The Different Clinicopathological Features of Remnant Gastric Cancer Depending on Initial Disease of Partial Gastrectomy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria and Clinical Characteristics
2.2. Pathological Analysis
2.3. Immunohistochemistry Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
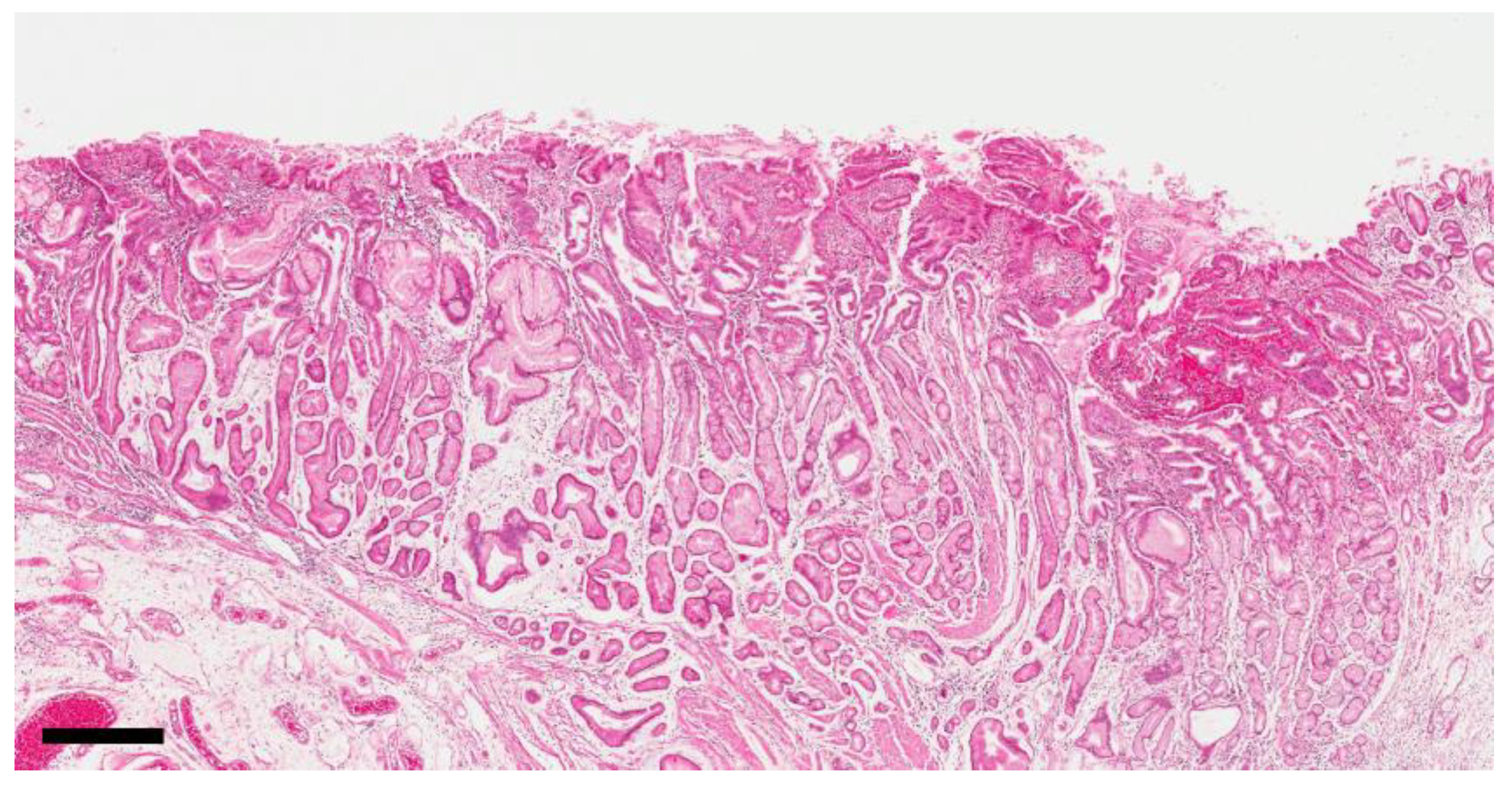
3.2. Distribution of Reflux-Induced Mucosal Changes in the Anastomosis Area
3.3. Differences in the Mucosa of the Anastomosis Area between Benign and Malignant Cases
3.4. Differences in the Background Mucosa around the Carcinoma between Benign and Malignant Cases
