Investigating Patterns of Immune Interaction in Ovarian Cancer: Probing the O-glycoproteome by the Macrophage Galactose-Like C-Type Lectin (MGL)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant MGL Protein
2.2. Cell Lines
2.3. Immunofluorescence
2.4. Flow Cytometry
2.5. MGL–LWAC Column
2.6. Tumor Samples
2.7. Sample Preparation and Lectin Enrichment
2.8. Mass Spectrometry and Data Analysis
2.9. Immunohistochemistry
3. Results
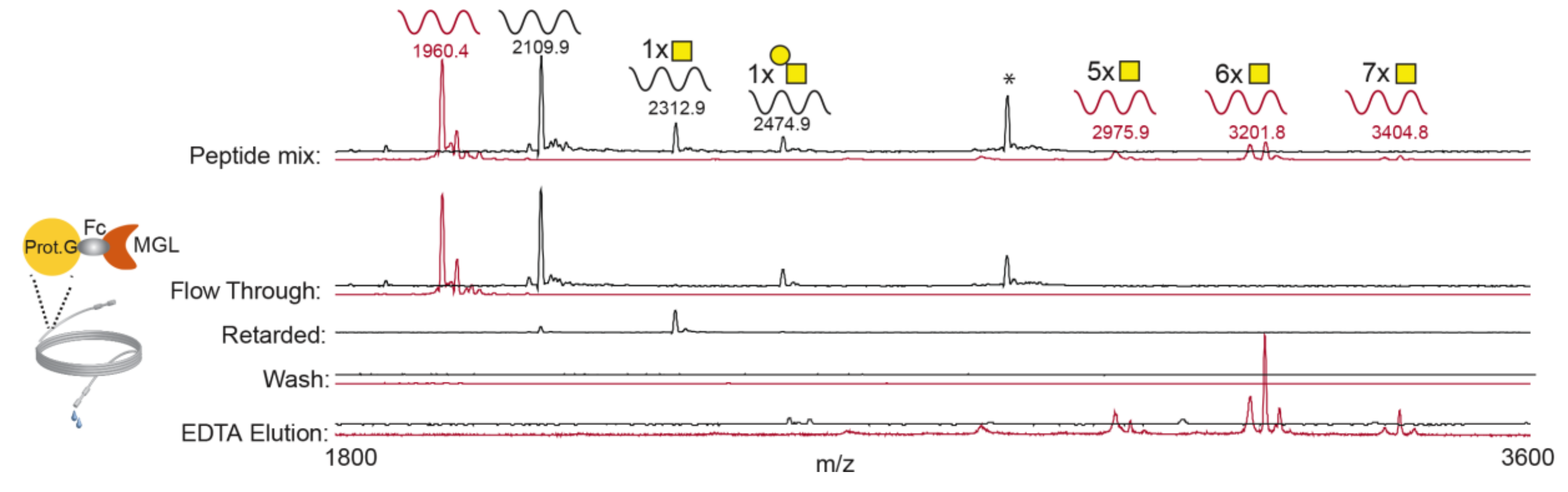
3.1. rhMGL Specifically Binds Tn Carrying Glycopeptides in Lectin Weak Affinity Chromatography (LWAC) Column
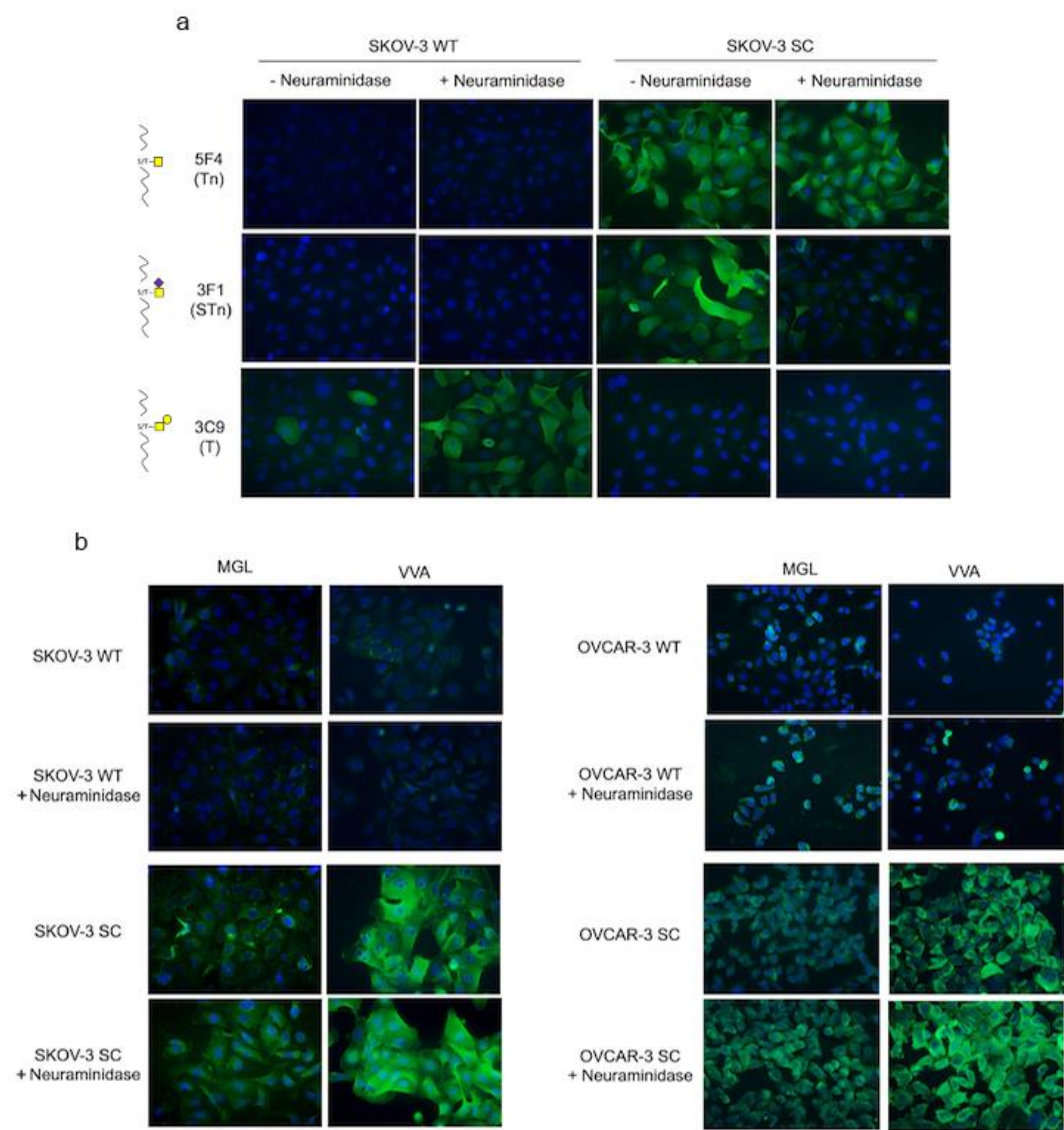
3.2. rhMGL Binds Tumor Glycan Tn on Ovarian Cancer Cells
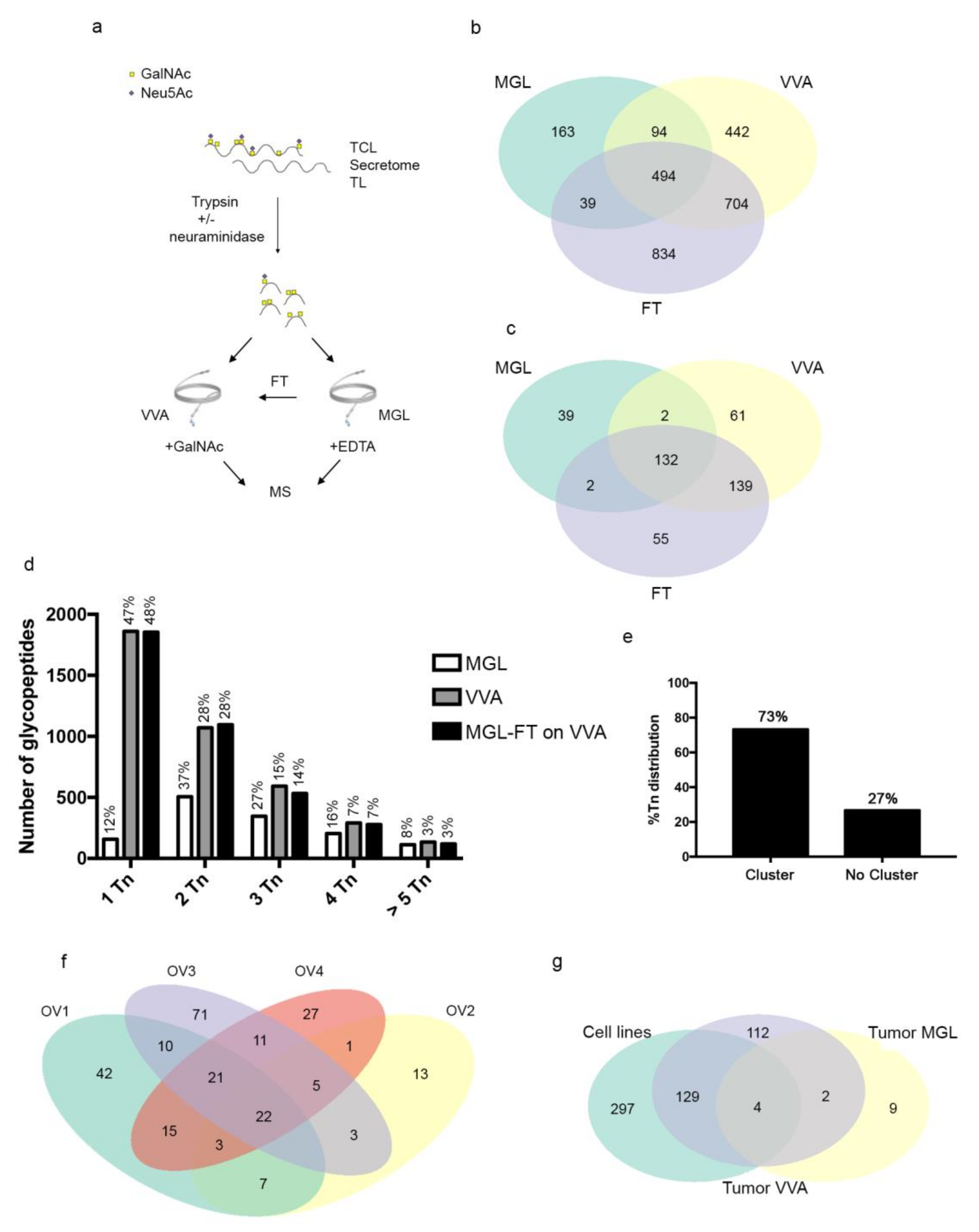
3.3. MGL Preferentially Recognizes Ovarian Cancer Associated Glycoproteins Carrying Multiple Tn Carbohydrate Moieties Clustered on Adjacent Amino Acid Sites
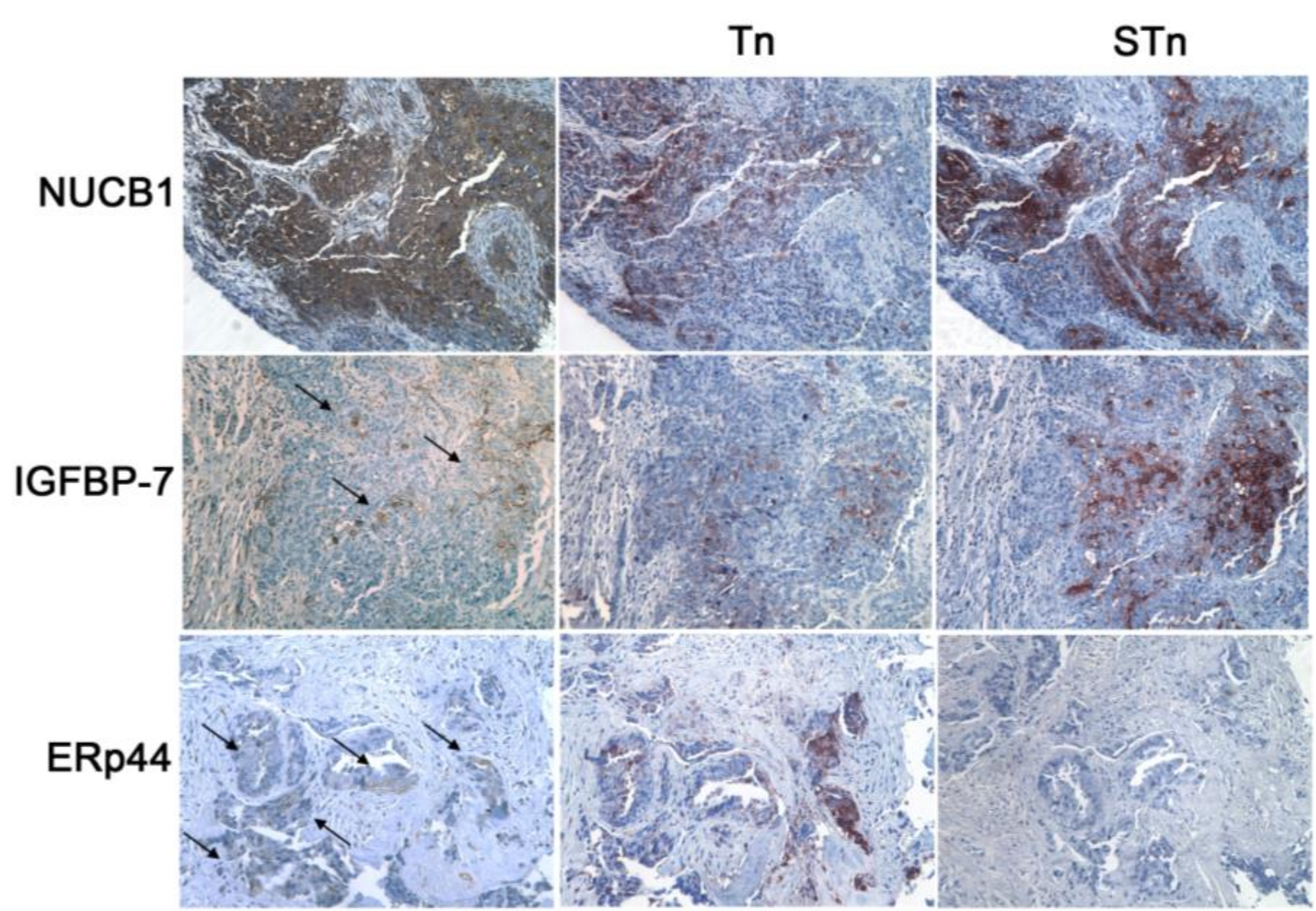
3.4. MGL Recognizes Intracellular and Extracellular Glycoproteins in Ovarian Tumor Tissues
3.5. The MGL Binding Motif Is Carried by Surface, Intracellular and Extracellular Matrix Glycoproteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, H.; Li, S.; Shen, Y.; Wang, H.; Shan, W.; Qiu, J.; Zhang, J.; Cheng, W. Identification of Potential Biomarkers in Association with Progression and Prognosis in Epithelial Ovarian Cancer by Integrated Bioinformatics Analysis. Front. Genet. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Kulbe, H.; Otto, R.; Darb-Esfahani, S.; Lammert, H.; Abobaker, S.; Welsch, G.; Chekerov, R.; Schäfer, R.; Dragun, D.; Hummel, M.; et al. Discovery and Validation of Novel Biomarkers for Detection of Epithelial Ovarian Cancer. Cells 2019, 8, 713. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Rodríguez, E.; Schetters, S.T.T.; Van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef]
- Ju, T.; Aryal, R.P.; Kudelka, M.R.; Wang, Y.; Cummings, R.D. The COSMC connection to the Tn antigen in cancer. Cancer Biomark. 2014, 14, 63–81. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Chia, J.; Ros, M.; Hui, K.M.; Saltel, F.; Bard, F. Organelle Specific O-Glycosylation Drives MMP14 Activation, Tumor Growth, and Metastasis. Cancer Cell 2017, 32, 639–653. [Google Scholar] [CrossRef]
- Posey, A.D.; Clausen, H.; June, C.H. Distinguishing Truncated and Normal MUC1 Glycoform Targeting from Tn-MUC1-Specific CAR T Cells: Specificity Is the Key to Safety. Immunity 2016, 45, 947–948. [Google Scholar] [CrossRef]
