FGF2-Derived PeptibodyF2-MMAE Conjugate for Targeted Delivery of Cytotoxic Drugs into Cancer Cells Overexpressing FGFR1
Simple Summary
Abstract
1. Introduction
2. Results
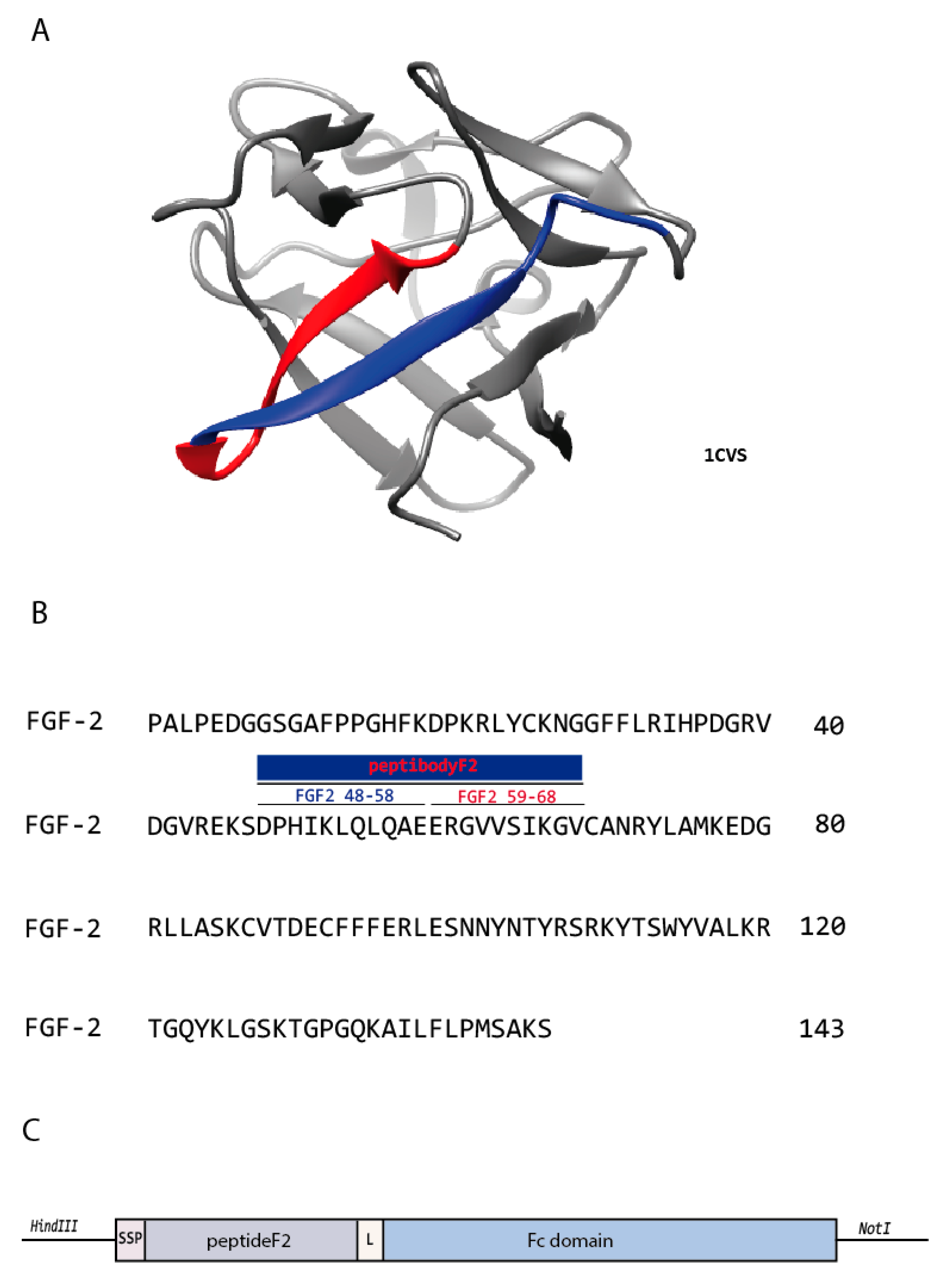
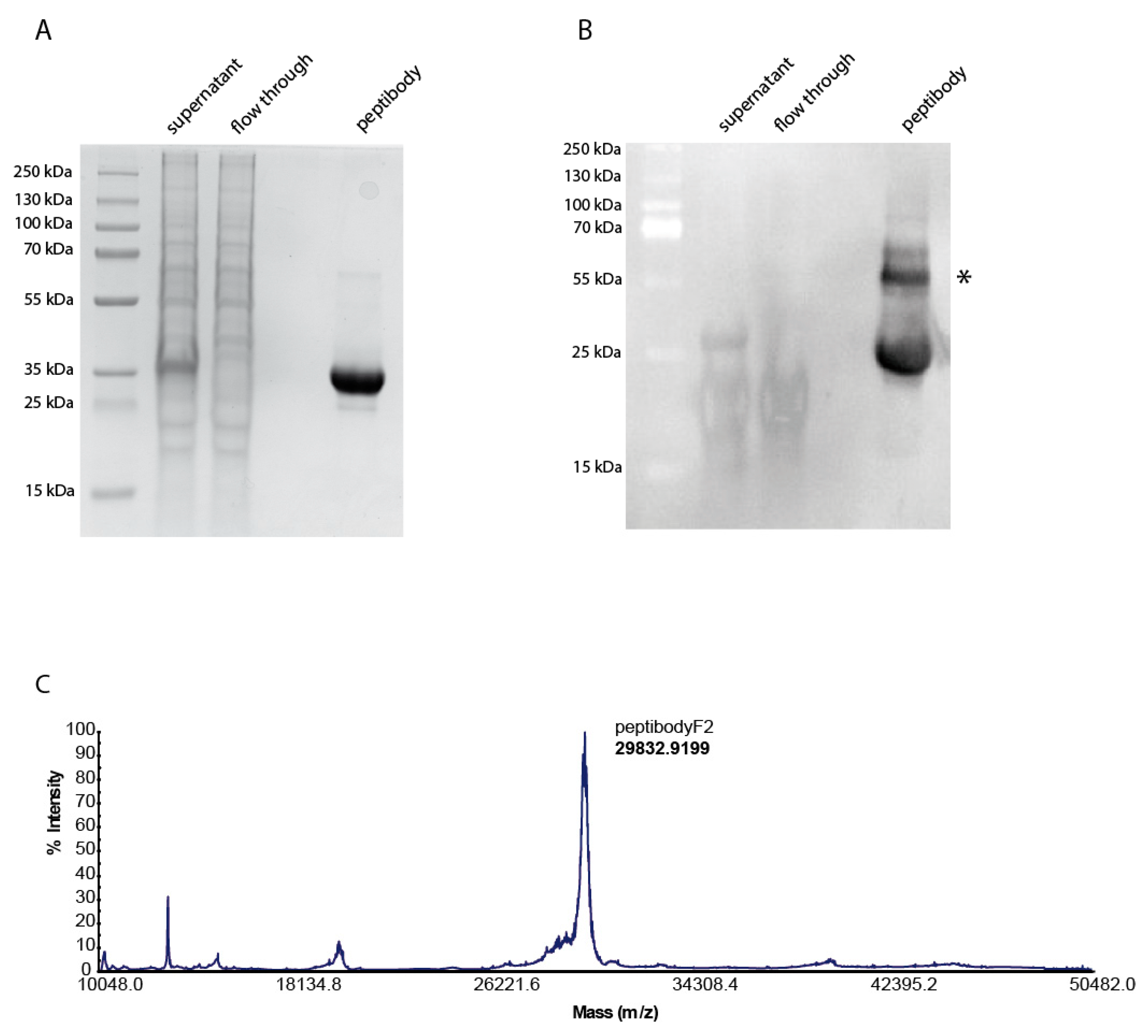
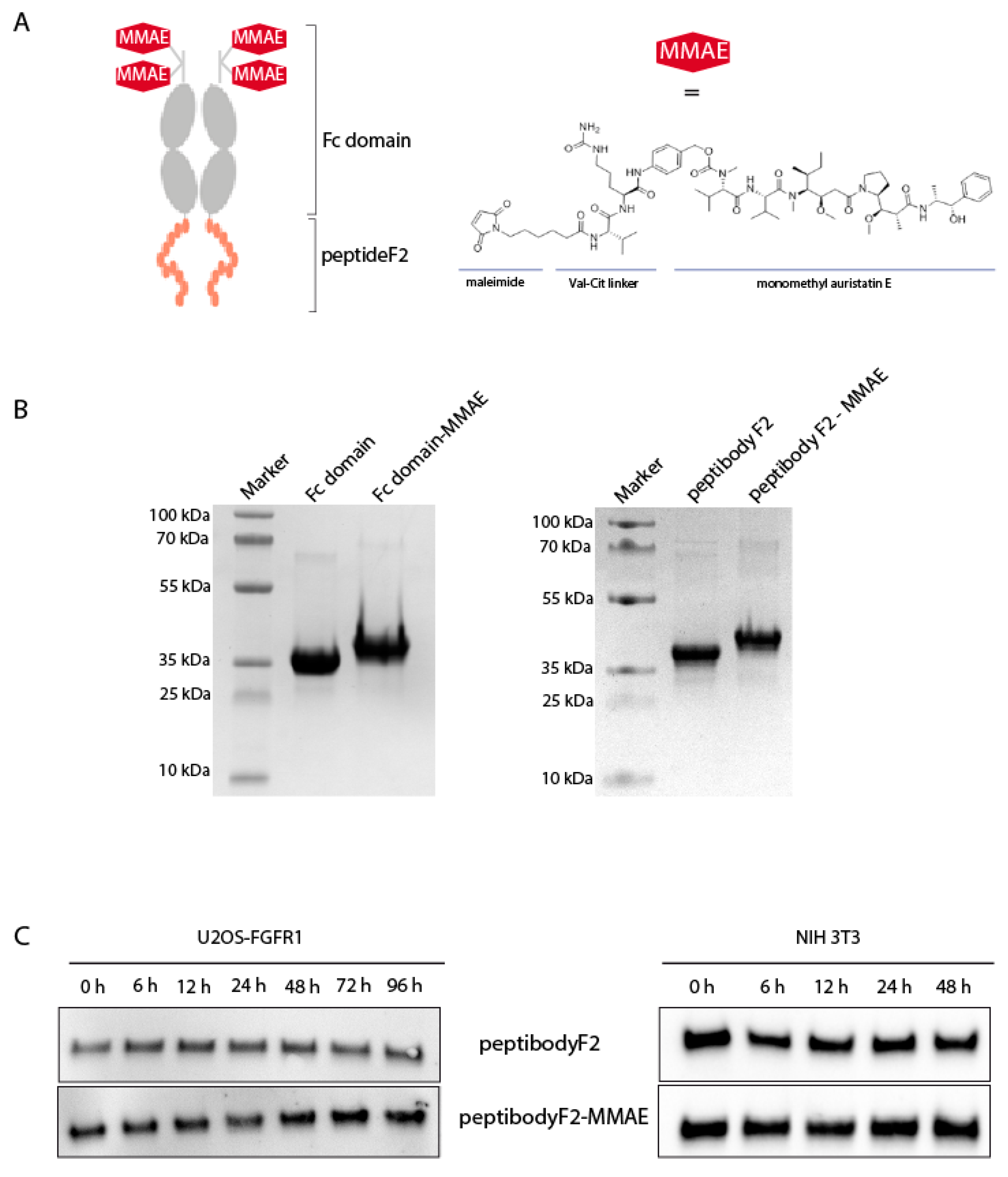
2.1. Design, Expression and Purification of FGFR-Targeting Peptibody
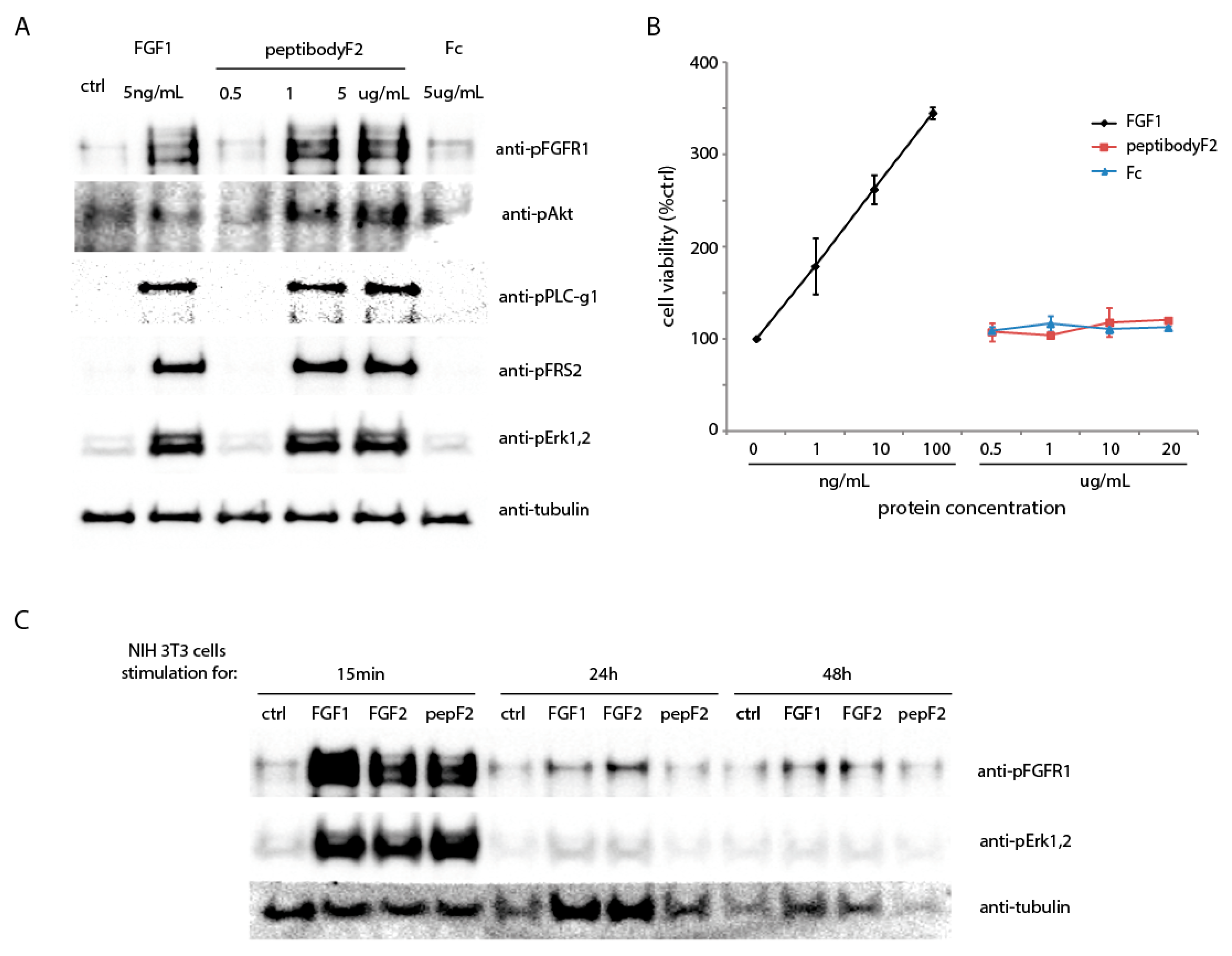
2.2. PeptibodyF2 Binds FGFR1 and Induces Receptor Activation, but Not Cell Proliferation
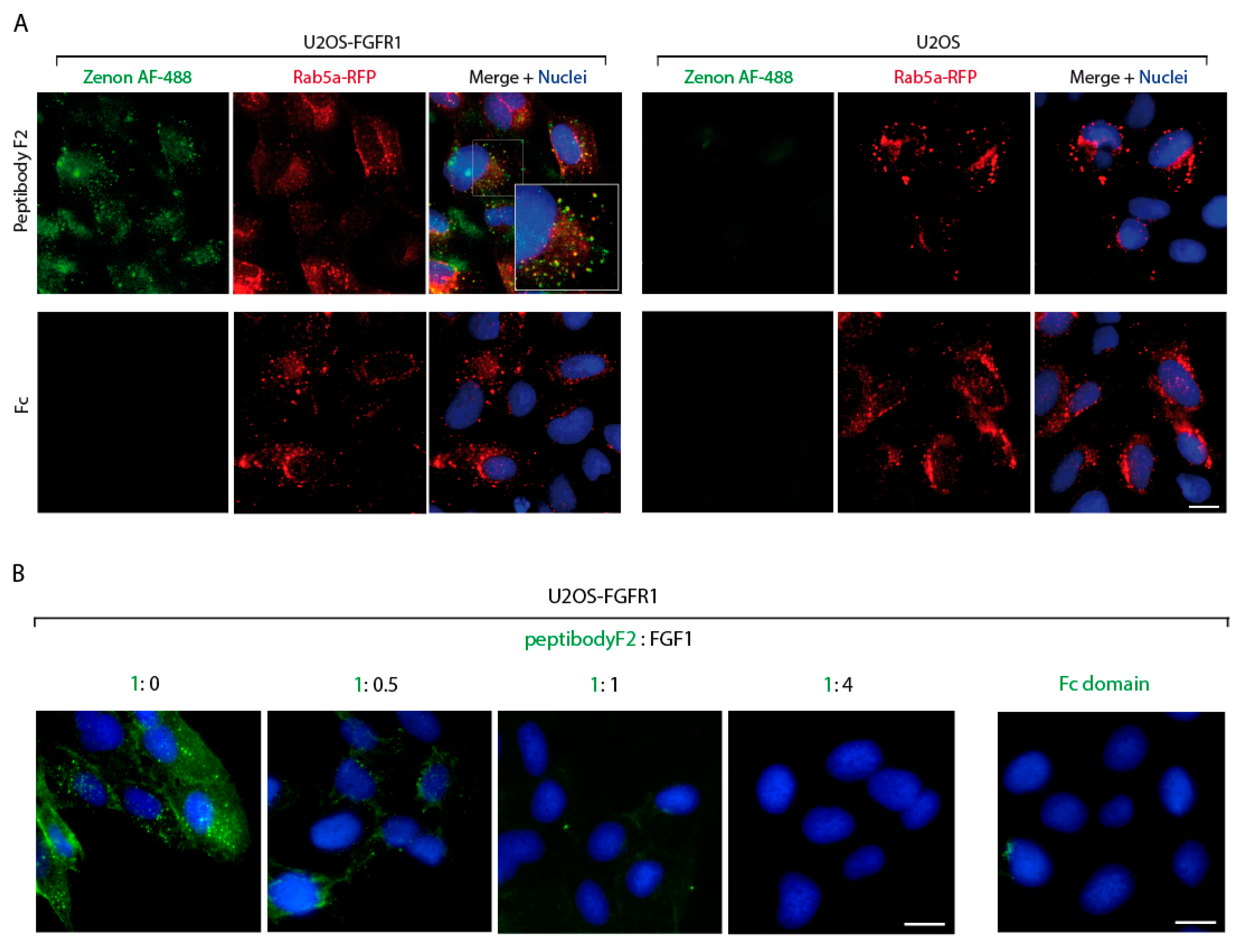
2.3. PeptibodyF2 Is Specifically Internalized by FGFR-Expressing Cells
2.4. Site-Specific Conjugation of Monomethyl Auristatin E to the PeptibodyF2
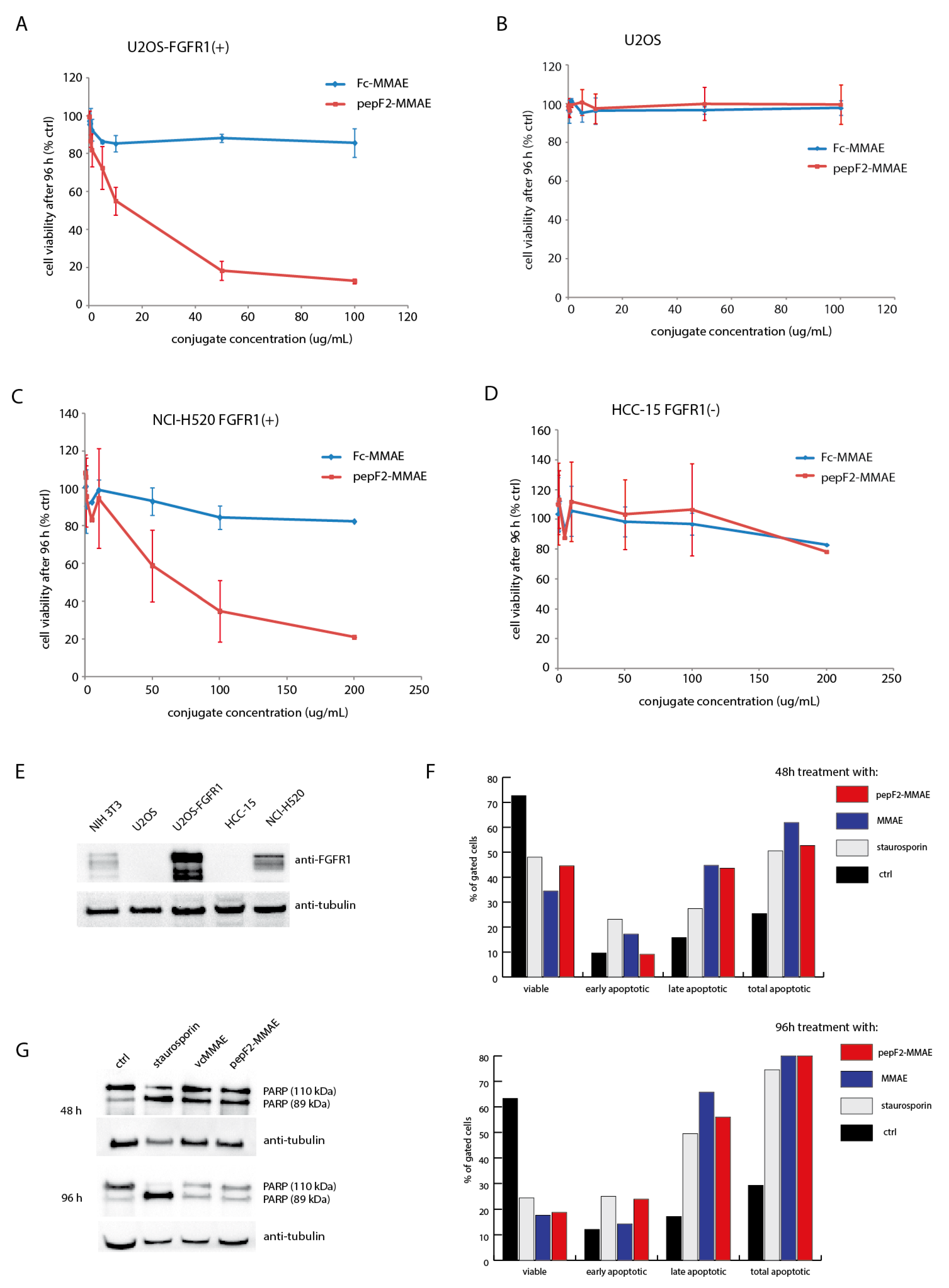
2.5. Cytotoxicity of PeptibodyF2-MMAE Conjugates
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Expression and Purification of Peptibody and Fc Fragment
4.3. Activation of FGFR1-Dependent Pathways in NIH 3T3 Cells
4.4. FGF1-Induced Proliferative Response
4.5. Fluorescence Microscopy
4.6. Conjugation of PeptibodyF2 and Fc Fragment with MMAE
4.7. Protein Stability in Cell-Conditioned Medium
4.8. Cytotoxicity Assay
4.9. Annexin V-7AAD Assay
4.10. PARP Cleavage
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindblom, A. Regular review: Tumour markers in malignancies. BMJ 2000, 320, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Bertucci, F.; Adélaïde, J.; Parc, P.; Coulier, F.; Jacquemier, J.; Birnbaum, D.; Delapeyrière, O. Expression of fgf and fgf receptor genes in human breast cancer. Int. J. Cancer 1995, 61, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. New