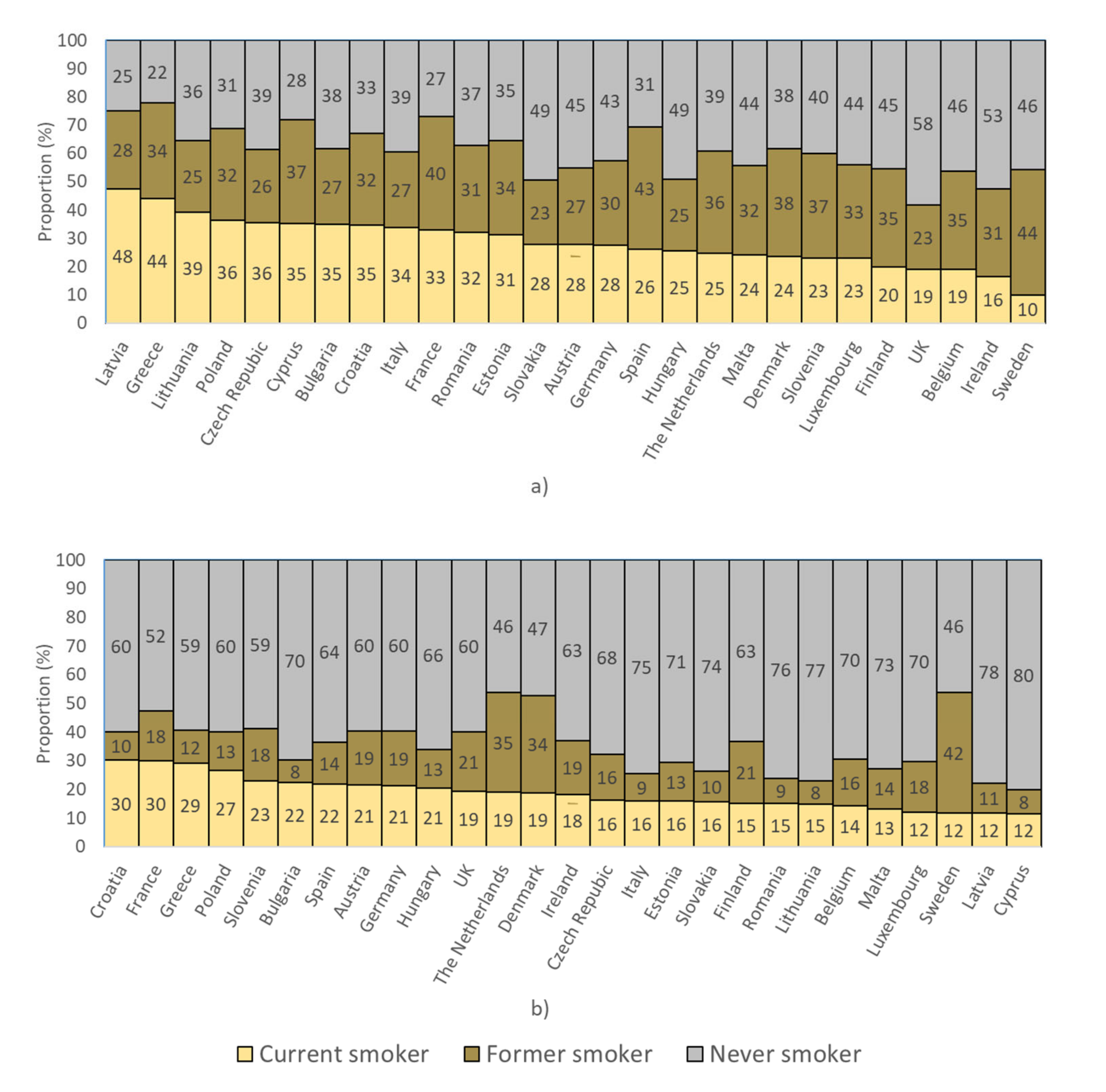
2.1. Smoking Prevalence in European Countries
2.2. Populations Eligible for Screening
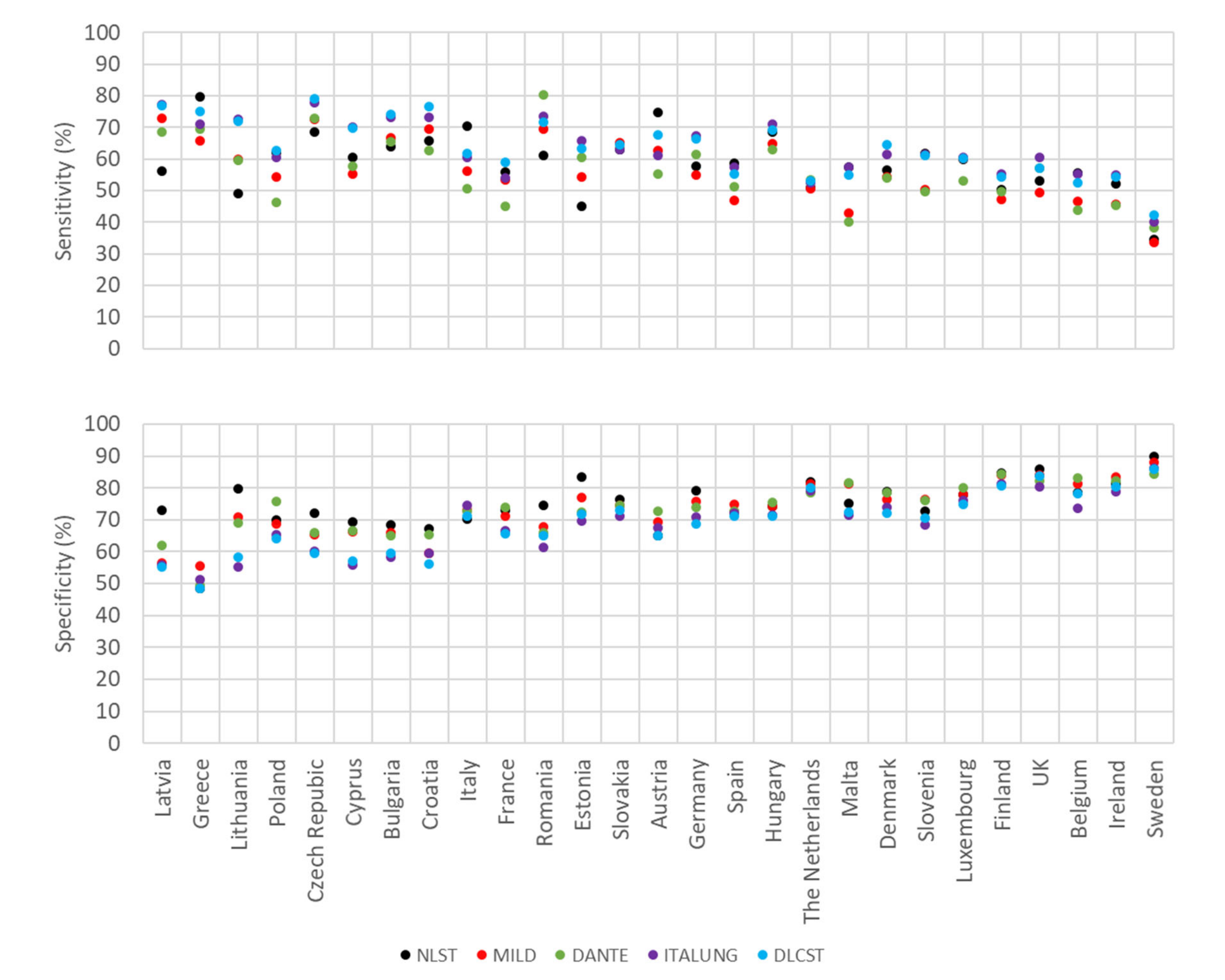
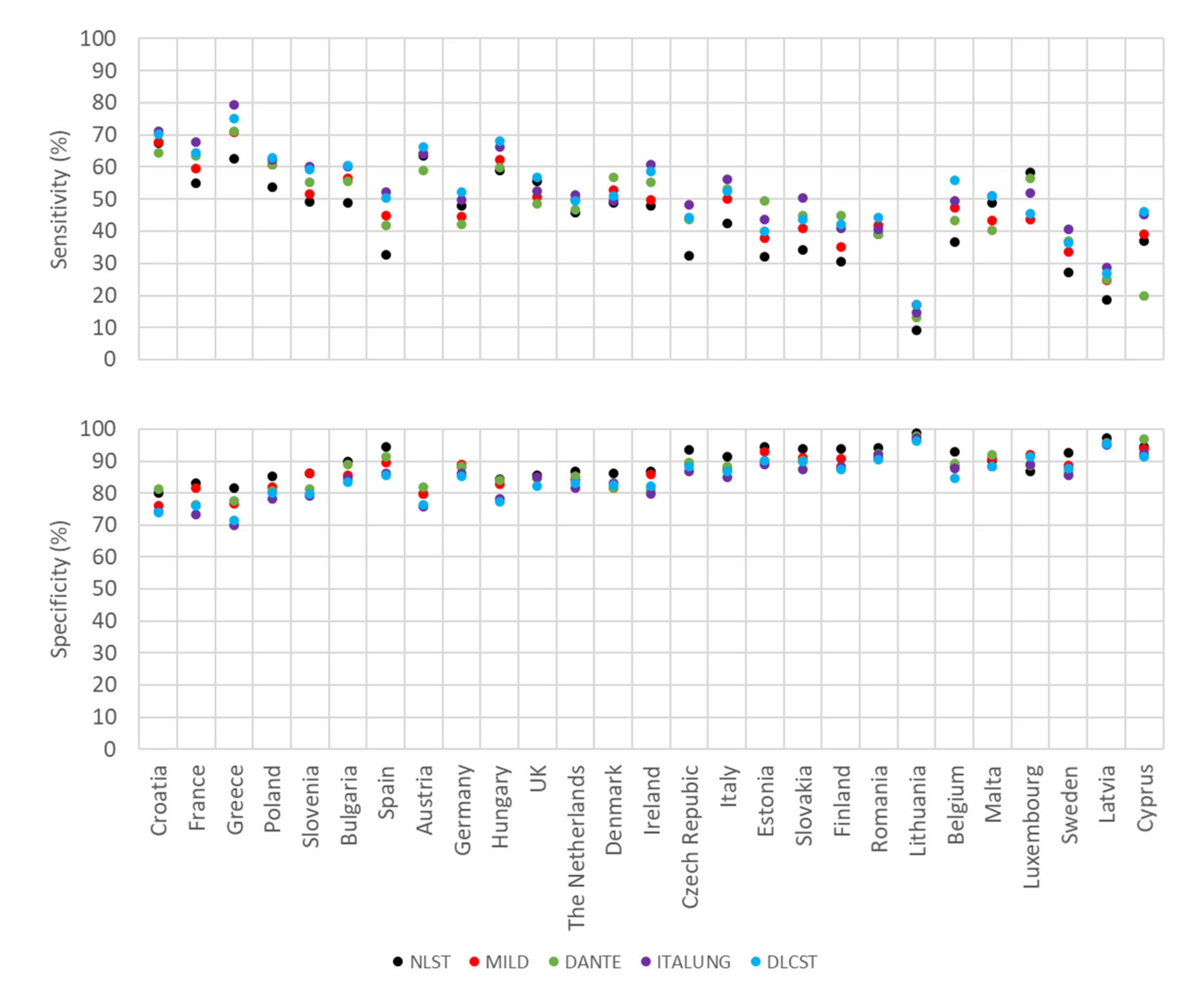
2.3. Estimates of Sensitivity and Specificity for Smoking History and Age
3. Discussion
4. Materials and Methods
4.1. Pre-Screening Criteria of Heavy Smoking Assessed in this Study
4.2. Strength of the Association between Smoking and Lung Cancer
4.3. Prevalence of Smoking in European Countries
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
Appendix D
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–426. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A. Preventive Services Task Force. Screening for lung cancer: US preventive services task force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). Available online: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274&N (accessed on 30 September 2020).
- McRonald, F.E.; Yadegarfar, G.; Baldwin, D.R.; Devaraj, A.; Brain, K.E.; Eisen, T.; Holemans, J.A.; Ledson, M.; Screaton, N.; Rintoul, R.C.; et al. The UK Lung Screen (UKLS): Demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev. Res. (Phila) 2014, 7, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Rossi, M.; Rosato, V.; Marchiano, A.; Sverzellati, N.; Morosi, C.; Fabbri, A.; Galeone, C.; Negri, E.; Sozzi, G.; et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur. J. Cancer Prev. 2012, 21, 308–315. [Google Scholar] [CrossRef]
- Infante, M.; Cavuto, S.; Lutman, F.R.; Passera, E.; Chiarenza, M.; Chiesa, G.; Brambilla, G.; Angeli, E.; Aranzulla, G.; Chiti, A.; et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am. J. Respir. Crit. Care Med. 2015, 191, 1166–1175. [Google Scholar] [CrossRef]
- Paci, E.; Puliti, D.; Pegna, A.L.; Carrozzi, L.; Picozzi, G.; Falaschi, F.; Pistelli, F.; Aquilini, F.; Ocello, C.; Zappa, M.; et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017, 72, 825–831. [Google Scholar] [CrossRef]