- Sheta, R.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Popa, I.; Bachvarov, D. Altered expression of different GalNAc-Transferases is associated with disease progression and poor prognosis in women with high-grade serous ovarian cancer. Int. J. Oncol. 2017, 51, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gentry-Maharaj, A.; Burnell, M.; Steentoft, C.; Marcos-Silva, L.; Mandel, U.; Jacobs, I.; Dawnay, A.; Menon, U.; Blixt, O. Microarray glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. J. Proteome Res. 2013, 12, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Salminen, L.; Nadeem, N.; Jain, S.; Grènman, S.; Carpén, O.; Hietanen, S.; Oksa, S.; Lamminmäki, U.; Pettersson, K.; Gidwani, K.; et al. A longitudinal analysis of CA125 glycoforms in the monitoring and follow up of high grade serous ovarian cancer. Gynecol. Oncol. 2020, 156, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, I.G.; Napoletano, C.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Pierelli, L.; Nuti, M.; Rughetti, A. MGL Receptor and Immunity: When the Ligand Can Make the Difference. J. Immunol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Heger, L.; Balk, S.; Lühr, J.J.; Heidkamp, G.F.; Lehmann, C.H.K.; Hatscher, L.; Purbojo, A.; Hartmann, A.; Garcia-Martin, F.; Nishimura, S.-I.; et al. CLEC10A Is a specific marker for human CD1c+ dendritic cells and enhances their toll-like receptor 7/8-Induced cytokine secretion. Front. Immunol. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Zaal, A.; Li, R.J.E.; Lübbers, J.; Bruijns, S.C.M.; Kalay, H.; van Kooyk, Y.; van Vliet, S.J. Activation of the C-Type Lectin MGL by Terminal GalNAc Ligands Reduces the Glycolytic Activity of Human Dendritic Cells. Front. Immunol. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, C.; Rughetti, A.; Agervig Tarp, M.P.; Coleman, J.; Bennett, E.P.; Picco, G.; Sale, P.; Denda-Nagai, K.; Irimura, T.; Mandel, U.; et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res. 2007, 67, 8358–8367. [Google Scholar] [CrossRef]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Van Der Horst, J.C.; Spaans, V.; Kenter, G.; De Kroon, C.; Bosse, T.; Van Vliet, S.J.; Jordanova, E.S. MGL ligand expression is correlated to lower survival and distant metastasis in cervical squamous cell and adenosquamous carcinoma. Front. Oncol. 2019. [Google Scholar] [CrossRef]