Drug Development and Clinical Pharmacology Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Ray, M.E.; Yang, Z.Q.; Albertson, D.; Kleer, C.G.; Washburn, J.G.; Macoska, J.A.; Ethier, S.P. Genomic and Expression Analysis of the 8p11–12 Amplicon in Human Breast Cancer Cell Lines. Cancer Res. 2004, 64, 40–47. [Google Scholar] [CrossRef]
- Haugsten, E.M.; Wiedlocha, A.; Olsnes, S.; Wesche, J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol. Cancer Res. 2010, 8, 1439–1452. [Google Scholar] [CrossRef]
- Babina, I.S.; Turner, N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef]
- Fearon, A.E.; Gould, C.R.; Grose, R.P. FGFR signalling in women’s cancers. Int. J. Biochem. Cell Biol. 2013, 45, 2832–2842. [Google Scholar] [CrossRef]
- Wesche, J.; Haglund, K.; Haugsten, E.M. Fibroblast growth factors and their receptors in cancer. Biochem. J. 2011, 437, 199–213. [Google Scholar] [CrossRef]
- Markham, A. Erdafitinib: First Global Approval. Drugs 2019, 79, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Pemigatinib: First Approval. Drugs 2020, 80, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, R.M.; Goldenberg, D.M. Targeted Therapy of Cancer: New Prospects for Antibodies and Immunoconjugates. CA Cancer J. Clin. 2006, 56, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Jazirehi, A.R.; Bonavida, B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: Implications in chemosensitization and therapeutic intervention. Oncogene 2005, 24, 2121–2143. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal antibodies in cancer therapy. Cancer Immun. Arch. 2012, 12, 14. [Google Scholar]
