(2R,3S)-Dihydroxybutanoic Acid Synthesis as a Novel Metabolic Function of Mutant Isocitrate Dehydrogenase 1 and 2 in Acute Myeloid Leukemia
Simple Summary
Abstract
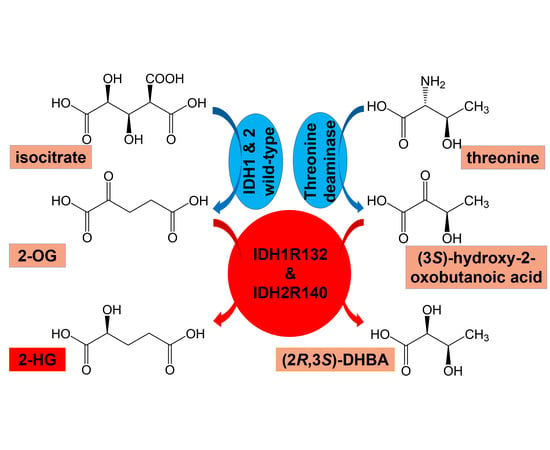
1. Introduction
2. Results
2.1. Metabolomic Analysis
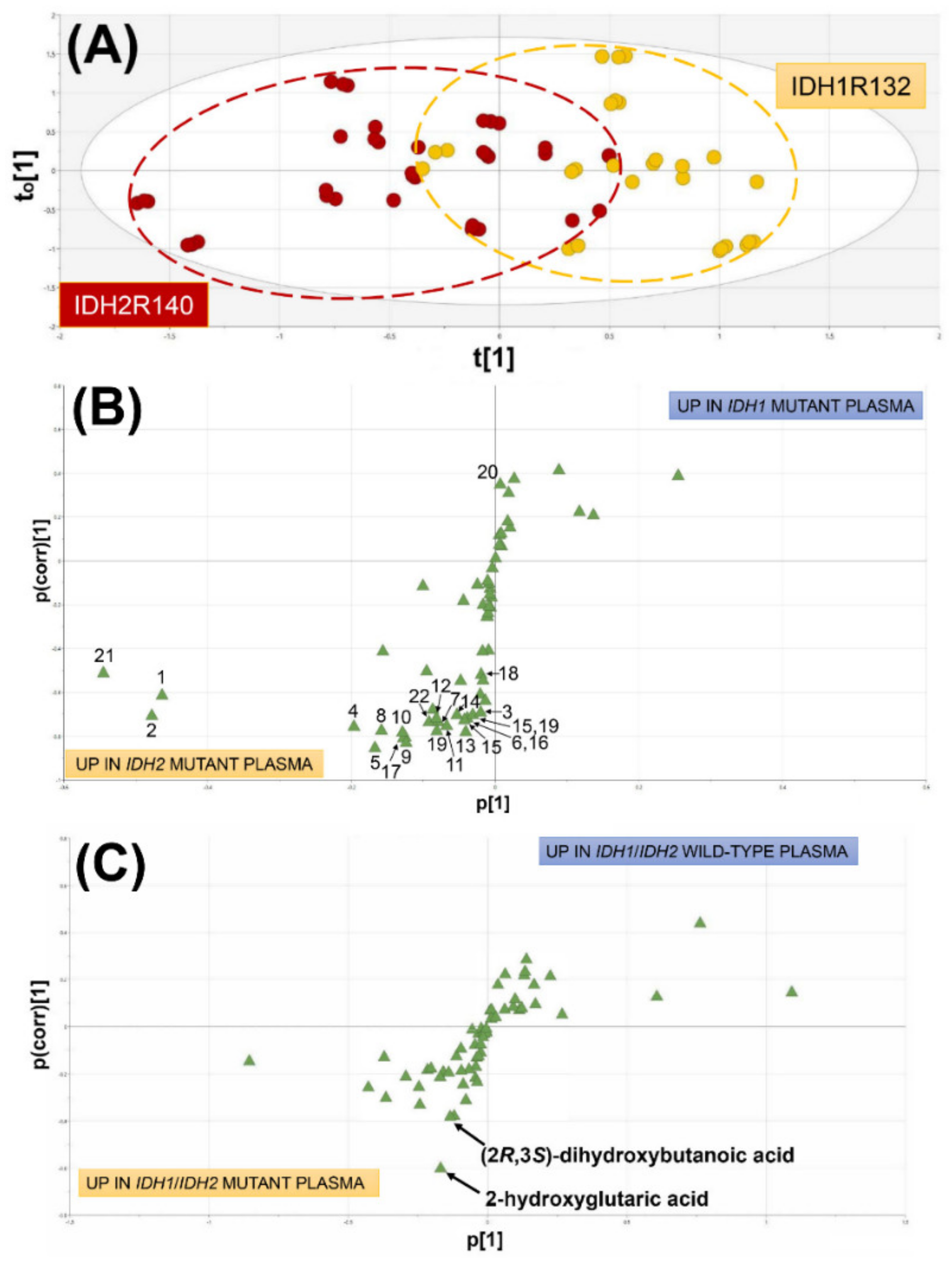
2.1.1. Comparison of AML Patients with WT IDH1/2, Mutated IDH1 and Mutated IDH2
2.1.2. Comparison of AML Patients with Wild-Type IDH1/2 and Mutated IDH1/IDH2
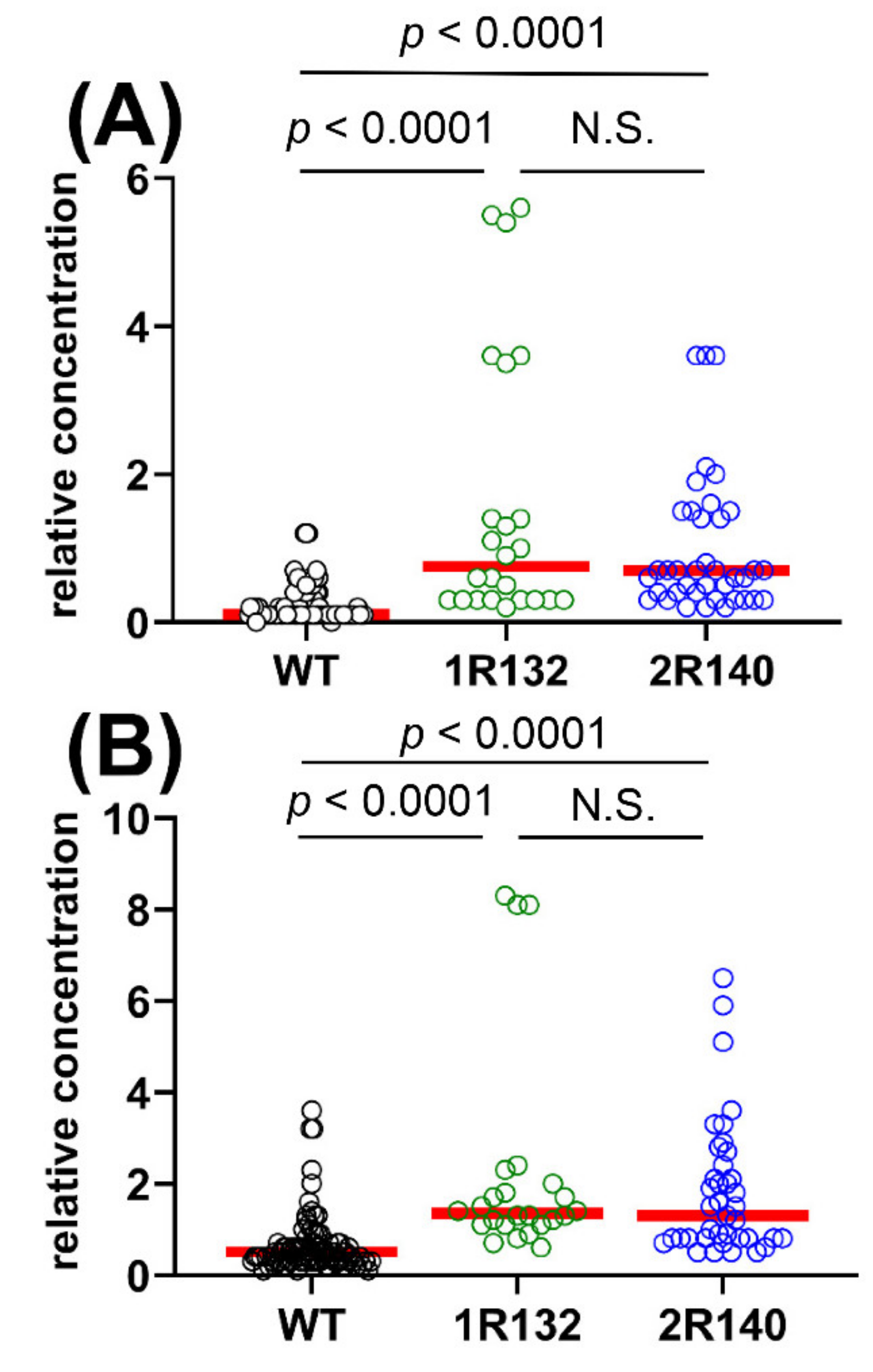
2.1.3. Comparison of WT vs. IDH1R132 and WT vs. IDH2R140 and the Effect of D2HGDH Polymorphism on 2R-HG and 2,3-DHBA Plasma Levels
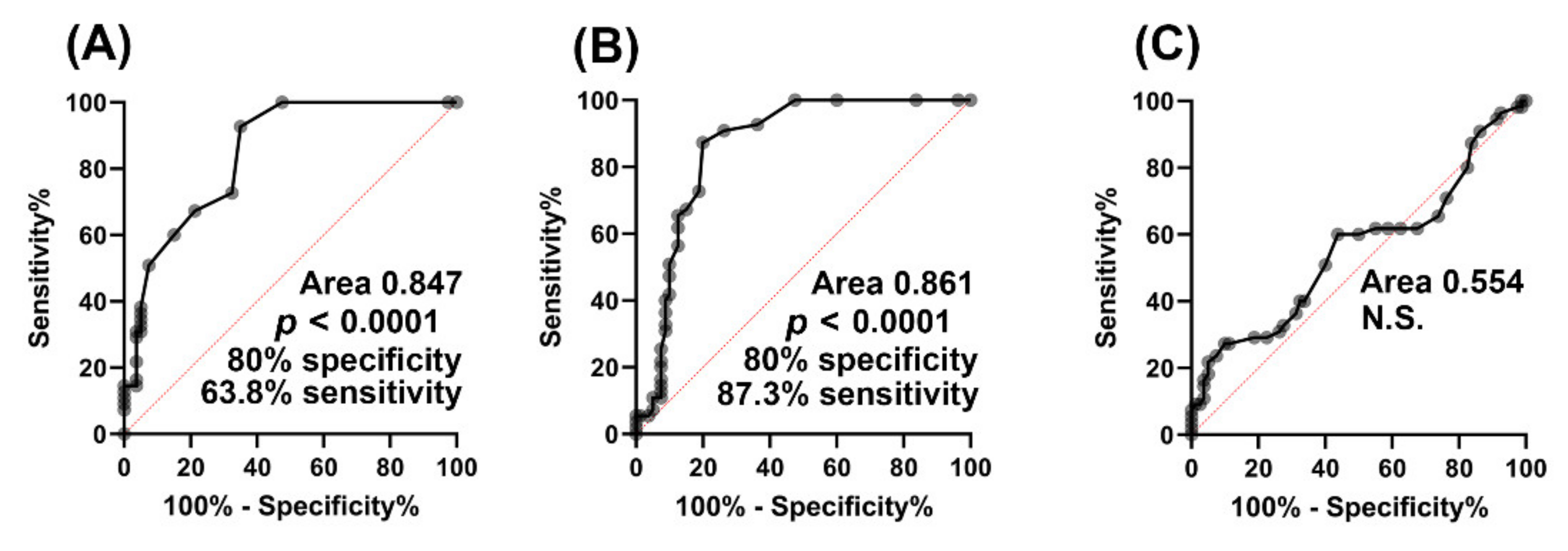
2.1.4. Biomarker Evaluation
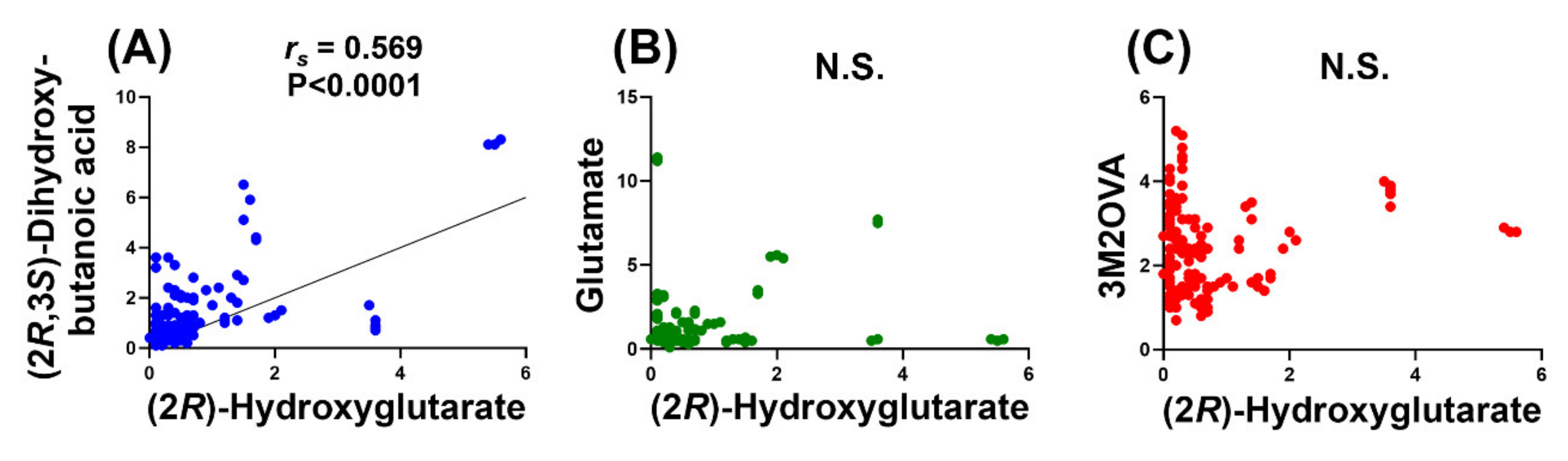
2.1.5. Relationship of (2R)-Hydroxyglutaric Acid and (2R,3S)-Dihydroxybutanoic Acid to Clinical Variables of AML
3. Discussion
4. Materials and Methods
4.1. Investigation of AML Patients
4.2. Genotyping IDH1,2 and D2HGDH
4.3. Gene Expression Analysis Using Taqman Assays
4.4. GC–MS Metabolomics of AML Plasmas
4.5. Multivariate Data Analysis and Univariate Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburg, O.; Posener, K.; Negelein, E. Ueber den Stoffwechsel der Tumoren. Biochem. Z 1924, 152, 319–344. [Google Scholar]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Pollard, P.J.; Briere, J.J.; Alam, N.A.; Barwell, J.; Barclay, E.; Wortham, N.C.; Hunt, T.; Mitchell, M.; Olpin, S.; Moat, S.J.; et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 2005, 14, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- McKenney, A.S.; Levine, R.L. Isocitrate dehydrogenase mutations in leukemia. J. Clin. Investig. 2013, 123, 3672–3677. [Google Scholar] [CrossRef]
- Kao, H.W.; Liang, D.C.; Wu, J.H.; Kuo, M.C.; Wang, P.N.; Yang, C.P.; Shih, Y.S.; Lin, T.H.; Huang, Y.H.; Shih, L.Y. Gene mutation patterns in patients with minimally differentiated acute myeloid leukemia. Neoplasia 2014, 16, 481–488. [Google Scholar] [CrossRef]
- Lin, J.; Yao, D.M.; Qian, J.; Chen, Q.; Qian, W.; Li, Y.; Yang, J.; Wang, C.Z.; Chai, H.Y.; Qian, Z.; et al. IDH1 and IDH2 mutation analysis in Chinese patients with acute myeloid leukemia and myelodysplastic syndrome. Ann. Hematol. 2012, 91, 519–525. [Google Scholar] [CrossRef]