- Dusoswa, S.A.; Verhoeff, J.; Abels, E.; Méndez-Huergo, S.P.; Croci, D.O.; Kuijper, L.H.; de Miguel, E.; Wouters, V.M.C.J.; Best, M.G.; Rodriguez, E.; et al. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc. Natl. Acad. Sci. USA 2020, 117, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Eggink, L.L.; Roby, K.F.; Cote, R.; Kenneth Hoober, J. An innovative immunotherapeutic strategy for ovarian cancer: CLEC10A and glycomimetic peptides. J. Immunother. Cancer 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.B.G.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, Y.; Joshi, H.J.; Yang, Z.; Gomes, C.; Chen, Y.H.; Lorenzetti, F.C.; Furukawa, S.; Schjoldager, K.T.; Hansen, L.; Clausen, H.; et al. A validated gRNA library for CRISPR/Cas9 targeting of the human glycosyltransferase genome. Glycobiology 2018, 28, 295–305. [Google Scholar] [CrossRef]
- Yang, Z.; Steentoft, C.; Hauge, C.; Hansen, L.; Thomsen, A.L.; Niola, F.; Vester-Christensen, M.B.; Frödin, M.; Clausen, H.; Wandall, H.H.; et al. Fast and sensitive detection of indels induced by precise gene targeting. Nucleic Acids Res. 2015, 43, e59. [Google Scholar] [CrossRef]
- Narimatsu, Y.; Joshi, H.J.; Nason, R.; Van Coillie, J.; Karlsson, R.; Sun, L.; Ye, Z.; Chen, Y.-H.; Schjoldager, K.T.; Steentoft, C.; et al. An Atlas of Human Glycosylation Pathways Enables Display of the Human Glycome by Gene Engineered Cells. Mol. Cell 2019, 75, 394–407. [Google Scholar] [CrossRef]
- Kong, Y.; Joshi, H.J.; Schjoldager, K.T.B.G.; Madsen, T.D.; Gerken, T.A.; Vester-Christensen, M.B.; Wandall, H.H.; Bennett, E.P.; Levery, S.B.; Vakhrushev, S.Y.; et al. Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis. Glycobiology 2015, 25, 55–65. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Joshi, H.J.; Kong, Y.; Goth, C.K.; King, S.L.; Wandall, H.H.; Bennett, E.P.; Vakhrushev, S.Y.; Clausen, H. Deconstruction of O-glycosylation—Gal NA c-T isoforms direct distinct subsets of the O-glycoproteome. EMBO Rep. 2015, 16, 1713–1722. [Google Scholar] [CrossRef]
- Iida, S.; Yamamoto, K.; Irimura, T. Interaction of human macrophage C-type lectin with O-linked N-acetylgalactosamine residues on mucin glycopeptides. J. Biol. Chem. 1999, 274, 10697–10705. [Google Scholar] [CrossRef]