3.5. Results of Immunohistochemistry and Molecular Classification
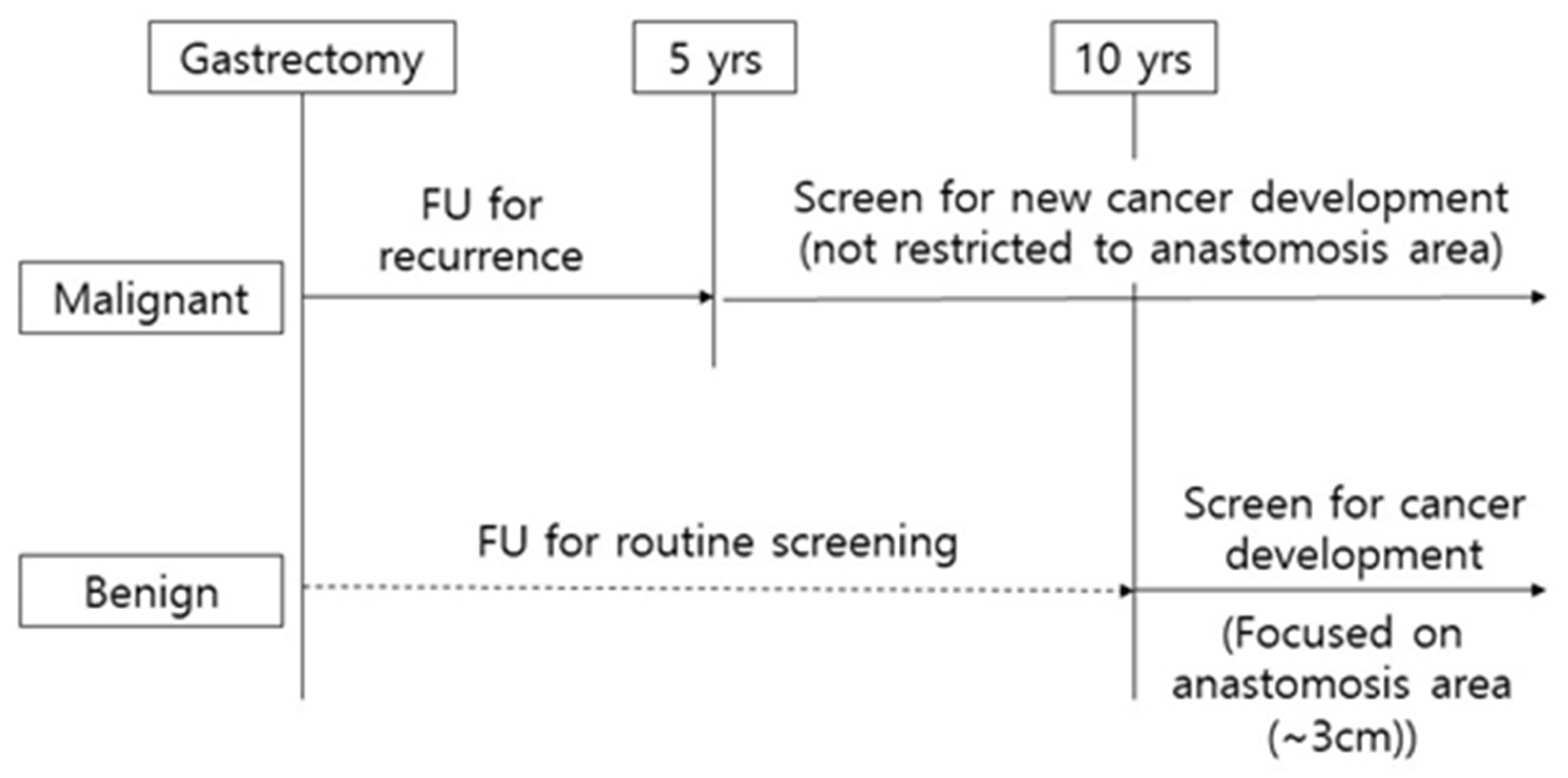
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J. Gastric Cancer 2016, 16, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Katai, H.; Ishikawa, T.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Oda, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; Fukagawa, T.; et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer 2017, 14, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Development Working Group & Review Panel Guideline Committee of the Korean Gastric Cancer Association (KGCA) Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48.