- Flach, J.; Shumilov, E.; Joncourt, R.; Porret, N.; Novak, U.; Pabst, T.; Bacher, U. Current concepts and future directions for hemato-oncologic diagnostics. Crit. Rev. Oncol. Hematol. 2020, 151, 102977. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Cairns, R.A.; Minden, M.D.; Driggers, E.M.; Bittinger, M.A.; Jang, H.G.; Sasaki, M.; Jin, S.; Schenkein, D.P.; Su, S.M.; et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J. Exp. Med. 2010, 207, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Chowdhury, R.; Yeoh, K.K.; Tian, Y.M.; Hillringhaus, L.; Bagg, E.A.; Rose, N.R.; Leung, I.K.; Li, X.S.; Woon, E.C.; Yang, M.; et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011, 12, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Conway O’Brien, E.; Prideaux, S.; Chevassut, T. The epigenetic landscape of acute myeloid leukemia. Adv. Hematol. 2014, 2014, 103175. [Google Scholar] [CrossRef]
- Guo, C.; Pirozzi, C.J.; Lopez, G.Y.; Yan, H. Isocitrate dehydrogenase mutations in gliomas: Mechanisms, biomarkers and therapeutic target. Curr. Opin. Neurol. 2011, 24, 648–652. [Google Scholar] [CrossRef]
- Graca, G.; Lau, C.E.; Goncalves, L.G. Exploring Cancer Metabolism: Applications of Metabolomics and Metabolic Phenotyping in Cancer Research and Diagnostics. Adv. Exp. Med. Biol. 2020, 1219, 367–385. [Google Scholar] [CrossRef]
- Beyoglu, D.; Zhou, Y.; Chen, C.; Idle, J.R. Mass isotopomer-guided decluttering of metabolomic data to visualize endogenous biomarkers of drug toxicity. Biochem. Pharmacol. 2018, 156, 491–500. [Google Scholar] [CrossRef]
- Beyoglu, D.; Idle, J.R. Metabolomic and Lipidomic Biomarkers for Premalignant Liver Disease Diagnosis and Therapy. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Pabst, T.; Kortz, L.; Fiedler, G.M.; Ceglarek, U.; Idle, J.R.; Beyoglu, D. The plasma lipidome in acute myeloid leukemia at diagnosis in relation to clinical disease features. BBA Clin. 2017, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Chen, W.L.; Wang, J.H.; Li, N.; Li, J.M.; Mi, J.Q.; Zhang, W.N.; Li, Y.; Wu, S.F.; et al. Rapid diagnosis and prognosis of de novo acute myeloid leukemia by serum metabonomic analysis. J. Proteome Res. 2013, 12, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Wang, J.H.; Zhao, A.H.; Xu, X.; Wang, Y.H.; Chen, T.L.; Li, J.M.; Mi, J.Q.; Zhu, Y.M.; Liu, Y.F.; et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 2014, 124, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Naz, A. SERUM metabolomics of acute lymphoblastic leukaemia and acute myeloid leukaemia for probing biomarker molecules. Hematol. Oncol. 2017, 35, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Stefanko, A.; Thiede, C.; Ehninger, G.; Simons, K.; Grzybek, M. Lipidomic approach for stratification of acute myeloid leukemia patients. PLoS ONE 2017, 12, e0168781. [Google Scholar] [CrossRef]