- Mortezai, N.; Behnken, H.N.; Kurze, A.K.; Ludewig, P.; Buck, F.; Meyer, B.; Wagener, C. Tumor-associated Neu5Ac-Tn and Neu5Gc-Tn antigens bind to C-type lectin CLEC10A (CD301, MGL). Glycobiology 2013, 23, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Freitas, D.; Gomes, J.; Magalhães, A.; Steentoft, C.; Gomes, C.; Vester-Christensen, M.B.; Ferreira, J.A.; Afonso, L.P.; Santos, L.L.; et al. Probing the O-glycoproteome of gastric cancer cell lines for biomarker discovery. Mol. Cell. Proteom. 2015, 14, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.B.G.; Vakhrushev, S.Y.; Kong, Y.; Steentoft, C.; Nudelman, A.S.; Pedersen, N.B.; Wandall, H.H.; Mandel, U.; Bennett, E.P.; Levery, S.B.; et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. Proc. Natl. Acad. Sci. USA 2012, 109, 9893–9898. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Westerlind, U.; Pett, C.; Schorlemer, M.; Rüetschi, U.; Brinkmalm, G.; Sihlbom, C.; Lengqvist, J.; Larson, G.; Nilsson, J. Assignment of Saccharide Identities through Analysis of Oxonium Ion Fragmentation Profiles in LC–MS/MS of Glycopeptides. J. Proteome Res. 2014, 13, 6024–6032. [Google Scholar] [CrossRef] [PubMed]
- Levery, S.B.; Steentoft, C.; Halim, A.; Narimatsu, Y.; Clausen, H.; Vakhrushev, S.Y. Advances in mass spectrometry driven O-glycoproteomics. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Tołoczko, A.; Hein, A.; Bouligny, A.L.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar]
- Truxova, I.; Kasikova, L.; Hensler, M.; Skapa, P.; Laco, J.; Pecen, L.; Belicova, L.; Praznovec, I.; Halaska, M.J.; Brtnicky, T.; et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I.G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int. J. Oncol. 2016, 48, 1369–1378. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef]
- Pedersen, M.; Westergaard, M.C.W.; Milne, K.; Nielsen, M.; Borch, T.H.; Poulsen, L.G.; Hendel, H.W.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: A pilot study. Oncoimmunology 2018, 7, e1502905. [Google Scholar] [CrossRef]
- Polastro, L.; Closset, C.; Kerger, J. Immunotherapy in gynecological cancers: Where are we? Curr. Opin. Oncol. 2020. [Google Scholar] [CrossRef]
