High Levels of miR-7-5p Potentiate Crizotinib-Induced Cytokilling and Autophagic Flux by Targeting RAF1 in NPM-ALK Positive Lymphoma Cells
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Downregulation of miR-7-5p Expression Upon NPM-ALK Inactivation
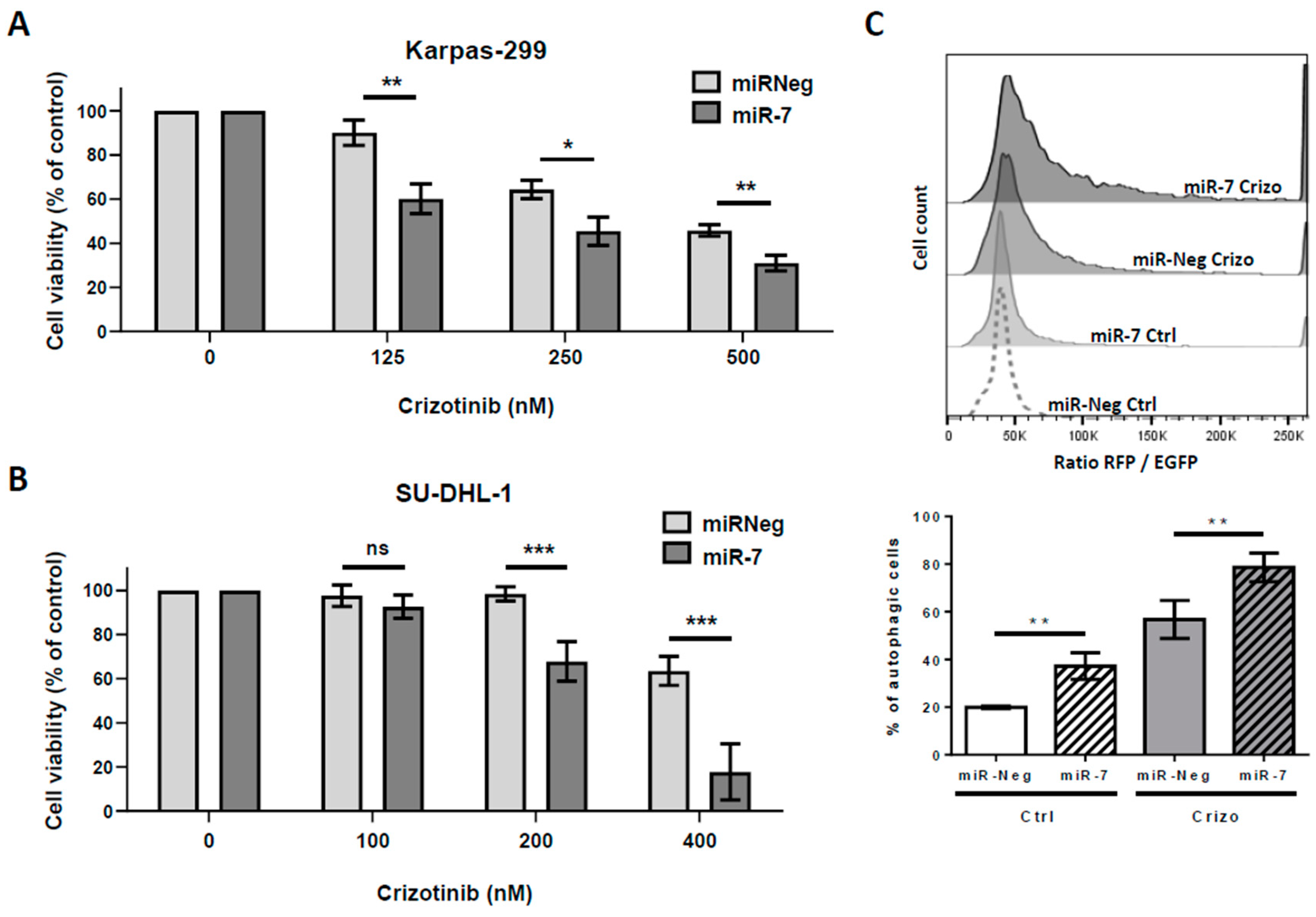
2.2. Enhanced Levels of miR-7-5p Potentiates Crizotinib-Induced Cytotoxic Effects and Autophagic Flux
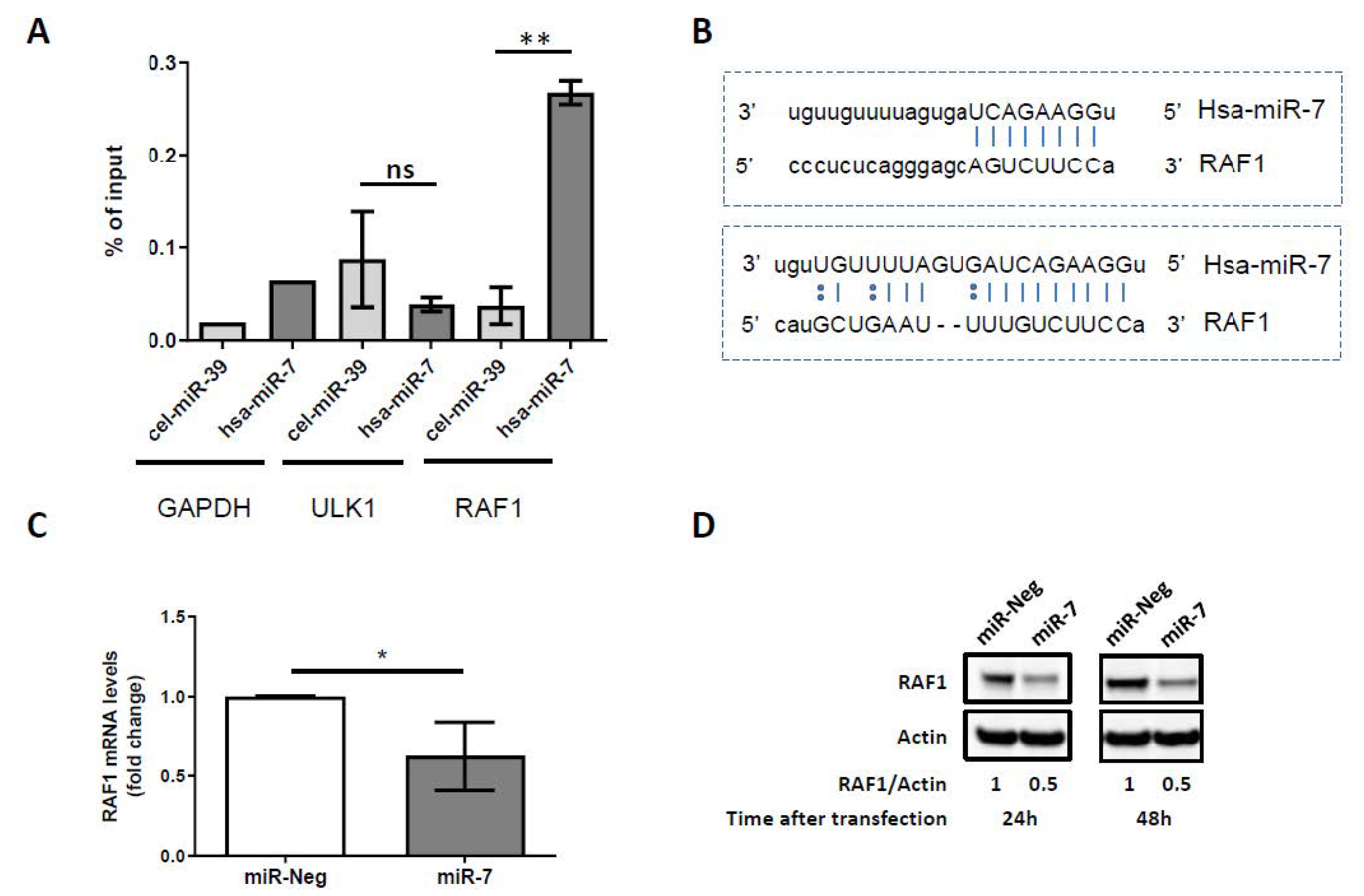
2.3. miR-7-5p Directly Targets RAF1 and Downregulates Its Expression
2.4. RAF1 Inhibition Mimics miR-7-5p Cellular Effects
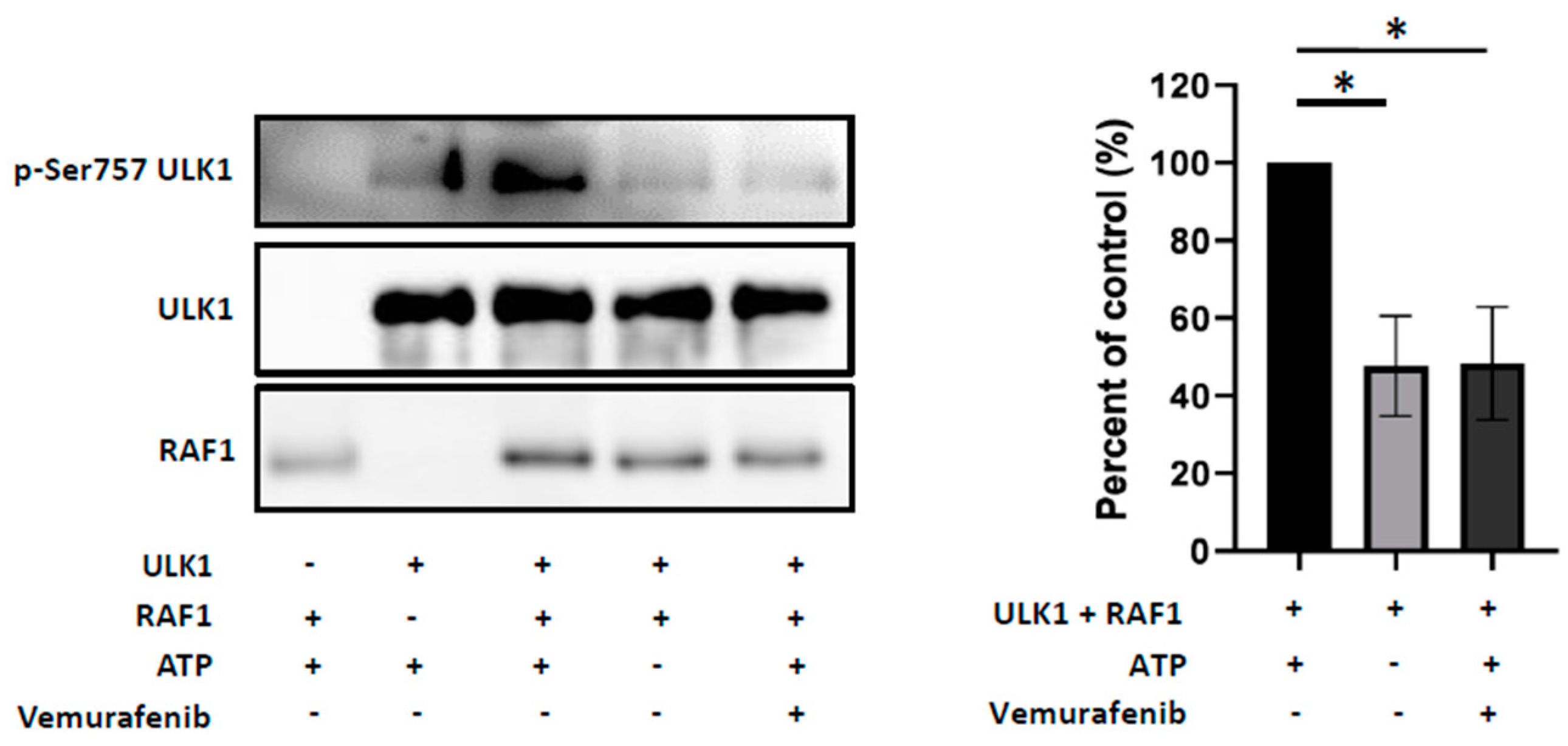
2.5. RAF1 Controls the Autophagic Process in Crizotinib-Treated Karpas-299 Cells through Phosphorylation of ULK1 at Serine757 Inhibitory Residue
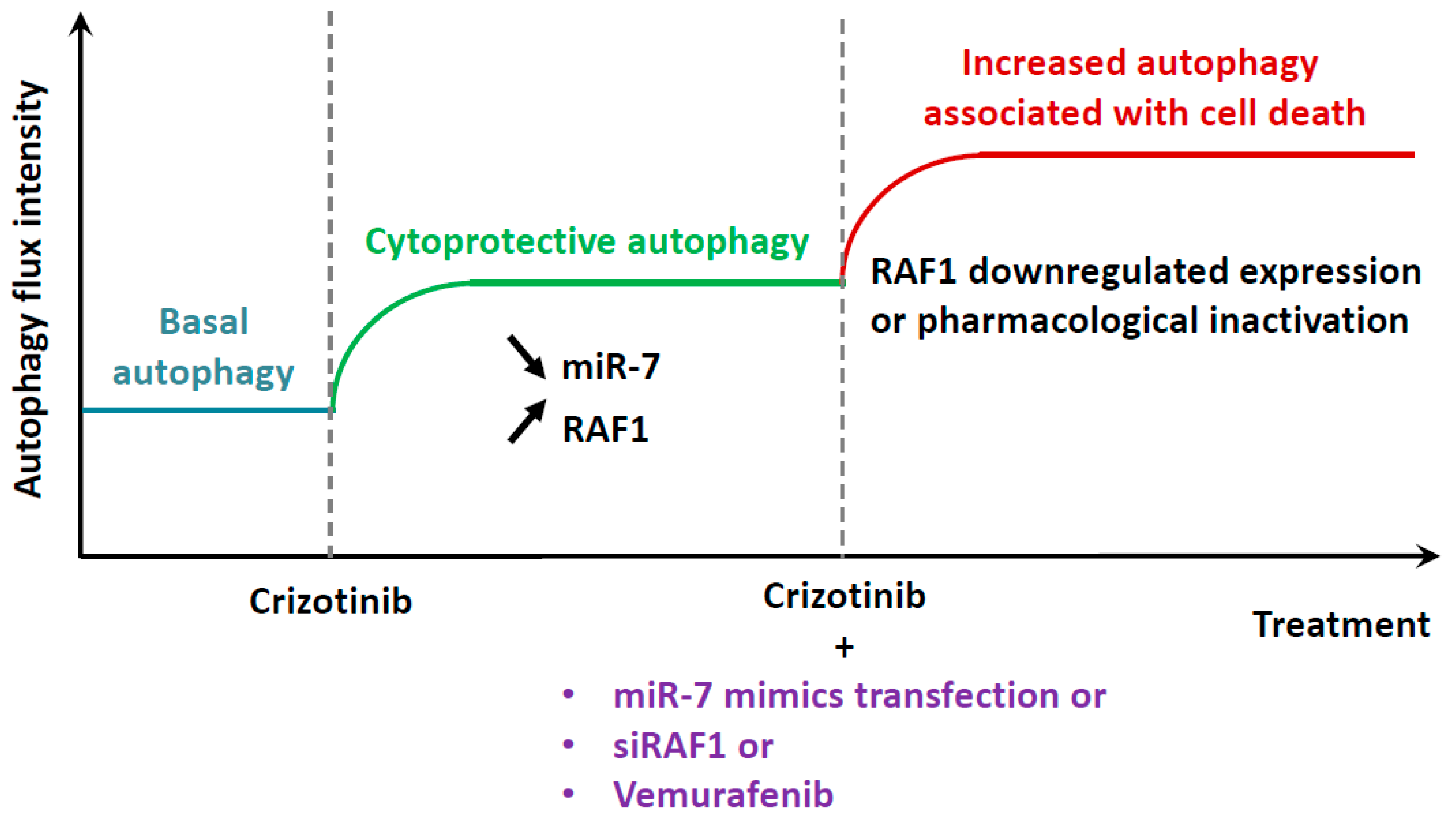
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture Conditions
4.2. Generation of RFP-GFP-LC3 Karpas-299 Cells
4.3. Generation of Karpas-299 Cells Knocked Down for RAF1
4.4. Reagents
4.5. MiRNA Expression Profiling Assay
4.6. RNA Extraction
4.7. Reverse Transcription
4.8. qPCR
4.9. miRNA Mimics and siRNA Transfection
4.10. Viability Assays
4.11. Apoptosis Measurement
4.12. Western-Blotting
4.13. Measure of the Autophagic Flux by Flow Cytometry
4.14. Immunofluorescence Staining
4.15. RNA Pulldown Using Biotinylated miRNA
4.16. In Vitro Kinase Assay
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, S.D.; Lamant, L.; Kenner, L.; Brugières, L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br. J. Haematol. 2016, 560–572. [Google Scholar] [CrossRef]
- Chiarle, R.; Voena, C.; Ambrogio, C.; Piva, R.; Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer 2008, 8, 11–23. [Google Scholar] [PubMed]
- Lai, R.; Ingham, R.J. The pathobiology of the oncogenic tyrosine kinase NPM-ALK: A brief update. Ther. Adv. Hematol. 2013, 4, 119–131. [Google Scholar]
- Werner, M.T.; Zhao, C.; Zhang, Q.; Wasik, M.A. Nucleophosmin-anaplastic lymphoma kinase: The ultimate oncogene and therapeutic target. Blood 2017, 129, 823–831. [Google Scholar]
- Lowe, E.J.; Lim, M.S. Potential therapies for anaplastic lymphoma kinase-driven tumors in children: Progress to date. Pediatr. Drugs 2013, 15, 163–169. [Google Scholar] [CrossRef]
- Prokoph, N.; Larose, H.; Lim, M.S.; Burke, G.A.A.; Turner, S.D. Treatment options for paediatric anaplastic large cell lymphoma (ALCL): Current standard and beyond. Cancers 2018, 10, 99. [Google Scholar]
- Christensen, J.G.; Zou, H.Y.; Arango, M.E.; Li, Q.; Lee, J.H.; McDonnell, S.R.; Yamazaki, S.; Alton, G.R.; Mroczkowski, B.; Los, G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007, 6, 3314–3322. [Google Scholar]
- Gambacorti Passerini, C.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl. Cancer Inst. 2014, 106, djt378. [Google Scholar]
- Ceccon, M.; Mologni, L.; Bisson, W.; Scapozza, L.; Gambacorti-Passerini, C. Crizotinib-resistant NPM-ALK mutants confer differential sensitivity to unrelated Alk inhibitors. Mol. Cancer Res. 2013, 11, 122–132. [Google Scholar]