- Wille, M.M.; Dirksen, A.; Ashraf, H.; Saghir, Z.; Bach, K.S.; Brodersen, J.; Clementsen, P.F.; Hansen, H.; Larsen, K.R.; Mortensen, J.; et al. Results of the randomized danish lung cancer screening trial with focus on high-risk profiling. Am. J. Respir. Crit. Care Med. 2016, 193, 542–551. [Google Scholar] [CrossRef]
- van Iersel, C.A.; de Koning, H.J.; Draisma, G.; Mali, W.P.; Scholten, E.T.; Nackaerts, K.; Prokop, M.; Habbema, J.D.; Oudkerk, M.; van Klaveren, R.J. Risk-based selection from the general population in a screening trial: Selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int. J. Cancer 2007, 120, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Motsch, E.; Trotter, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Kauczor, H.U.; Maldonado, S.G.; Miller, A.B.; Kaaks, R.; et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int. J. Cancer 2020, 146, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Blanchon, T.; Brechot, J.M.; Grenier, P.A.; Ferretti, G.R.; Lemarie, E.; Milleron, B.; Chague, D.; Laurent, F.; Martinet, Y.; Beigelman-Aubry, C.; et al. Baseline results of the depiscan study: A French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007, 58, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Schumer, E.M.; Trivedi, J.R.; van Berkel, V.; Black, M.C.; Li, M.; Fu, X.A.; Bousamra, M., II. High sensitivity for lung cancer detection using analysis of exhaled carbonyl compounds. J. Thorac Cardiovasc. Surg. 2015, 150, 1517–1522. [Google Scholar] [CrossRef]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; di Natale, C.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016, 10, 16007. [Google Scholar] [CrossRef]
- Tirzite, M.; Bukovskis, M.; Strazda, G.; Jurka, N.; Taivans, I. Detection of lung cancer in exhaled breath with an electronic nose using support vector machine analysis. J. Breath Res. 2017, 11, 36009. [Google Scholar] [CrossRef]