- Pirro, M.; Rombouts, Y.; Stella, A.; Neyrolles, O.; Burlet-Schiltz, O.; van Vliet, S.J.; de Ru, A.H.; Mohammed, Y.; Wuhrer, M.; van Veelen, P.A.; et al. Characterization of Macrophage Galactose-type Lectin (MGL) ligands in colorectal cancer cell lines. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129513. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Schoof, E.; Van Vliet, S.J.; Rombouts, Y.; Stella, A.; De Ru, A.; Mohammed, Y.; Wuhrer, M.; Van Veelen, P.A.; Hensbergen, P.J. Glycoproteomic Analysis of MGL-Binding Proteins on Acute T-Cell Leukemia Cells. J. Proteome Res. 2019, 18, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; Gringhuis, S.I.; Geijtenbeek, T.B.H.; van Kooyk, Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 2006, 7, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, I.G.; Martufi, P.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Bellati, F.; Nuti, M.; Rughetti, A.; Napoletano, C. The macrophage galactose-type C-type lectin (MGL) modulates regulatory T cell functions. PLoS ONE 2015, 10, e0132617. [Google Scholar] [CrossRef] [PubMed]
- Artigas, G.; Monteiro, J.T.; Hinou, H.; Nishimura, S.I.; Lepenies, B.; Garcia-Martin, F. Glycopeptides as Targets for Dendritic Cells: Exploring MUC1 Glycopeptides Binding Profile toward Macrophage Galactose-Type Lectin (MGL) Orthologs. J. Med. Chem. 2017, 60, 9012–9021. [Google Scholar] [CrossRef] [PubMed]
- Wandall, H.H.; Blixt, O.; Tarp, M.A.; Pedersen, J.W.; Bennett, E.P.; Mandel, U.; Ragupathi, G.; Livingston, P.O.; Hollingsworth, M.A.; Taylor-Papadimitriou, J.; et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010, 70, 1306–1313. [Google Scholar] [CrossRef]
- Gambaro, K.; Quinn, M.C.J.; Cáceres-Gorriti, K.Y.; Shapiro, R.S.; Provencher, D.; Rahimi, K.; Mes-Masson, A.M.; Tonin, P.N. Low levels of IGFBP7 expression in high-grade serous ovarian carcinoma is associated with patient outcome. BMC Cancer 2015, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Pattnaik, S.; Aradhyam, G.K. Molecular evolution guided functional analyses reveals Nucleobindin-1 as a canonical E-box binding protein promoting Epithelial-to-Mesenchymal transition (EMT). Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 765–775. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 4002. [Google Scholar] [CrossRef]
- Garranzo-Asensio, M.; San Segundo-Acosta, P.; Povés, C.; Fernández-Aceñero, M.J.; Martínez-Useros, J.; Montero-Calle, A.; Solís-Fernández, G.; Sanchez-Martinez, M.; Rodríguez, N.; Cerón, M.Á.; et al. Identification of tumor-associated antigens with diagnostic ability of colorectal cancer by in-depth immunomic and seroproteomic analysis. J. Proteom. 2020, 214, 103635. [Google Scholar] [CrossRef] [PubMed]