- Hlavackova, A.; Vydra, J.; Chrastinova, L.; Kotlin, R.; Stikarova, J.; Suttnar, J.; Dyr, J.E. Targeted metabolomic profiling in acute myeloid leukemia with IDH2R140 and IDH2R172 mutations. Blood 2018, 132, 1470. [Google Scholar] [CrossRef]
- Stockard, B.; Garrett, T.; Guingab-Cagmat, J.; Meshinchi, S.; Lamba, J. Distinct Metabolic features differentiating FLT3-ITD AML from FLT3-WT childhood Acute Myeloid Leukemia. Sci. Rep. 2018, 8, 5534. [Google Scholar] [CrossRef]
- Stockard, B.; Wu, H.; Guingab, J.D.; Garrett, T.J.; Rubnitz, J.; Pounds, S.; Lamba, J.K. Metabolomics profing reveals markers for chemosensitivity and clinical outcomes in pediatric AML patients. Blood 2018, 132, 1536. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, M.; Wang, Q.; Aa, J.; Cao, B.; Li, J. Metabolomics analysis identifies lysine and taurine as candidate prognostic biomarkers for AML-M2 patients. Int. J. Hematol. 2020, 111, 761–770. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Choudhary, M.I.; Rahman, A.U. Serum metabonomics of acute leukemia using nuclear magnetic resonance spectroscopy. Sci. Rep. 2016, 6, 30693. [Google Scholar] [CrossRef]
- Wojcicki, A.V.; Kasowski, M.M.; Sakamoto, K.M.; Lacayo, N. Metabolomics in acute myeloid leukemia. Mol. Genet. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, J.; Guallar, V.; Krilov, G.; Kantrowitz, E.R. Mechanism of thermal decomposition of carbamoyl phosphate and its stabilization by aspartate and ornithine transcarbamoylases. Proc. Natl. Acad. Sci. USA 2008, 105, 16918–16923. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.P. Proteome-pI: Proteome isoelectric point database. Nucleic Acids Res. 2017, 45, D1112–D1116. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Conversano, L.; Petti, M.C.; Torelli, G.F.; Cascino, A.; Mecarocci, S.; Annicchiarico, M.A.; Rossi Fanelli, F. Plasma amino acid concentrations in patients with acute myelogenous leukemia. Nutrition 1999, 15, 195–199. [Google Scholar] [CrossRef]