- Phillips, M.; Bauer, T.L.; Pass, H.I. A volatile biomarker in breath predicts lung cancer and pulmonary nodules. J. Breath Res. 2019, 13, 36013. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, M.; Weng, Y.; Zhang, F.; Meng, D.; Song, J.; Zhou, H.; Xie, Z. Circulating MACC1 as a novel diagnostic and prognostic biomarker for nonsmall cell lung cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 1353–1361. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yu, Z.B.; Yuan, S.; Xie, W.J.; Li, C.Y.; Hu, Z.Y.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef]
- Jin, X.C.; Chen, Y.F.; Chen, H.B.; Fei, S.R.; Chen, D.D.; Cai, X.N.; Liu, L.; Lin, B.C.; Su, H.F.; Zhao, L.H.; et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef]
- Abdollahi, A.; Rahmati, S.; Ghaderi, B.; Sigari, N.; Nikkhoo, B.; Sharifi, K.; Abdi, M. A combined panel of circulating micro-RNA as a diagnostic tool for detection of the non-small cell lung cancer. QJM Int. J. Med. 2019, 112, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F. Lung cancer screening with low-dose CT: A world-wide view. Transl. Lung Cancer Res. 2018, 7, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of cancer through exhaled breath: A systematic review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef]
- Hamling, J.S.; Coombs, K.J.; Lee, P.N. Misclassification of smoking habits: An updated review of the literature. World J. Meta-Anal. 2019, 7, 31–50. [Google Scholar] [CrossRef]
- Bilano, V.; Gilmour, S.; Moffiet, T.; d’Espaignet, E.T.; Stevens, G.A.; Commar, A.; Tuyl, F.; Hudson, I.; Shibuya, K. Global trends and projections for tobacco use, 1990–2025: An analysis of smoking indicators from the WHO comprehensive information systems for tobacco control. Lancet 2015, 385, 966–976. [Google Scholar] [CrossRef]
- Pesch, B.; Kendzia, B.; Gustavsson, P.; Jockel, K.H.; Johnen, G.; Pohlabeln, H.; Olsson, A.; Ahrens, W.; Gross, I.M.; Bruske, I.; et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer 2012, 131, 1210–1219. [Google Scholar] [CrossRef]