- Rughetti, A.; Rahimi, H.; Belleudi, F.; Napoletano, C.; Battisti, F.; Zizzari, I.G.; Antonilli, M.; Bellati, F.; Wandall, H.H.; Benedetti Panici, P.; et al. Microvesicle cargo of tumor-associated MUC1 to dendritic cells allows cross-presentation and specific carbohydrate processing. Cancer Immunol. Res. 2014, 2, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Marcos-Silva, L.; Pereira, D.; Pinto, R.; Almeida, R.; Söderberg, O.; Mandel, U.; Clausen, H.; Felix, A.; Lunet, N.; et al. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol. Oncol. 2015, 9, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.J.; Tham, K.M.; Chia, J.; Wang, S.C.; Steentoft, C.; Clausen, H.; Bard-Chapeau, E.A.; Bard, F.A. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2013, 110, E3152–E3161. [Google Scholar] [CrossRef] [PubMed]
- Kurze, A.-K.; Buhs, S.; Eggert, D.; Oliveira-Ferrer, L.; Müller, V.; Niendorf, A.; Wagener, C.; Nollau, P. Immature O-glycans recognized by the macrophage glycoreceptor CLEC10A (MGL) are induced by 4-hydroxy-tamoxifen, oxidative stress and DNA-damage in breast cancer cells. Cell Commun. Signal. 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Bin, X.; Hongwen, Y.; Nenan, L.; Bin, L.; Jia, G.; Supig, G.; Naijiun, H.; Jun, Q.; Kaiti, Z.; et al. The ovarian cancer-derived secretory/releasing proteome: A repertoire of tumor markers. Proteomics 2012, 12, 1883–1891. [Google Scholar]
- Torky, H.A.; Sherif, A.; Abo-Louz, A.; Ali, M.; Ahmed, A.; Ali, A. Evaluation of serum nidogen-2 as a screening and diagnostic tool for ovarian cancer. Gynecol. Obstet. Invest. 2018, 83, 461–465. [Google Scholar] [CrossRef]
- Millstein, J.; Budden, T.; Goode, E.; Anglesio, M.; Talhouk, A.; Intermaggio, M.; Leong, H.; Al, E. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. 2020, 31, 1240–1250. [Google Scholar] [CrossRef]
- Mitra, S.; Tiwari, K.; Podicheti, R.; Pandhiri, T.; Rusch, D.B.; Bonetto, A.; Zhang, C.; Mitra, A.K. Transcriptome profiling reveals matrisome alteration as a key feature of ovarian cancer progression. Cancers (Basel) 2019, 11, 1513. [Google Scholar] [CrossRef]
- Van Vliet, S.J.; Paessens, L.C.; Broks-van den Berg, V.C.M.; Geijtenbeek, T.B.H.; van Kooyk, Y. The C-Type Lectin Macrophage Galactose-Type Lectin Impedes Migration of Immature APCs. J. Immunol. 2008, 181, 3148–3155. [Google Scholar] [CrossRef]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef] [PubMed]