- Shimamoto, G.; Gegg, C.; Boone, T.; Quéva, C. A flexible alternative format to antibodies. mAbs 2012, 4, 586–591. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Kivimäe, S.; Szoka, F.C. Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice. PLoS ONE 2014, 9, e102566. [Google Scholar] [CrossRef]
- Fayaz, S.; Fard-Esfahani, P.; Golkar, M.; Allahyari, M.; Sadeghi, S. Expression, purification and biological activity assessment of romiplostim biosimilar peptibody. DARU J. Pharm. Sci. 2016, 24, 1–5. [Google Scholar] [CrossRef]
- Lu, S.C.; Atangan, L.; Kim, K.W.; Chen, M.M.; Komorowski, R.; Chu, C.; Han, J.; Hu, S.; Gu, W.; Véniant, M.; et al. An apoA-I mimetic peptibody generates HDL-like particles and increases alpha-1 HDL subfraction in mice. J. Lipid Res. 2012, 53, 643–652. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, X.; Zhu, L.; Gan, Y.; Baiwu, R.; Wei, J.; Li, Z.; Li, R.; Sun, J. A Novel BLyS Peptibody Down-Regulates B Cell and T Helper Cell Subsets In Vivo and Ameliorates Collagen-Induced Arthritis. Inflammation 2016, 39, 839–848. [Google Scholar] [CrossRef]
- Molineux, G.; Newland, A. Development of romiplostim for the treatment of patients with chronic immune thrombocytopenia: From bench to bedside: Review. Br. J. Haematol. 2010, 150, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Trujillo, J.M. Dulaglutide: The Newest GLP-1 Receptor Agonist for the Management of Type 2 Diabetes. Ann. Pharmacother. 2015, 49, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; Baird, A.; Gage, F.H. A 10-amino acid sequence of fibroblast growth factor 2 is sufficient for its mitogenic activity on neural progenitor cells. Proc. Natl. Acad. Sci. USA 1997, 94, 7047–7052. [Google Scholar] [CrossRef] [PubMed]