- Cascino, A.; Muscaritoli, M.; Cangiano, C.; Conversano, L.; Laviano, A.; Ariemma, S.; Meguid, M.M.; Rossi Fanelli, F. Plasma amino acid imbalance in patients with lung and breast cancer. Anticancer Res. 1995, 15, 507–510. [Google Scholar]
- McBrayer, S.K.; Mayers, J.R.; DiNatale, G.J.; Shi, D.D.; Khanal, J.; Chakraborty, A.A.; Sarosiek, K.A.; Briggs, K.J.; Robbins, A.K.; Sewastianik, T.; et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell 2018, 175, 101–116. [Google Scholar] [CrossRef]
- Kassel, D.B.; Martin, M.; Schall, W.; Sweeley, C.C. Urinary metabolites of L-threonine in type 1 diabetes determined by combined gas chromatography/chemical ionization mass spectrometry. Biomed. Environ. Mass Spectrom. 1986, 13, 535–540. [Google Scholar] [CrossRef]
- Appiah-Amponsah, E.; Shanaiah, N.; Nagana Gowda, G.A.; Owusu-Sarfo, K.; Ye, T.; Raftery, D. Identification of 4-deoxythreonic acid present in human urine using HPLC and NMR techniques. J. Pharm. Biomed. Anal. 2009, 50, 878–885. [Google Scholar] [CrossRef]
- Thompson, J.A.; Markey, S.P.; Fennessey, P.V. Gas-chromatographic/mass-spectrometric identification and quantitation of tetronic and deoxytetronic acids in urine from normal adults and neonates. Clin. Chem. 1975, 21, 1892–1898. [Google Scholar] [CrossRef]
- Malinovsky, A.V. Reason for Indispensability of Threonine in Humans and Other Mammals in Comparative Aspect. Biochem. (Mosc.) 2017, 82, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L. Amino acid synthesis. In Reference Module in Biomedical Sciences; Academic Press: Cambridge, MA, USA, 2009; pp. 2–17. [Google Scholar]
- Lin, A.P.; Abbas, S.; Kim, S.W.; Ortega, M.; Bouamar, H.; Escobedo, Y.; Varadarajan, P.; Qin, Y.; Sudderth, J.; Schulz, E.; et al. D2HGDH regulates alpha-ketoglutarate levels and dioxygenase function by modulating IDH2. Nat. Commun 2015, 6, 7768. [Google Scholar] [CrossRef] [PubMed]
- Thirumal Kumar, D.; Jerushah Emerald, L.; George Priya Doss, C.; Sneha, P.; Siva, R.; Charles Emmanuel Jebaraj, W.; Zayed, H. Computational approach to unravel the impact of missense mutations of proteins (D2HGDH and IDH2) causing D-2-hydroxyglutaric aciduria 2. Metab. Brain Dis. 2018, 33, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Smith, C.P.; Wilbur, R.R.; Cain, C.P.; Kallu, S.R.; Valasapalli, S.; Sahoo, A.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. An overview of MCT1 and MCT4 in GBM: Small molecule transporters with large implications. Am. J. Cancer Res. 2018, 8, 1967–1976. [Google Scholar]