- Sharma, G.G.; Mota, I.; Mologni, L.; Patrucco, E.; Gambacorti-Passerini, C.; Chiarle, R. Tumor resistance against ALK targeted therapy—Where it comes from and where it goes. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Mologni, L. Inhibitors of the anaplastic lymphoma kinase. Expert Opin. Investig. Drugs 2012, 21, 985–994. [Google Scholar] [CrossRef]
- Crescenzo, R.; Inghirami, G. Anaplastic lymphoma kinase inhibitors. Curr. Opin. Pharmacol. 2015, 23, 39–44. [Google Scholar] [CrossRef]
- Redaelli, S.; Ceccon, M.; Antolini, L.; Rigolio, R.; Pirola, A.; Peronaci, M.; Gambacorti-Passerini, C.; Mologni, L. Synergistic activity of ALK and mTOR inhibitors for the treatment of NPM-ALK positive lymphoma. Oncotarget 2016. [Google Scholar] [CrossRef]
- Larose, H.; Burke, G.A.A.; Lowe, E.J.; Turner, S.D. From bench to bedside: The past, present and future of therapy for systemic paediatric ALCL, ALK+. Br. J. Haematol. 2019, 185, 1043–1054. [Google Scholar]
- Mitou, G.; Frentzel, J.; Desquesnes, A.; Le Gonidec, S.; AlSaati, T.; Beau, I.; Lamant, L.; Meggetto, F.; Espinos, E.; Codogno, P.; et al. Targeting autophagy enhances the anti-tumoral action of crizotinib in ALK-positive anaplastic large cell lymphoma. Oncotarget 2015, 6, 30149–30164. [Google Scholar] [CrossRef] [PubMed]
- Frentzel, J.; Sorrentino, D.; Giuriato, S. Targeting autophagy in ALK-associated cancers. Cancers 2017, 9, 161. [Google Scholar] [CrossRef]
- Torossian, A.; Broin, N.; Frentzel, J.; Daugrois, C.; Gandarillas, S.; Al Saati, T.; Lamant, L.; Brousset, P.; Giuriato, S.; Espinos, E. Blockade of crizotinib-induced BCL2 elevation in ALK-positive anaplastic large cell lymphoma triggers autophagy associated with cell death. Haematologica 2019, 104, 1428–1439. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12 (Suppl. 2), 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar]
- Chan, E.Y.; Kir, S.; Tooze, S.A. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 2007, 282, 25464–25474. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Beau, I.; Mehrpour, M.; Codogno, P. Autophagosomes and human diseases. Int. J. Biochem. Cell Biol. 2011, 43, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; Ryan, K.M. The role of autophagy in tumour development and cancer therapy. Expert Rev. Mol. Med. 2009, 11, e36. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Pedro, J.M.B.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Joffre, C.; Djavaheri-Mergny, M.; Pattingre, S.; Giuriato, S. The yin and the yang of autophagy in cancer cells. Med. Sci. 2017, 33. [Google Scholar] [CrossRef]
- Tompkins, K.D.; Thorburn, A. Regulation of apoptosis by autophagy to enhance cancer therapy. Yale J. Biol. Med. 2019, 92, 707–718. [Google Scholar]