- Facchiano, A.; Russo, K.; Facchiano, A.M.; De Marchis, F.; Facchiano, F.; Ribatti, D.; Aguzzi, M.S.; Capogrossi, M.C. Identification of a novel domain of fibroblast growth factor 2 controlling its angiogenic properties. J. Biol. Chem. 2003, 278, 8751–8760. [Google Scholar] [CrossRef]
- Sokolowska-Wedzina, A.; Borek, A.; Chudzian, J.; Jakimowicz, P.; Zakrzewska, M.; Otlewski, J. Efficient production and purification of extracellular domain of human FGFR-Fc fusion proteins from Chinese hamster ovary cells. Protein Expr. Purif. 2014, 99, 50–57. [Google Scholar] [CrossRef]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef]
- Borek, A.; Sokolowska-Wedzina, A.; Chodaczek, G.; Otlewski, J. Generation of high-affinity, internalizing anti-fgfr2 single-chain variable antibody fragment fused with fc for targeting gastrointestinal cancers. PLoS ONE 2018, 13, e0192194. [Google Scholar] [CrossRef]
- Doronina, S.O.; Mendelsohn, B.A.; Bovee, T.D.; Cerveny, C.G.; Alley, S.C.; Meyer, D.L.; Oflazoglu, E.; Toki, B.E.; Sanderson, R.J.; Zabinski, R.F.; et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: Effects of linker technology on efficacy and toxicity. Bioconjug. Chem. 2006, 17, 114–124. [Google Scholar] [CrossRef]