- Green, C.L.; Evans, C.M.; Zhao, L.; Hills, R.K.; Burnett, A.K.; Linch, D.C.; Gale, R.E. The prognostic significance of IDH2 mutations in AML depends on the location of the mutation. Blood 2011, 118, 409–412. [Google Scholar] [CrossRef]
- Kotredes, K.P.; Razmpour, R.; Lutton, E.; Alfonso-Prieto, M.; Ramirez, S.H.; Gamero, A.M. Characterization of cancer-associated IDH2 mutations that differ in tumorigenicity, chemosensitivity and 2-hydroxyglutarate production. Oncotarget 2019, 10, 2675–2692. [Google Scholar] [CrossRef]
- Berger, M.D.; Heini, A.D.; Seipel, K.; Mueller, B.; Angelillo-Scherrer, A.; Pabst, T. Increased fibrinogen levels at diagnosis are associated with adverse outcome in patients with acute myeloid leukemia. Hematol. Oncol. 2017, 35, 789–796. [Google Scholar] [CrossRef]
- Dai, C.; Arceo, J.; Arnold, J.; Sreekumar, A.; Dovichi, N.J.; Li, J.; Littlepage, L.E. Metabolomics of oncogene-specific metabolic reprogramming during breast cancer. Cancer Metab. 2018, 6, 5. [Google Scholar] [CrossRef]
- Ortmayr, K.; Dubuis, S.; Zampieri, M. Metabolic profiling of cancer cells reveals genome-wide crosstalk between transcriptional regulators and metabolism. Nat. Commun. 2019, 10, 1841. [Google Scholar] [CrossRef]
- Sun, C.; Li, T.; Song, X.; Huang, L.; Zang, Q.; Xu, J.; Bi, N.; Jiao, G.; Hao, Y.; Chen, Y.; et al. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc. Natl. Acad. Sci. USA 2019, 116, 52–57. [Google Scholar] [CrossRef]
- Vriend, J.; Hoogstraten, C.A.; Venrooij, K.R.; van den Berge, B.T.; Govers, L.P.; van Rooij, A.; Huigen, M.; Schirris, T.J.J.; Russel, F.G.M.; Masereeuw, R.; et al. Organic anion transporters 1 and 3 influence cellular energy metabolism in renal proximal tubule cells. Biol. Chem. 2019, 400, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Chaumeil, M.M.; Radoul, M.; Najac, C.; Eriksson, P.; Viswanath, P.; Blough, M.D.; Chesnelong, C.; Luchman, H.A.; Cairncross, J.G.; Ronen, S.M. Hyperpolarized (13)C MR imaging detects no lactate production in mutant IDH1 gliomas: Implications for diagnosis and response monitoring. Neuroimage Clin. 2016, 12, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Tian, Y.; Zhu, G.; Qin, Y.; Chen, X.; Pi, L.; Wei, M.; Liu, G.; Li, Z.; et al. Six-gene signature for predicting survival in patients with head and neck squamous cell carcinoma. Aging (Albany NY) 2020, 12, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Struys, E.A. 2-Hydroxyglutarate is not a metabolite; D-2-hydroxyglutarate and L-2-hydroxyglutarate are! Proc. Natl. Acad. Sci. USA 2013, 110, E4939. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.E.; Nelson, T.; Fales, H.M.; Levin, D.M. Isolation and characterization of a hydroxyacid-oxoacid transhydrogenase from rat kidney mitochondria. J. Biol. Chem. 1988, 263, 16872–16879. [Google Scholar]
- Berger, R.S.; Ellmann, L.; Reinders, J.; Kreutz, M.; Stempfl, T.; Oefner, P.J.; Dettmer, K. Degradation of D-2-hydroxyglutarate in the presence of isocitrate dehydrogenase mutations. Sci. Rep. 2019, 9, 7436. [Google Scholar] [CrossRef]
- Maeda, K.; Shiraishi, S.; Sakamoto, N.; Ohki, T.; Hosoi, M.; Ohta, K.; Yamanaka, N. Identification of Escherichia coli by detection of hydroquinone and uracil in the urine system. J. Chromatogr. 1985, 345, 11–18. [Google Scholar] [CrossRef]
- Diaz, S.O.; Barros, A.S.; Goodfellow, B.J.; Duarte, I.F.; Carreira, I.M.; Galhano, E.; Pita, C.; Almeida Mdo, C.; Gil, A.M. Following healthy pregnancy by nuclear magnetic resonance (NMR) metabolic profiling of human urine. J. Proteome Res. 2013, 12, 969–979. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhou, C.J.; Liu, Z.; Fu, Y.Y.; Zheng, P.; Yang, D.Y.; Li, Q.; Mu, J.; Wei, Y.D.; Zhou, J.J.; et al. Divergent Urinary Metabolic Phenotypes between Major Depressive Disorder and Bipolar Disorder Identified by a Combined GC-MS and NMR Spectroscopic Metabonomic Approach. J. Proteome Res. 2015, 14, 3382–3389. [Google Scholar] [CrossRef]
- Chamberlin, B.A.; Sweeley, C.C. Metabolic profiles of urinary organic acids recovered from absorbent filter paper. Clin. Chem. 1987, 33, 572–576. [Google Scholar] [CrossRef]
- Tuchman, M.; Bowers, L.D.; Fregien, K.D.; Crippin, P.J.; Krivit, W. Capillary gas chromatographic separation of urinary organic acids. Retention indices of 101 urinary acids on a 5% phenylmethyl silicone capillary column. J. Chromatogr. Sci. 1984, 22, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, H.; Wang, Y.; Wang, Y.; Liu, J.; Zhou, Z.; Chu, H.; Zhuang, P.; Zhang, Y. Application of GC/MS-based metabonomic profiling in studying the therapeutic effects of Huangbai-Zhimu herb-pair (HZ) extract on streptozotocin-induced type 2 diabetes in mice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 997, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hamoudeh, E.; Zeidan, A.M.; Barbarotta, L.; Rosano, N. The Interactions Between Diabetes Mellitus and Myelodysplastic Syndromes: Current State of Evidence and Future Directions. Curr. Diabetes Rev. 2016, 12, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Klepin, H.D. Myelodysplastic Syndromes and Acute Myeloid Leukemia in the Elderly. Clin. Geriatr. Med. 2016, 32, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, F.; Lecuit, M.; Broutin, S.; Saada, S.; Jeanson, A.; Penard-Lacronique, V.; de Botton, S. Ivosidenib to treat adult patients with relapsed or refractory acute myeloid leukemia. Drugs Today (Barc.) 2020, 56, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Wang, H.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Watts, J.M.; Pollyea, D.A.; et al. Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv. 2020, 4, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Dai, D.; DiNardo, C.D.; Stein, E.; de Botton, S.; Attar, E.C.; Liu, H.; Liu, G.; Lemieux, I.; Agresta, S.V.; et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib in patients with advanced hematologic malignancies with an IDH1 mutation. Cancer Chemother. Pharmacol. 2020, 85, 959–968. [Google Scholar] [CrossRef]
- Keogh, A.; Senkardes, S.; Idle, J.R.; Kucukguzel, S.G.; Beyoglu, D. A Novel Anti-Hepatitis C Virus and Antiproliferative Agent Alters Metabolic Networks in HepG2 and Hep3B Cells. Metabolites 2017, 7. [Google Scholar] [CrossRef]
- Semmo, N.; Weber, T.; Idle, J.R.; Beyoglu, D. Metabolomics reveals that aldose reductase activity due to AKR1B10 is upregulated in hepatitis C virus infection. J. Viral Hepat. 2015, 22, 617–624. [Google Scholar] [CrossRef]
- Beyoglu, D.; Imbeaud, S.; Maurhofer, O.; Bioulac-Sage, P.; Zucman-Rossi, J.; Dufour, J.F.; Idle, J.R. Tissue metabolomics of hepatocellular carcinoma: Tumor energy metabolism and the role of transcriptomic classification. Hepatology 2013, 58, 229–238. [Google Scholar] [CrossRef]
- Fahrner, R.; Beyoglu, D.; Beldi, G.; Idle, J.R. Metabolomic markers for intestinal ischemia in a mouse model. J. Surg. Res. 2012, 178, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Beyoglu, D.; Idle, J.R. Metabolomics and its potential in drug development. Biochem. Pharmacol. 2013, 85, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G. Simultaneous Statistical Inference; Springer: New York, NY, USA, 1966. [Google Scholar]