- Fulda, S.; Kögel, D. Cell death by autophagy: Emerging molecular mechanisms and implications for cancer therapy. Oncogene 2015, 34, 5105–5113. [Google Scholar] [CrossRef]
- Macintosh, R.L.; Ryan, K.M. Autophagy in tumour cell death. Semin. Cancer Biol. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Long, J.S.; Ryan, K.M. New frontiers in promoting tumour cell death: Targeting apoptosis, necroptosis and autophagy. Oncogene 2012, 31, 5045–5060. [Google Scholar] [CrossRef]
- Pietrocola, F.; Bravo-San Pedro, J.M.; Galluzzi, L.; Kroemer, G. Autophagy in natural and therapy-driven anticancer immunosurveillance. Autophagy 2017, 13, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Hoareau-Aveilla, C.; Merkel, O.; Meggetto, F. MicroRNA and ALK-positive anaplastic large cell lymphoma. Front. Biosci. Schol. Ed. 2015, 7, 217–225. [Google Scholar] [PubMed]
- Li, M.; Li, J.; Ding, X.; He, M.; Cheng, S.-Y. microRNA and cancer. AAPS J. 2010, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hoareau-Aveilla, C.; Meggetto, F. Crosstalk between microRNA and DNA methylation offers potential biomarkers and targeted therapies in ALK-positive lymphomas. Cancers 2017, 9, 100. [Google Scholar] [CrossRef]
- Hauptman, N.; Glavac, D. MicroRNAs and long non-coding RNAs: Prospects in diagnostics and therapy of cancer. Radiol. Oncol. 2013, 47, 311–318. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017. [Google Scholar] [CrossRef]
- Gozuacik, D.; Akkoc, Y.; Gulfem Ozturk, D.; Kocak, M. Autophagy-Regulating microRNAs and Cancer. Front. Oncol. 2017. [Google Scholar] [CrossRef]
- Frankel, L.B.; Lund, A.H. MicroRNA regulation of autophagy. Carcinogenesis 2012, 33, 2018–2025. [Google Scholar] [CrossRef]
- Kalinowski, F.C.; Brown, R.A.M.; Ganda, C.; Giles, K.M.; Epis, M.R.; Horsham, J.; Leedman, P.J. MicroRNA-7: A tumor suppressor miRNA with therapeutic potential. Int. J. Biochem. Cell Biol. 2014, 54, 312–317. [Google Scholar] [CrossRef]
- Webster, R.J.; Giles, K.M.; Price, K.J.; Zhang, P.M.; Mattick, J.S.; Leedman, P.J. Regulation of epidermal growth factor receptor signaling in human cancer cells by MicroRNA-7. J. Biol. Chem. 2009. [Google Scholar] [CrossRef]
- Rai, K.; Takigawa, N.; Ito, S.; Kashihara, H.; Ichihara, E.; Yasuda, T.; Shimizu, K.; Tanimoto, M.; Kiura, K. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol. Cancer Ther. 2011. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Huang, J.; Huang, S.; Li, Y.; Yu, S.; Yu, S.; Liu, X. miR-7 inhibits glioblastoma growth by simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK pathways. Int. J. Oncol. 2014. [Google Scholar] [CrossRef]
- Horsham, J.; Kalinowski, F.; Epis, M.; Ganda, C.; Brown, R.; Leedman, P. Clinical potential of microRNA-7 in cancer. J. Clin. Med. 2015, 4, 1668–1687. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Novel therapeutic approaches for pancreatic cancer by combined targeting of RAF→MEK→ERK signaling and autophagy survival response. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Piva, R.; Chiarle, R.; Manazza, A.D.; Taulli, R.; Simmons, W.; Ambrogio, C.; D’Escamard, V.; Pellegrino, E.; Ponzetto, C.; Palestro, G.; et al. Ablation of oncogenic ALK is a viable therapeutic approach for anaplastic large-cell lymphomas. Blood 2006, 107, 689–697. [Google Scholar] [CrossRef]