- Doronina, S.O.; Bovee, T.D.; Meyer, D.W.; Miyamoto, J.B.; Anderson, M.E.; Morris-Tilden, C.A.; Senter, P.D. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug. Chem. 2008, 19, 1960–1963. [Google Scholar] [CrossRef]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural basis of microtubule destabilization by potent auristatin anti-mitotics. PLoS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef]
- Cavaco, M.; Castanho, M.A.R.B.; Neves, V. Peptibodies: An elegant solution for a long-standing problem. Pept. Sci. 2018, 110. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; Freund, B.; Nielsen, P.; Jung, C.; Reimer, R.; Hohenberg, H.; Zangemeister-Wittke, U.; Wester, H.J.; Lüers, G.H.; Schumacher, U. High interstitial fluid pressure is associated with low tumour penetration of diagnostic monoclonal antibodies applied for molecular imaging purposes. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Christiansen, J.; Rajasekaran, A.K. Biological impediments to monoclonal antibody–based cancer immunotherapy. Mol. Cancer Ther. 2004, 3, 1493–1501. [Google Scholar]
- McGregor, D.P. Discovering and improving novel peptide therapeutics. Curr. Opin. Pharmacol. 2008, 8, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 1–15. [Google Scholar] [CrossRef]
- Chang, S.T.; Ghosh, D.; Kirschner, D.E.; Linderman, J.J. Peptide length-based prediction of peptide—MHC class II binding. Bioinformatics 2006, 22, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xia, A.; Qi, X. Identification of novel peptoid agonists of fibroblast growth factor receptors using microarray-based screening. Medchemcomm 2016, 7, 1183–1189. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kanematsu-Yamaki, Y.; Kamada, Y.; Oka, M.; Ohnishi, T.; Miwa, M.; Asami, T.; Inooka, H. Identification of ligand-selective peptidic ActRIIB-antagonists using phage display technology. Biochem. Biophys. Rep. 2017, 11, 33–39. [Google Scholar] [CrossRef]