- European Commission and European Parliament. Eurobarometer 87.1; TNS opinion, Brussels [producer]; GESIS Data Archive: Cologne, Germany, 2017; ZA6861 Data file Version 1.2.0. [Google Scholar] [CrossRef]
| Trial/Recommendation (Country) | Reference | Smoking History (Current and Former Smokers) | Restrictions for Former Smokers | Target Age |
|---|---|---|---|---|
| NLST (U.S.) | [3] | ≥30 pack-years | quit within 15 years | 55–74 |
| U.S. Preventive Services Task Force 1 (U.S.) | [5] | ≥30 pack-years | quit within 15 years | 55–80 |
| Centers for Medicare and Medicaid Services 1 (U.S.) | [6] | ≥30 pack-years | quit within 15 years | 55–77 |
| MILD (Italy) | [8] | ≥20 pack-years | quit within 10 years | 49+ |
| DANTE (Italy) | [9] | ≥20 pack-years | quit within 10 years | 60–74 |
| ITALUNG (Italy) | [10] | ≥20 pack-years | quit within 10 years | 55–69 |
| DLCST (Denmark) | [11] | ≥20 pack-years | quit within 10 years | 50–70 |
| LUSI (Germany) | [13] | Either ≥15 cigarettes per day for at least 25 years or ≥10 cigarettes per day for at least 30 years | quit within 10 years | 50–69 |
| NELSON (Netherlands/Belgium) | [12] | Either ≥15 cigarettes per day for at least 25 years or ≥10 cigarettes per day for at least 30 years | quit within 10 years | 50–75 |
| DEPISCAN (France) | [14] | ≥15 cigarettes per day for at least 20 years | quit within 15 years | 50–74 |
| UKLS (UK) | [7] | Individual risk for lung cancer assessed with the Liverpool Lung Project (LLP) risk model | n/a | 50–75 |
| Smoking Status | Pack-Years | N. Cases | N. Controls | Odds Ratio (OR) (95% CI) 1 |
|---|---|---|---|---|
| Men | ||||
| Never smokers | - | 220 | 2883 | 1.0 (reference) |
| Current smokers | >1–<20 | 646 | 885 | 8.9 (7.4–10.6) |
| 20–<30 | 1213 | 880 | 17.1 (14.4–20.2) | |
| 30–<40 | 1527 | 800 | 24.6 (20.8–29.0) | |
| 40–<50 | 1324 | 582 | 32.4 (26.7–39.5) | |
| 50–<60 | 770 | 243 | 46.3 (37.0–58.1) | |
| ≥60 | 1245 | 414 | 47.7 (38.5–59.0) | |
| Women | ||||