- Ohyama, S.; Tokunaga, M.; Hiki, N.; Fukunaga, T.; Fujisaki, J.; Seto, Y.; Yamaguchi, T. A clinicopathological study of gastric stump carcinoma following proximal gastrectomy. Gastric Cancer 2009, 12, 88–94. [Google Scholar] [CrossRef]
- Lee, Y.; Tokunaga, A.; Tajiri, T.; Masuda, G.; Okuda, T.; Fujita, I.; Kiyama, T.; Yoshiyuki, T.; Kato, S.; Matsukura, N.; et al. Inflammation of the gastric remnant after gastrectomy: Mucosal erythema is associated with bile reflux and inflammatory cellular infiltration is associated with Helicobacter pylori infection. J. Gastroenterol. 2004, 39, 520–526. [Google Scholar] [CrossRef]
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.-J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Chan, D.-C.; Fan, Y.-M.; Lin, C.-K.; Chen, C.-J.; Chen, C.-Y.; Chao, Y.-C. Roux-en-Y Reconstruction after Distal Gastrectomy to Reduce Enterogastric Reflux and Helicobacter pylori Infection. J. Gastrointest. Surg. 2007, 11, 1732–1740. [Google Scholar] [CrossRef]
- Dixon, M.F.; O’Connor, H.J.; Axon, A.T.; King, R.F.; Johnston, D. Reflux gastritis: Distinct histopathological entity? J. Clin. Pathol. 1986, 39, 524–530. [Google Scholar] [CrossRef]
- Onoda, N.; Maeda, K.; Sawada, T.; Wakasa, K.; Arakawa, T.; Chung, K.H.-Y.-S. Prevalence of Helicobacter pylori infection in gastric remnant after distal gastrectomy for primary gastric cancer. Gastric Cancer 2001, 4, 87–92. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Setia, N.; Agoston, A.T.; Han, H.S.; Mullen, J.T.; Duda, D.G.; Clark, J.W.; Deshpande, V.; Mino-Kenudson, M.; Srivastava, A.; Lennerz, J.K.; et al. A protein and mRNA expression-based classification of gastric cancer. Mod. Pathol. 2016, 29, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Goldenring, J.R.; Kaminishi, M.; Lee, J.R. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig. Dis. Sci. 2002, 47, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.H.-X.; Kalantar, J.S.; Talley, N.J.; Wyatt, J.M.; Adams, S.; Chueng, K.; Mitchell, H.M.; Cheung, K. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): A link between Helicobacter pylori infection and intestinal metaplasia? Am. J. Gastroenterol. 2000, 95, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kook, M.-C.; Shin, O.-R.; Kim, H.S.; Bae, H.-I.; Park, Y.; Choi, I.J.; Kim, Y.-I.; Nam, B.H.; Kim, S.; et al. Factors to improve the interobserver agreement for gastric atrophy and intestinal metaplasia: Consensus of definition and criteria. Histopathology 2018, 72, 838–845. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef]
- Sowa, M.; Kato, Y.; Onoda, N.; Kubo, T.; Maekawa, H.; Yoshikawa, K.; Nishimura, M.; Nakanishi, I.; Chung, Y.S. Early cancer of the gastric remnant with special reference to the importance of follow-up of gastrectomized patients. Eur. J. Surg. Oncol. 1993, 19, 43–49. [Google Scholar]
- Ohira, M.; Toyokawa, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Onoda, N.; Hirakawa, K. Current status in remnant gastric cancer after distal gastrectomy. World J. Gastroenterol. 2016, 22, 2424–2433. [Google Scholar] [CrossRef]