- Wiezorek, J.; Holland, P.; Graves, J. Death Receptor Agonists as a Targeted Therapy for Cancer. Clin. Cancer Res. 2010, 16, 1701–1708. [Google Scholar] [CrossRef]
- Huang, Z.; Tan, Y.; Gu, J.; Liu, Y.; Song, L.; Niu, J.; Li, Y.; Zhao, L.; Lin, Q.; Srinivasan, L.; et al. Uncoupling the Mitogenic and Metabolic Functions of FGF1 by Tuning FGF1-FGF Receptor Dimer Stability. Cell Rep. 2017, 20, 1717–1728. [Google Scholar] [CrossRef]
- Shen, B.Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody–drug conjugates. Nat. Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Oflazoglu, E.; Stone, I.J.; Gordon, K.; Wood, C.G.; Repasky, E.A.; Grewal, I.S.; Law, C.L.; Gerber, H.P. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin. Cancer Res. 2008, 14, 6171–6180. [Google Scholar] [CrossRef]
- King, H.D.; Dubowchik, G.M.; Mastalerz, H.; Willner, D.; Hofstead, S.J.; Firestone, R.A.; Lasch, S.J.; Trail, P.A. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: Inhibition of aggregation by methoxytriethyleneglycol chains. J. Med. Chem. 2002, 45, 4336–4343. [Google Scholar] [CrossRef]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Bagheri, N.; Sineh Sepehr, K.; Habibi-Anbouhi, M.; Kobarfard, F.; Balalaie, S.; Foroumadi, A.; Abbaszadeh-Goudarzi, G.; Abbaszadeh-Goudarzi, K.; et al. Trastuzumab-monomethyl auristatin E conjugate exhibits potent cytotoxic activity in vitro against HER2-positive human breast cancer. J. Cell. Physiol. 2019, 234, 2693–2704. [Google Scholar] [CrossRef]