| Ovarian Cancer Patient | NUCB1 | IGFBP7 | ERP44 | Tn | STn | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Positive Cancer Cells | Intensity | % Positive Cancer Cells | Intensity | % Positive Cancer Cells | Intensity | % Positive Cancer Cells | Intensity | % Positive Cancer Cells | Intensity | |
| OV11 | 10 | low | 30–35 | strong | <1 | NA | 40 | int./strong | 50–60 | strong |
| OV12 | 50–55 | low | <1 | NA | <1 | NA | 35 | strong | 60 | strong |
| OV13 | <1 | NA | 5–10 | strong | <1 | NA | 20 | int. | 40 | strong |
| OV14 | 90 | strong | 5–10 | int. | <1 | NA | 50–60 | strong | 70–80 | strong |
| OV15 | 65 | int. | <1 | NA | <1 | NA | 40 | strong | 50 | int. |
| OV16 | 60 | low | <1 | NA | 50 | low | 30–40 | int./strong | <1 | NA |
| OV17 | 30–40 | low | <1 | NA | <1 | NA | 20 | strong | 40 | int. |
| OV18 | 70–80 | low | <1 | NA | 5 | low | 60 | strong | 70–80 | strong |
| OV19 | 90 | Int. | <1 | NA | 70–80 | low | 50–60 | strong | 70–80 | strong |
| OV20 | 50 | low | 1–5 | strong | <1 | NA | 30 | int. | 50 | strong |
| OV21 | 10 | low | 1–5 | strong | 60 | low | 20 | strong | 60 | int. |
| Function | Gene | Protein | Glycopeptide Sequences | Glycan Moieties | Glycosylated Sites * |
|---|---|---|---|---|---|
| Mucins | MUC1 | Mucin-1 | SGHASSTPGGEKETSATQR | 4 | S6; T7; T14; S15 |
| SSTPGGEKETSATQR | 4 | S2; T3; T10; S11 | |||
| MUC16 | Mucin-16 | FLHSEMTTLMSR | 2 | T7; T8 | |
| IHPSSNTPVVNVGTVIYK | 4 | S4; S5; T7; T14 | |||
| ISTPDHDKSTVPP | 4 | S2; T3; S9; T10 | |||
| MUC24 | Mucin-24 | VTTPAPETCEGR | 2 | T2; T3 | |
| Protein folding/cell metabolism | ERP44 | Endoplamic Reticulum Resident Protein 44 | EFHHGPDPTDTAPGEQAQDVASSPPESSFQK | 2 | S22; S23 |
| LAMP1 | Lysosome-associated membrane glycoprotein 1 | CEQDRPSPTTAPPAPPSPSPSPVPK | 5 | T9; T10; S17; S19; S21 | |
| RPSPTTAPPAPPSPSPSPVPK | 5 | T5; T6; S13; S15; S17 | |||
| LAMP2 | Lysosome-associated membrane glycoprotein 2 | TSTVAPTIHTTVPSPTTTPTPK | 7 | T3; T7; T10; T11; T16; T17; T18 | |
| QSOX1 | Sulphydryl oxidase 1 | EAAQTTVAPTTANK | 4 | T5; T6; T10; T11 | |
| Protein folding/cell metabolism | SEL1L | Protein Sel-1 homolog 1 | TTLTSDESVKDHTTAGR | 2 | T13; T14 |
| LRR8CD | Leucin-rich repeated-containing protein 8D | AHTPPGNAEVTTNIPK | 3 | T3; T11; T12 | |
| Matrix/cell interaction | AGRN | Agrin | ATTASRLPSSAVTPR | 4 | T2; T3; S10; T13 |
| APHPSHTSQPVAKTTAAPTTR | 5 | T7; S8; T14; T15; T19 | |||