- Giuriato, S.; Foisseau, M.; Dejean, E.; Felsher, D.W.; Al Saati, T.; Demur, C.; Ragab, A.; Kruczynski, A.; Schiff, C.; Delsol, G.; et al. Conditional TPM3-ALK and NPM-ALK transgenic mice develop reversible ALK-positive early B-cell lymphoma/leukemia. Blood 2010, 115, 4061–4070. [Google Scholar] [CrossRef]
- Orang, A.V.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-specific upregulation. Int. J. Genom. 2014. [Google Scholar] [CrossRef]
- Lovly, C.M.; Heuckmann, J.M.; de Stanchina, E.; Chen, H.; Thomas, R.K.; Liang, C.; Pao, W. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011, 71, 4920–4931. [Google Scholar] [CrossRef]
- Turturro, F.; Frist, A.Y.; Arnold, M.D.; Seth, P.; Pulford, K. Biochemical differences between SUDHL-1 and KARPAS 299 cells derived from t(2;5)-positive anaplastic large cell lymphoma are responsible for the different sensitivity to the antiproliferative effect of p27(Kip1). Oncogene 2001, 20, 4466–4475. [Google Scholar] [CrossRef]
- Akar, U.; Chaves-Reyez, A.; Barria, M.; Tari, A.; Sanguino, A.; Kondo, Y.; Kondo, S.; Arun, B.; Lopez-Berestein, G.; Ozpolat, B. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy 2008, 4, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Takahashi, Y.; Cheng, E.; Liu, J.; Terranova, P.F.; Zhao, B.; Thrasher, J.B.; Wang, H.G.; Li, B. GSK-3β promotes cell survival by modulating Bif-1-dependent autophagy and cell death. J. Cell Sci. 2010, 128, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.; Ngo, V.N.; Emre, N.C.T.; Shaffer, A.L.; Yang, Y.; Tian, E.; Nair, V.; Kruhlak, M.J.; Zingone, A.; Landgren, O.; et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell 2013, 23, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Gump, J.M.; Thorburn, A. Sorting cells for basal and induced autophagic flux by quantitative ratiometric flow cytometry. Autophagy 2014, 10, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, H.; Yano, S.; Yoshida, R.; Yamasaki, Y.; Sasaki, T.; Hashimoto, Y.; Kuroda, S.; Ouchi, M.; Onishi, T.; Uno, F.; et al. Genetically engineered oncolytic adenovirus induces autophagic cell death through an E2F1-microRNA-7-epidermal growth factor receptor axis. Int. J. Cancer 2012, 131, 2939–2950. [Google Scholar] [CrossRef]
- Cai, S.; Shi, G.S.; Cheng, H.Y.; Zeng, Y.N.; Li, G.; Zhang, M.; Song, M.; Zhou, P.K.; Tian, Y.; Cui, F.M.; et al. Exosomal miR-7 mediates bystander autophagy in lung after focal brain irradiation in mice. Int. J. Biol. Sci. 2017. [Google Scholar] [CrossRef]