- Currier, N.V.; Ackerman, S.E.; Kintzing, J.R.; Chen, R.; Interrante, M.F.; Steiner, A.; Sato, A.K.; Cochran, J.R. Targeted drug delivery with an integrin-binding Knottin-Fc-MMAF conjugate produced by cell- free protein synthesis. Mol. Cancer Ther. 2016, 15, 1291–1300. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Hao, G.; Zhang, H.; Yang, H.; Chen, H.; Qian, P. Fusion of pseudorabies virus glycoproteins to IgG Fc enhances protective immunity against pseudorabies virus. Virology 2019, 536, 49–57. [Google Scholar] [CrossRef]
- Yao, H.P.; Feng, L.; Suthe, S.R.; Chen, L.H.; Weng, T.H.; Hu, C.Y.; Jun, E.S.; Wu, Z.G.; Wang, W.L.; Kim, S.C.; et al. Correction: Therapeutic efficacy, pharmacokinetic profiles, and toxicological activities of humanized antibody–drug conjugate Zt/g4-MMAE targeting RON receptor tyrosine kinase for cancer therapy. J. Immunother. Cancer 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Szlachcic, A.; Lobocki, M. Design and characteristics of cytotoxic fibroblast growth factor 1 conjugate for fibroblast growth factor receptor-targeted cancer therapy. Drug Des. Dev. Ther. 2016, 10, 2547–2560. [Google Scholar] [CrossRef]
- Krzyscik, M.A.; Zakrzewska, M.; Sørensen, V.; Sokolowska-Wedzina, A.; Lobocki, M.; Swiderska, K.W.; Krowarsch, D.; Wiedlocha, A.; Otlewski, J. Cytotoxic Conjugates of Fibroblast Growth Factor 2 (FGF2) with Monomethyl Auristatin e for Effective Killing of Cells Expressing FGF Receptors. ACS Omega 2017, 2, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska-Wedzina, A.; Chodaczek, G.; Chudzian, J.; Borek, A.; Zakrzewska, M.; Otlewski, J. High-affinity internalizing human scFv-Fc antibody for targeting FGFR1-overexpressing lung cancer. Mol. Cancer Res. 2017, 15, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jendryczko, K.; Chudzian, J.; Skinder, N.; Opaliński, Ł.; Rzeszótko, J.; Wiedlocha, A.; Otlewski, J.; Szlachcic, A. FGF2-Derived PeptibodyF2-MMAE Conjugate for Targeted Delivery of Cytotoxic Drugs into Cancer Cells Overexpressing FGFR1. Cancers 2020, 12, 2992. https://doi.org/10.3390/cancers12102992
Jendryczko K, Chudzian J, Skinder N, Opaliński Ł, Rzeszótko J, Wiedlocha A, Otlewski J, Szlachcic A. FGF2-Derived PeptibodyF2-MMAE Conjugate for Targeted Delivery of Cytotoxic Drugs into Cancer Cells Overexpressing FGFR1. Cancers. 2020; 12(10):2992. https://doi.org/10.3390/cancers12102992
Chicago/Turabian StyleJendryczko, Karolina, Julia Chudzian, Natalia Skinder, Łukasz Opaliński, Jakub Rzeszótko, Antoni Wiedlocha, Jacek Otlewski, and Anna Szlachcic. 2020. "FGF2-Derived PeptibodyF2-MMAE Conjugate for Targeted Delivery of Cytotoxic Drugs into Cancer Cells Overexpressing FGFR1" Cancers 12, no. 10: 2992. https://doi.org/10.3390/cancers12102992
APA StyleJendryczko, K., Chudzian, J., Skinder, N., Opaliński, Ł., Rzeszótko, J., Wiedlocha, A., Otlewski, J., & Szlachcic, A. (2020). FGF2-Derived PeptibodyF2-MMAE Conjugate for Targeted Delivery of Cytotoxic Drugs into Cancer Cells Overexpressing FGFR1. Cancers, 12(10), 2992. https://doi.org/10.3390/cancers12102992