- Kimura, K. Chronological Transition of the Fundic-Pyloric Border Determined by Stepwise Biopsy of the Lesser and Greater Curvatures of the Stomach. Gastroenterology 1972, 63, 584–592. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N.A. Spasmolytic Polypeptide-Expressing Metaplasia and Intestinal Metaplasia: Time for Reevaluation of Metaplasias and the Origins of Gastric Cancer. Gastroenterology 2010, 138, 2207–2210.e1. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, S.-J.; Beom, S.-H.; Jung, M.; Choi, Y.Y.; Son, T.; Kim, H.-I.; Cheong, J.-H.; Hyung, W.J.; Noh, S.H.; et al. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: Implications for individualized therapy. Oncotarget 2016, 7, 44608–44620. [Google Scholar] [CrossRef] [PubMed]
- Birkman, E.-M.; Mansuri, N.; Kurki, S.; Ålgars, A.; Lintunen, M.; Ristamäki, R.; Sundström, J.; Carpén, O. Gastric cancer: Immunohistochemical classification of molecular subtypes and their association with clinicopathological characteristics. Virchows Arch. 2017, 472, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.-H.; Han, S.-H.; An, J.-S.; Lee, E.-S.; Kim, Y.-S. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. 2009, 24, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Pfeiffer, R.; Camargo, M.C.; Rabkin, C.S. Meta-analysis Shows That Prevalence of Epstein–Barr Virus-Positive Gastric Cancer Differs Based on Sex and Anatomic Location. Gastroenterology 2009, 137, 824–833. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, H.; Zhou, W.; Wan, X.; Li, L.; Yu, C. Epstein–Barr virus infection and genome polymorphisms on gastric remnant carcinoma: A meta-analysis. Cancer Cell Int. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Almeida, R.; Silva, E.; Santos-Silva, F.; Silberg, D.G.; Wang, J.; De Bolós, C.; David, L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J. Pathol. 2002, 199, 36–40. [Google Scholar] [CrossRef]
- Mizoshita, T.; Inada, K.-I.; Tsukamoto, T.; Kodera, Y.; Yamamura, Y.; Hirai, T.; Kato, T.; Joh, T.; Itoh, M.; Tatematsu, M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa--with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 2001, 4, 185–191. [Google Scholar] [CrossRef]
- Mizoshita, T.; Tsukamoto, T.; Inada, K.-I.; Ogasawara, N.; Hirata, A.; Kato, S.; Joh, T.; Itoh, M.; Yamamura, Y.; Tatematsu, M. Immunohistochemically detectable Cdx2 is present in intestinal phenotypic elements in early gastric cancers of both differentiated and undifferentiated types, with no correlation to non-neoplastic surrounding mucosa. Pathol. Int. 2004, 54, 392–400. [Google Scholar] [CrossRef]
| Category | Variables | Benign (n = 24) | Malignancy (n = 35) | p Value |
|---|---|---|---|---|
| Age (years) | 63.9 ± 8.7 | 65.1 ± 11.5 | 0.66 | |
| Sex | Male | 24 (100%) | 27 (77.1%) | 0.02 |
| Female | 0 (0%) | 8 (22.9%) | ||
| Interval (years) | 30.1 ± 8.0 | 13.4 ± 6.8 | <0.001 | |
| Initial operation | Billroth I | 0 (0%) | 7 (20%) | 0.06 |
| Billroth II | 24 (100%) | 28 (80%) | ||
| Pathologic stage of initial adenocarcinoma | I | - | 24 (68.6%) | |
| II | 3 (8.6.%) | |||
| III | 1 (2.9%) | |||
| IV | 0 (0%) | |||
| unknown | 7 (20.0%) | |||
| Pathologic Stage of remnant cancer | I | 13 (54.2%) | 19 (54.3%) | 0.71 |
| II | 6 (25.0%) | 10 (28.6%) | ||
| III | 4 (16.7%) | 6 (17.1%) | ||
| IV | 1 (4.2%) | 0 (0%) | ||
| LN metastasis | Negative | 15 (62.5%) | 25 (71.4%) | 0.47 |
| Positive | 9 (37.5%) | 10 (28.6%) | ||
| Tumor size | 4.6 ± 2.1 | 4.4 ± 2.4 | 0.52 | |