- Song, M.; Wang, Y.; Shang, Z.F.; Liu, X.D.; Xie, D.F.; Wang, Q.; Guan, H.; Zhou, P.K. Bystander autophagy mediated by radiation-induced exosomal MIR-7-5p in non-targeted human bronchial epithelial cells. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Zhu, H.; Gan, X.; Jiang, X.; Diao, S.; Wu, H.; Hu, J. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J. Exp. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Sun, Y.; Zhang, Y.; Dong, L.; Shen, C.; Yang, L.; Yang, M.; Li, Y.; Shen, G.; et al. miR-7 reverses the resistance to BRAFi in melanoma by targeting EGFR/IGF-1R/CRAF and inhibiting the MAPK and PI3K/AKT signaling pathways. Oncotarget 2016, 7, 53558–53570. [Google Scholar] [CrossRef]
- Shingu, T.; Holmes, L.; Henry, V.; Wang, Q.; Latha, K.; Gururaj, A.E.; Gibson, L.A.; Doucette, T.; Lang, F.F.; Rao, G.; et al. Suppression of RAF/MEK or PI3K synergizes cytotoxicity of receptor tyrosine kinase inhibitors in glioma tumor-initiating cells. J. Transl. Med. 2016. [Google Scholar] [CrossRef]
- Ceteci, F.; Xu, J.; Ceteci, S.; Zanucco, E.; Thakur, C.; Rapp, U.R. Conditional expression of oncogenic C-RAF in mouse pulmonary epithelial cells reveals differential tumorigenesis and induction of autophagy leading to tumor regression. Neoplasia 2011, 13, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Lieberman, J. Capture and identification of mirna targets by biotin pulldown and RNA-seq. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1358, pp. 211–228. [Google Scholar]
- Hoareau-Aveilla, C.; Valentin, T.; Daugrois, C.; Quelen, C.; Mitou, G.; Quentin, S.; Jia, J.; Spicuglia, S.; Ferrier, P.; Ceccon, M.; et al. Reversal of microRNA-150 silencing disadvantages crizotinib-resistant NPM-ALK(+) cell growth. J. Clin. Investig. 2015, 125, 3505–3518. [Google Scholar] [CrossRef] [PubMed]
- Piva, R.; Pellegrino, E.; Mattioli, M.; Agnelli, L.; Lombardi, L.; Boccalatte, F.; Costa, G.; Ruggeri, B.A.; Cheng, M.; Chiarle, R.; et al. Functional validation of the anaplastic lymphoma kinase signature identifies CEBPB and BCl2A1 as critical target genes. J. Clin. Investig. 2006. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Halasa, K.; Liu, X.; Wang, H.Y.; Cheng, M.; Baldwin, D.; Tobias, J.W.; Schuster, S.J.; Woetmann, A.; Zhang, Q.; et al. Malignant transformation of CD4+ T lymphocytes mediated by oncogenic kinase NPM/ALK recapitulates IL-2-induced cell signaling and gene expression reprogramming. J. Immunol. 2013, 191, 6200–6207. [Google Scholar] [CrossRef]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef]
- Heakal, Y.; Kester, M.; Savage, S. Vemurafenib (PLX4032): An orally available inhibitor of mutated BRAF for the treatment of metastatic melanoma. Ann. Pharmacother. 2011, 45, 1399–1405. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A children’s oncology group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef]