| Never smokers | - | 609 | 1902 | 1.0 (reference) |
| Current smokers | >1–<20 | 330 | 305 | 3.5 (2.9–4.3) |
| 20–<30 | 304 | 160 | 7.3 (5.8–9.2) | |
| 30–<40 | 291 | 97 | 12.9 (9.9–16.9) | |
| 40–<50 | 204 | 61 | 14.0 (9.3–21.1) | |
| 50–<60 | 129 | 31 | 17.9 (10.6–30.1) | |
| ≥60 | 151 | 24 | 25.7 (14.5–45.5) |
| Parameter | Notation | Source |
|---|---|---|
| Prevalence of lung cancer among never smokers 1 | LN | |
| Relative risk for pack-year category i (j) above (below) threshold compared to never smokers | RRi (RRj) | Pesch et al. [28]: Odds ratios in Table 2 |
| Proportions of various subgroups in target age range of screening | Eurobarometer data base [29] | |
| Never smokers | PN | |
| Current smokers in pack-year category i (j) above (below) threshold | PCi (PCj) | |
| Short-term quitters in pack-year category i (j) above (below) threshold 2 | PSi (PSj) | |
| Long-term quitters in pack-year category i (j) above (below) threshold 2 | PLi (PLj) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenner, H.; Krilaviciute, A. Commonly Applied Selection Criteria for Lung Cancer Screening May Have Strongly Varying Diagnostic Performance in Different Countries. Cancers 2020, 12, 3012. https://doi.org/10.3390/cancers12103012
Brenner H, Krilaviciute A. Commonly Applied Selection Criteria for Lung Cancer Screening May Have Strongly Varying Diagnostic Performance in Different Countries. Cancers. 2020; 12(10):3012. https://doi.org/10.3390/cancers12103012
Chicago/Turabian StyleBrenner, Hermann, and Agne Krilaviciute. 2020. "Commonly Applied Selection Criteria for Lung Cancer Screening May Have Strongly Varying Diagnostic Performance in Different Countries" Cancers 12, no. 10: 3012. https://doi.org/10.3390/cancers12103012
APA StyleBrenner, H., & Krilaviciute, A. (2020). Commonly Applied Selection Criteria for Lung Cancer Screening May Have Strongly Varying Diagnostic Performance in Different Countries. Cancers, 12(10), 3012. https://doi.org/10.3390/cancers12103012