| Histology | Differentiated | 15 (62.5%) | 16 (45.7%) | 0.2 |
| Undifferentiated | 9 (37.5%) | 19 (54.3%) | ||
| Lauren classification | Intestinal | 14 (58.3%) | 12 (34.3%) | 0.07 |
| Diffuse | 8 (33.3%) | 17 (48.6%) | ||
| Mixed | 2 (8.3%) | 6 (17.1%) | ||
| H. pylori infection | Negative | 8 (33.3%) | 21 (60.0%) | 0.17 |
| Positive | 15 (62.5%) | 11 (31.4%) | ||
| Unknown | 1 (4.2%) | 3 (8.6%) |
| Variables | Median | Maximum | 33 Percentile | 67 Percentile |
|---|---|---|---|---|
| Foveolar hyperplasia Pyloric metaplasia | 1.3 cm 1.5 cm | 5 cm 5 cm | 1.29 cm 0.8 cm | 2.2 cm 1.9 cm |
| Variables | Grade | Benign (n = 19) | Malignancy (n = 22) | p-Value |
|---|---|---|---|---|
| Distance from tumor center to anastomosis (cm) | 1.80 ± 0.57 | 2.98 ± 0.53 | 0.14 | |
| Length of PM * | 2.3 ± 0.27 | 0.85 ± 0.15 | <0.001 | |
| PM grade | Low (grade 0,1) | 1 (5.3%) | 14 (63.6%) | <0.001 |
| High (grade 2,3) | 18 (94.7%) | 8 (36.4%) | ||
| IM * grade | Low (grade 0,1) | 19 (100%) | 20 (91%) | 0.927 |
| High (grade 2,3) | 0 | 2 (9%) |
| Mucosal Type | Benign (n = 20) | Malignancy (n = 28) | p-Value |
|---|---|---|---|
| Normal mucosa Pyloric metaplasia Intestinal metaplasia | 7 (35.0%) 13 (65.0%) 0 (0%) | 11 (39.3%) 10 (35.7%) 7 (25.0%) | 0.028 |
| Variables | Classification | Benign (n = 22) | Malignancy (n = 33) | p Value |
|---|---|---|---|---|
| Molecular type | EBV type | 7 (31.8%) | 1 (3.0%) | 0.068 |
| MSI type | 0 | 3 (9.1%) | ||
| E-cadherin aberrant type | 1 (4.5%) | 1 (3.0%) | ||
| P 53 aberrant type | 6 (27.3%) | 10 (30.3%) | ||
| Other type * | 9 (42.9%) | 19 (57.6%) | ||
| HER-2 aberrant | Negative | 22 (100.0%) | 29 (87.9%) | 0.106 |
| Positive | 0 (0.0%) | 4 (12.1%) | ||
| MET aberrant | Normal | 12 (54.5%) | 23 (66.7%) | 0.404 |
| Abnormal | 10 (45.5%) | 10 (33.3%) | ||
| Mucin phenotype | Gastric type | 7 (31.8%) | 10 (30.3%) | 0.827 |
| Gastric-intestinal type | 5 (22.7%) | 10 (30.3%) | ||
| Intestinal type | 4 (18.2%) | 6 (18.2%) | ||
| Null type | 6 (27.2%) | 7 (21.3%) | ||
| CDX2 expression | Negative | 11 (50.0%) | 7 (21.2%) | 0.014 |
| Positive | 11 (50.0%) | 26 (78.8%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.H.; Eom, B.W.; Yoon, H.M.; Kim, Y.-W.; Kook, M.-C.; Ryu, K.W. The Different Clinicopathological Features of Remnant Gastric Cancer Depending on Initial Disease of Partial Gastrectomy. Cancers 2020, 12, 2847. https://doi.org/10.3390/cancers12102847
Han WH, Eom BW, Yoon HM, Kim Y-W, Kook M-C, Ryu KW. The Different Clinicopathological Features of Remnant Gastric Cancer Depending on Initial Disease of Partial Gastrectomy. Cancers. 2020; 12(10):2847. https://doi.org/10.3390/cancers12102847
Chicago/Turabian StyleHan, Won Ho, Bang Wool Eom, Hong Man Yoon, Young-Woo Kim, Myeong-Cherl Kook, and Keun Won Ryu. 2020. "The Different Clinicopathological Features of Remnant Gastric Cancer Depending on Initial Disease of Partial Gastrectomy" Cancers 12, no. 10: 2847. https://doi.org/10.3390/cancers12102847
APA StyleHan, W. H., Eom, B. W., Yoon, H. M., Kim, Y.-W., Kook, M.-C., & Ryu, K. W. (2020). The Different Clinicopathological Features of Remnant Gastric Cancer Depending on Initial Disease of Partial Gastrectomy. Cancers, 12(10), 2847. https://doi.org/10.3390/cancers12102847