- Brugières, L.; Houot, R.; Cozic, N.; de la Fouchardière, C.; Morschhauser, F.; Brice, P.; Arakelyan Laboure, N.; Auvrignon, A.; Aladjidi, N.; Kolb, B.; et al. Crizotinib in advanced ALK+ anaplastic large cell lymphoma in children and adults: Results of the Acs© phase II trial. Blood 2017, 130, 2831. [Google Scholar] [CrossRef]
- Werner, M.T.; Zhang, Q.; Wasik, M.A. From pathology to precision medicine in anaplastic large cell lymphoma expressing anaplastic lymphoma kinase (ALK+ ALCL). Cancers 2017, 9, 138. [Google Scholar] [CrossRef]
- Puissant, A.; Robert, G.; Auberger, P. Targeting autophagy to fight hematopoietic malignancies. Cell Cycle 2010, 9, 3470–3478. [Google Scholar] [CrossRef]
- Djavaheri-Mergny, M.; Giuriato, S.; Tschan, M.P.; Humbert, M. Therapeutic modulation of autophagy in leukaemia and lymphoma. Cells 2019, 8, 103. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Lippincott-Schwartz, J.; Yin, X.-M.; Weiss, W.A.; Takebe, N.; Timmer, W.; DiPaola, R.S.; Lotze, M.T.; White, E. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 2011, 17, 654–666. [Google Scholar] [CrossRef]
- Puissant, A.; Robert, G.; Fenouille, N.; Luciano, F.; Cassuto, J.P.; Raynaud, S.; Auberger, P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010, 70, 1042–1052. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef]
- Basit, F.; Cristofanon, S.; Fulda, S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013, 20, 1161–1173. [Google Scholar] [CrossRef]
- Roskoski, R.J. Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharmacol. Res. 2018, 135, 239–258. [Google Scholar] [CrossRef]
- Wang, W.; Dai, L.X.; Zhang, S.; Yang, Y.; Yan, N.; Fan, P.; Dai, L.; Tian, H.W.; Cheng, L.; Zhang, X.M.; et al. Regulation of epidermal growth factor receptor signaling by plasmid-based MicroRNA-7 inhibits human malignant gliomas growth and metastasis in vivo. Neoplasma 2013. [Google Scholar] [CrossRef]
- Babae, N.; Bourajjaj, M.; Liu, Y.; van Beijnum, J.R.; Cerisoli, F.; Scaria, P.V.; Verheul, M.; van Berkel, M.P.; Pieters, E.H.E.; van Haastert, R.J.; et al. Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma. Oncotarget 2014. [Google Scholar] [CrossRef]
- Cui, X.; Sun, Y.; Shen, M.; Song, K.; Yin, X.; Di, W.; Duan, Y. Enhanced chemotherapeutic efficacy of paclitaxel nanoparticles co-delivered with MicroRNA-7 by inhibiting paclitaxel-induced EGFR/ERK pathway activation for ovarian cancer therapy. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Zhao, J.G.; Men, W.F.; Tang, J. MicroRNA-7 enhances cytotoxicity induced by gefitinib in non-small cell lung cancer via inhibiting the EGFR and IGF1R signalling pathways. Wspolczesna Onkol. 2015. [Google Scholar] [CrossRef]