| # | Metabolite | Median Relative Concentration Wild-Type IDH1/2 | Median Relative Concentration IDH1R132 | Median Relative Concentration IDH2R140 | p-Value |
|---|---|---|---|---|---|
| 1 | galactose | 541 | 372 | 595 | <0.0001 |
| 2 | mannose | 265 | 170 | 302 | <0.0001 |
| 3 | xylitol | 0.6 | 0.3 | 0.8 | <0.0001 |
| 4 | alanine | 14.7 | 7.7 | 23.5 | <0.0001 |
| 5 | valine | 32.4 | 26.4 | 47.6 | <0.0001 |
| 6 | leucine | 29.1 | 23.8 | 38.4 | <0.0001 |
| 7 | isoleucine | 10.2 | 7.9 | 14.0 | <0.0001 |
| 8 | proline | 17.2 | 9.3 | 20.3 | <0.0001 |
| 9 | tyrosine | 20.2 | 14.4 | 26.9 | <0.0001 |
| 10 | glutamine | 8.2 | 3.7 | 9.2 | <0.0001 |
| 11 | phenylalanine | 11.5 | 9.4 | 12.5 | <0.0001 |
| 12 | tryptophan | 12 | 8.6 | 16.5 | 0.0002 |
| 13 | methionine | 1.8 | 1.2 | 2.2 | <0.0001 |
| 14 | lysine | 0.6 | 0.3 | 1.1 | <0.0001 |
| 15 | glutamic acid | 0.8 | 0.5 | 1.2 | <0.0001 |
| 16 | ornithine | 0.2 | 0.1 | 0.4 | <0.0001 |
| 17 | ethanolamine | 14.5 | 9.2 | 20.2 | <0.0001 |
| 18 | carbamic acid 1 | 3.6 | 3.1 | 3.8 | <0.0001 |
| 19 | threonine | 6.2 | 4.0 | 7.7 | 0.0001 |
| 20 | hexanoic acid | 0.8 | 0.8 | 0.7 | 0.001 |
| 21 | urea | 965.4 | 285 | 1051 | 0.02 |
| 22 | serine | 7.2 | 3.9 | 8.9 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idle, J.R.; Seipel, K.; Bacher, U.; Pabst, T.; Beyoğlu, D. (2R,3S)-Dihydroxybutanoic Acid Synthesis as a Novel Metabolic Function of Mutant Isocitrate Dehydrogenase 1 and 2 in Acute Myeloid Leukemia. Cancers 2020, 12, 2842. https://doi.org/10.3390/cancers12102842
Idle JR, Seipel K, Bacher U, Pabst T, Beyoğlu D. (2R,3S)-Dihydroxybutanoic Acid Synthesis as a Novel Metabolic Function of Mutant Isocitrate Dehydrogenase 1 and 2 in Acute Myeloid Leukemia. Cancers. 2020; 12(10):2842. https://doi.org/10.3390/cancers12102842
Chicago/Turabian StyleIdle, Jeffrey R., Katja Seipel, Ulrike Bacher, Thomas Pabst, and Diren Beyoğlu. 2020. "(2R,3S)-Dihydroxybutanoic Acid Synthesis as a Novel Metabolic Function of Mutant Isocitrate Dehydrogenase 1 and 2 in Acute Myeloid Leukemia" Cancers 12, no. 10: 2842. https://doi.org/10.3390/cancers12102842
APA StyleIdle, J. R., Seipel, K., Bacher, U., Pabst, T., & Beyoğlu, D. (2020). (2R,3S)-Dihydroxybutanoic Acid Synthesis as a Novel Metabolic Function of Mutant Isocitrate Dehydrogenase 1 and 2 in Acute Myeloid Leukemia. Cancers, 12(10), 2842. https://doi.org/10.3390/cancers12102842