| APP | Amyloid beta A4 protein | GLTTRPGSGLTNIK | 3 | T3; T4; T11 | |
| DAG1 | Dystroglycan | IRTTTSGVPR | 4 | T3; T4; T5; S6 | |
| FN1 | Fibronectin | HTSVQTTSSGSGPFDVR | 2 | T6;T7 | |
| NID-2 | Nidogen-2 | SYPASGHTTPLSR | 2 | T8; T9 | |
| PODXL | Podocalyxin | SSHSVTTDLTSTK | 7 | S2; S4; T6; T7; T10; S11; T12 | |
| SDC3 | Syndecan-3 | LVSTATSRPR | 3 | S3; T4; S7 | |
| TPTPETFLTTIR | 3 | T6; T9; T10 | |||
| VCAN | Versican core protein | HLVTTVPKDPEAAEAR | 2 | T4; T5 | |
| YLSTTPFPSQHR | 2 | T4; T5 | |||
| QESSTTFVSDGSLEKHPEVPSAK | 3 | T5; T6; S21 | |||
| STILPTAEVEGTKAPVEKEEVK | 4 | S1; T2; T6; T12 | |||
| STILPTAEVEGTKAPVEK | 4 | S1; T2; T6; T12 | |||
| LHTTSAFKPSSAITK | 4 | T3; T4; S11; T14 | |||
| RKEEEGTTGTASTFEVYSSTQR | 4 | T7; S12; S18; S19 | |||
| TTDYSVLTTKK | 4 | T1; T2; T8; T9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoletano, C.; Steentoff, C.; Battisti, F.; Ye, Z.; Rahimi, H.; Zizzari, I.G.; Dionisi, M.; Cerbelli, B.; Tomao, F.; French, D.; et al. Investigating Patterns of Immune Interaction in Ovarian Cancer: Probing the O-glycoproteome by the Macrophage Galactose-Like C-Type Lectin (MGL). Cancers 2020, 12, 2841. https://doi.org/10.3390/cancers12102841
Napoletano C, Steentoff C, Battisti F, Ye Z, Rahimi H, Zizzari IG, Dionisi M, Cerbelli B, Tomao F, French D, et al. Investigating Patterns of Immune Interaction in Ovarian Cancer: Probing the O-glycoproteome by the Macrophage Galactose-Like C-Type Lectin (MGL). Cancers. 2020; 12(10):2841. https://doi.org/10.3390/cancers12102841
Chicago/Turabian StyleNapoletano, Chiara, Catharina Steentoff, Federico Battisti, Zilu Ye, Hassan Rahimi, Ilaria Grazia Zizzari, Marco Dionisi, Bruna Cerbelli, Federica Tomao, Deborah French, and et al. 2020. "Investigating Patterns of Immune Interaction in Ovarian Cancer: Probing the O-glycoproteome by the Macrophage Galactose-Like C-Type Lectin (MGL)" Cancers 12, no. 10: 2841. https://doi.org/10.3390/cancers12102841
APA StyleNapoletano, C., Steentoff, C., Battisti, F., Ye, Z., Rahimi, H., Zizzari, I. G., Dionisi, M., Cerbelli, B., Tomao, F., French, D., d’Amati, G., Panici, P. B., Vakhrushev, S., Clausen, H., Nuti, M., & Rughetti, A. (2020). Investigating Patterns of Immune Interaction in Ovarian Cancer: Probing the O-glycoproteome by the Macrophage Galactose-Like C-Type Lectin (MGL). Cancers, 12(10), 2841. https://doi.org/10.3390/cancers12102841