- Henson, E.; Chen, Y.; Gibson, S. EGFR family members’ regulation of autophagy is at a crossroads of cell survival and death in cancer. Cancers 2017, 9, 27. [Google Scholar] [CrossRef]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, N.; Athonvarangkul, D.; Mishall, P.; Sahu, S.; Singh, R. Autophagy proteins regulate ERK phosphorylation. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Kasprzycka, M.; Liu, X.; Raghunath, P.N.; Wlodarski, P.; Wasik, M.A. Oncogenic tyrosine kinase NPM/ALK induces activation of the MEK/ERK signaling pathway independently of c-Raf. Oncogene 2007, 26, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Ehrenreiter, K.; Kern, F.; Velamoor, V.; Meissl, K.; Galabova-Kovacs, G.; Sibilia, M.; Baccarini, M. Raf-1 addiction in ras-induced skin carcinogenesis. Cancer Cell 2009. [Google Scholar] [CrossRef]
- Doma, E.; Rupp, C.; Varga, A.; Kern, F.; Riegler, B.; Baccarini, M. Skin tumorigenesis stimulated by raf inhibitors relies upon raf functions that are dependent and independent of ERK. Cancer Res. 2013. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Guan, S.; Cao, W.; Wang, H.; Chen, Z.; Zhao, Y.; Yu, Y.; Zhang, H.; Pang, J.C.; et al. Novel ALK inhibitor AZD3463 inhibits neuroblastoma growth by overcoming crizotinib resistance and inducing apoptosis. Sci. Rep. 2016, 6, srep19423. [Google Scholar] [CrossRef]
- Megiorni, F.; McDowell, H.P.; Camero, S.; Mannarino, O.; Ceccarelli, S.; Paiano, M.; Losty, P.D.; Pizer, B.; Shukla, R.; Pizzuti, A.; et al. Crizotinib-induced antitumour activity in human alveolar rhabdomyosarcoma cells is not solely dependent on ALK and MET inhibition. J. Exp. Clin. Cancer Res. 2015, 34, 1–16. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Vega, F.; Medeiros, L.J.; Leventaki, V.; Atwell, C.; Cho-Vega, J.H.; Tian, L.; Claret, F.X.; Rassidakis, G.Z. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 2006, 66, 6589–6597. [Google Scholar] [CrossRef]
- Marzec, M.; Kasprzycka, M.; Liu, X.; El-Salem, M.; Halasa, K.; Raghunath, P.N.; Bucki, R.; Wlodarski, P.; Wasik, M.A. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene 2007, 26, 5606–5614. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, M.; Zhu, Y.; Gu, L.; Zhang, Y.; Li, Q.; Jia, C.; Ma, Z. Prognostic significance and therapeutic potential of the activation of anaplastic lymphoma kinase/protein kinase B/mammalian target of rapamycin signaling pathway in anaplastic large cell lymphoma. BMC Cancer 2013. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Noh, K.H.; Lee, Y.H.; Hong, S.O.; Song, K.H.; Lee, H.J.; Kim, S.; Kim, T.M.; Jeon, J.H.; Seo, J.H.; et al. Targeting stemness is an effective strategy to control EML4-ALK + non-small cell lung cancer cells. Oncotarget 2015. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALK F1174L mutation potentiates the oncogenic activity of MYCN in neuroblastoma overexpressing MYCN and suggest a strategy for improving targeted therapy for ALK-positive. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef]
- Moore, N.F.; Azarova, A.M.; Bhatnagar, N.; Ross, K.N.; Drake, L.E.; Frumm, S.; Liu, Q.S.; Christie, A.L.; Sanda, T.; Chesler, L.; et al. Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma. Oncotarget 2014. [Google Scholar] [CrossRef]
- Blessing, A.M.; Santiago-O’Farrill, J.M.; Mao, W.; Pang, L.; Ning, J.; Pak, D.; Bollu, L.R.; Rask, P.; Iles, L.K.; Yang, H.; et al. Elimination of dormant, autophagic ovarian cancer cells and xenografts through enhanced sensitivity to anaplastic lymphoma kinase inhibition. Cancer 2020. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef]
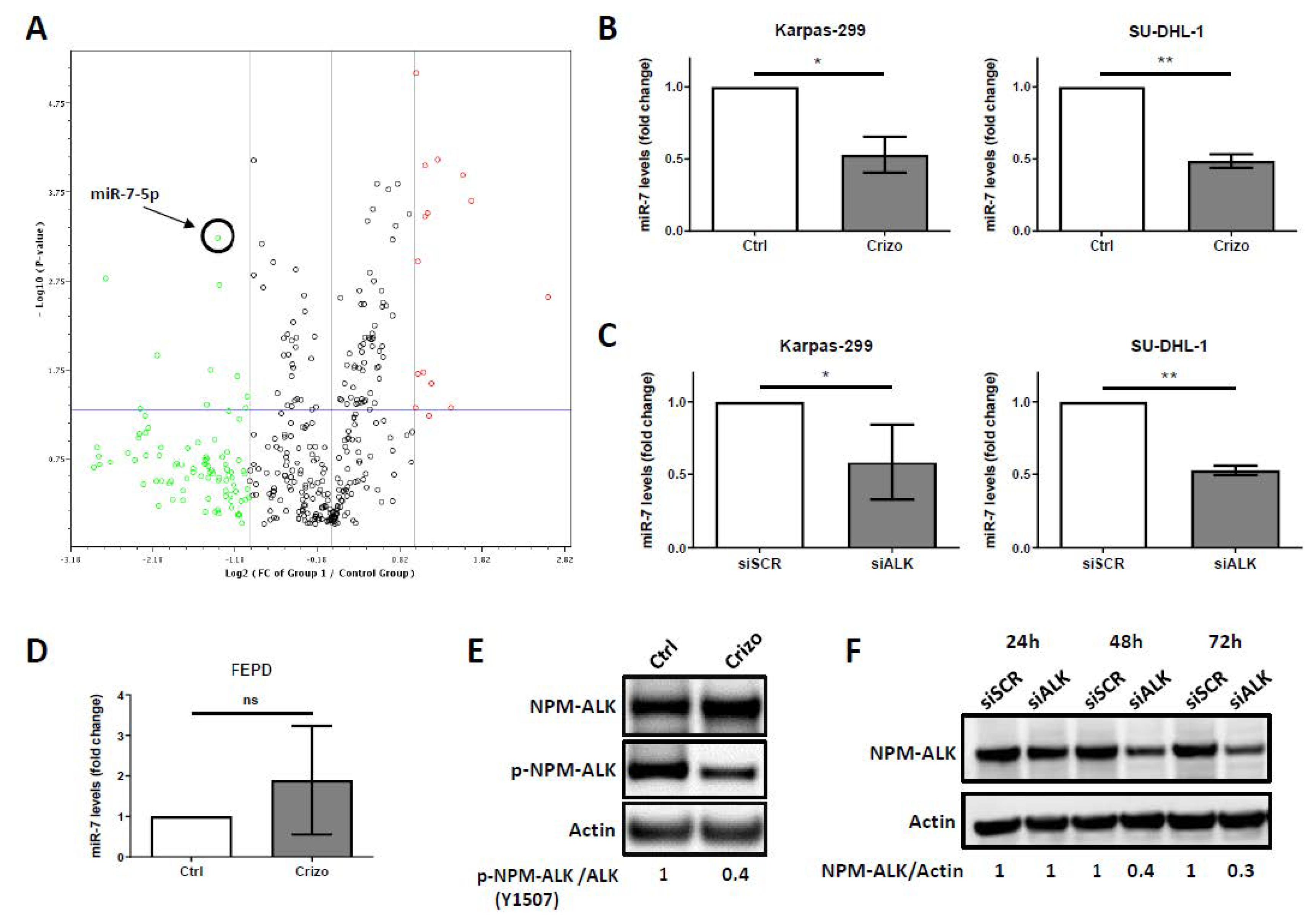


| Genes Under-Expressed in Crizotinib Treated Versus Untreated Cells | ||
| Name | Fold Regulation | p-Value |
| hsa-miR-7-5p | −2.6411 | 0.000594 |
| hsa-miR-222-5p | −6.7695 | 0.001684 |
| hsa-miR-221-5p | −2.6084 | 0.001993 |
| hsa-miR-19a-5p | −4.402 | 0.01234 |
| hsa-miR-92a-1-5p | −2.7998 | 0.017765 |
| hsa-miR-885-5p | −2.2267 | 0.021152 |
| hsa-miR-146a-5p | −2.0383 | 0.035913 |
| hsa-miR-363-5p | −2.8992 | 0.043604 |
| hsa-miR-143-3p | −2.0704 | 0.047823 |
| hsa-miR-10b-3p | −5.0548 | 0.049052 |
| Genes Over-Expressed in Crizotinib Treated Versus Untreated Cells | ||
| Name | Fold Regulation | p-Value |
| hsa-miR-15a-5p | 2.0232 | 0.000008 |
| hsa-miR-425-5p | 2.4347 | 0.000077 |
| hsa-miR-9-5p | 2.1883 | 0.000089 |
| hsa-miR-219a-5p | 3.0043 | 0.000116 |
| hsa-miR-31-5p | 3.2204 | 0.000226 |
| hsa-miR-26a-5p | 2.2241 | 0.000308 |
| hsa-miR-425-3p | 2.1882 | 0.000335 |
| hsa-miR-191-5p | 2.0509 | 0.001091 |
| hsa-miR-497-5p | 6.1522 | 0.002711 |
| hsa-miR-190a-5p | 2.1526 | 0.01914 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, D.; Frentzel, J.; Mitou, G.; Blasco, R.B.; Torossian, A.; Hoareau-Aveilla, C.; Pighi, C.; Farcé, M.; Meggetto, F.; Manenti, S.; et al. High Levels of miR-7-5p Potentiate Crizotinib-Induced Cytokilling and Autophagic Flux by Targeting RAF1 in NPM-ALK Positive Lymphoma Cells. Cancers 2020, 12, 2951. https://doi.org/10.3390/cancers12102951
Sorrentino D, Frentzel J, Mitou G, Blasco RB, Torossian A, Hoareau-Aveilla C, Pighi C, Farcé M, Meggetto F, Manenti S, et al. High Levels of miR-7-5p Potentiate Crizotinib-Induced Cytokilling and Autophagic Flux by Targeting RAF1 in NPM-ALK Positive Lymphoma Cells. Cancers. 2020; 12(10):2951. https://doi.org/10.3390/cancers12102951
Chicago/Turabian StyleSorrentino, Domenico, Julie Frentzel, Géraldine Mitou, Rafael B. Blasco, Avédis Torossian, Coralie Hoareau-Aveilla, Chiara Pighi, Manon Farcé, Fabienne Meggetto, Stéphane Manenti, and et al. 2020. "High Levels of miR-7-5p Potentiate Crizotinib-Induced Cytokilling and Autophagic Flux by Targeting RAF1 in NPM-ALK Positive Lymphoma Cells" Cancers 12, no. 10: 2951. https://doi.org/10.3390/cancers12102951
APA StyleSorrentino, D., Frentzel, J., Mitou, G., Blasco, R. B., Torossian, A., Hoareau-Aveilla, C., Pighi, C., Farcé, M., Meggetto, F., Manenti, S., Espinos, E., Chiarle, R., & Giuriato, S. (2020). High Levels of miR-7-5p Potentiate Crizotinib-Induced Cytokilling and Autophagic Flux by Targeting RAF1 in NPM-ALK Positive Lymphoma Cells. Cancers, 12(10), 2951. https://doi.org/10.3390/cancers12102951






