Biopathological Significance of PIWI–piRNA Pathway Deregulation in Invasive Breast Carcinomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. RNA Extraction
2.3. Real-Time RT–PCR
2.4. Immunohistochemistry
2.5. Statistical Analysis
2.6. In Silico DNA Methylation Data and mRNA Expression Levels Analysis
3. Results
3.1. The Purely Germinal or Mixed Germinal and Somatic Status of the Genes Encoding the PIWIL1-2-3-4 Proteins Depends on the Origin of Tissues
3.2. In Breast Tissue, only Mixed Somatic and Germinal Genes Encoding PIWIL2 and PIWIL4 Proteins Are Normally Expressed and Thus Participate in piRNAs Biogenesis and PIWI–piRNA Pathway Activity
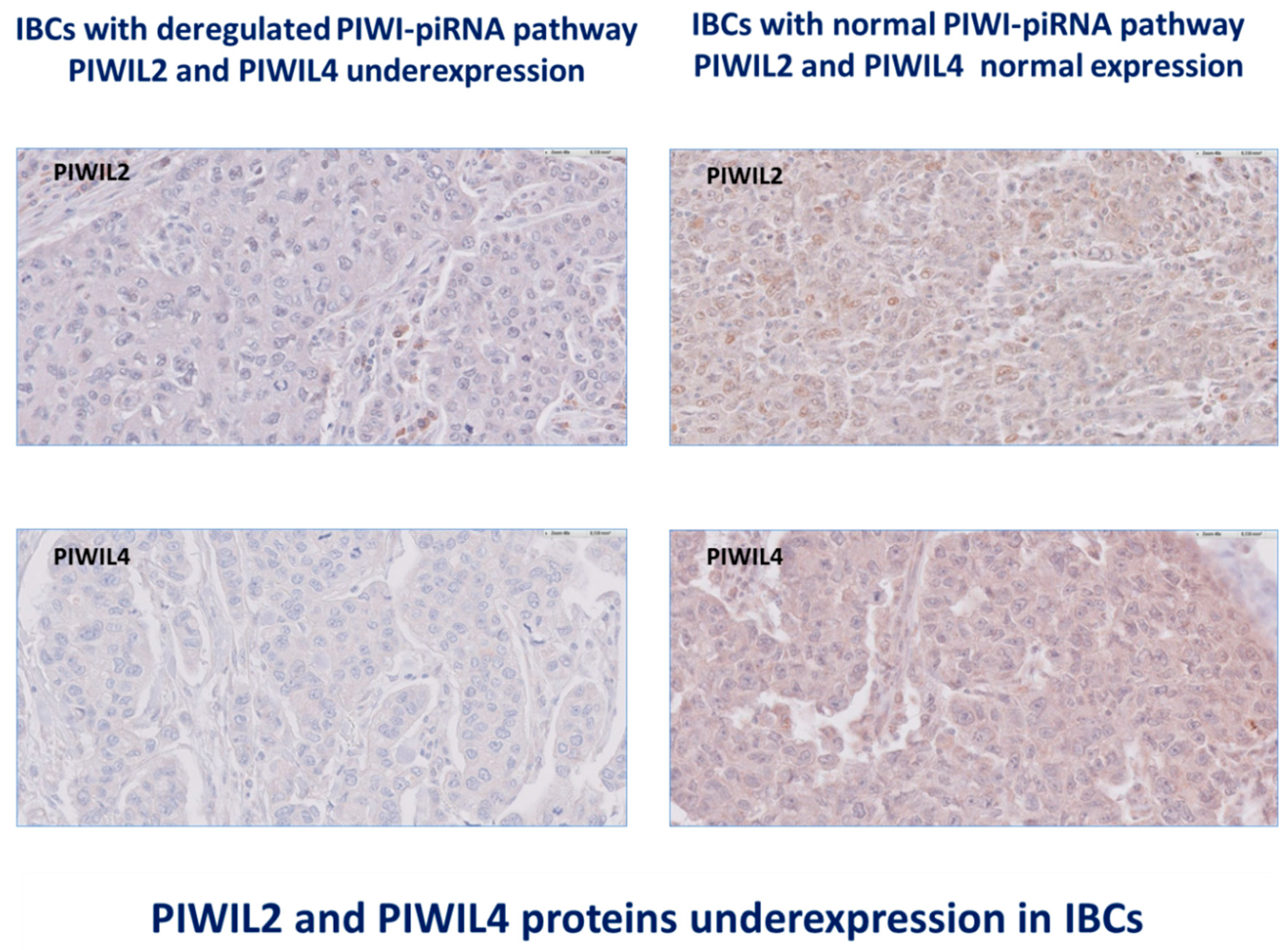
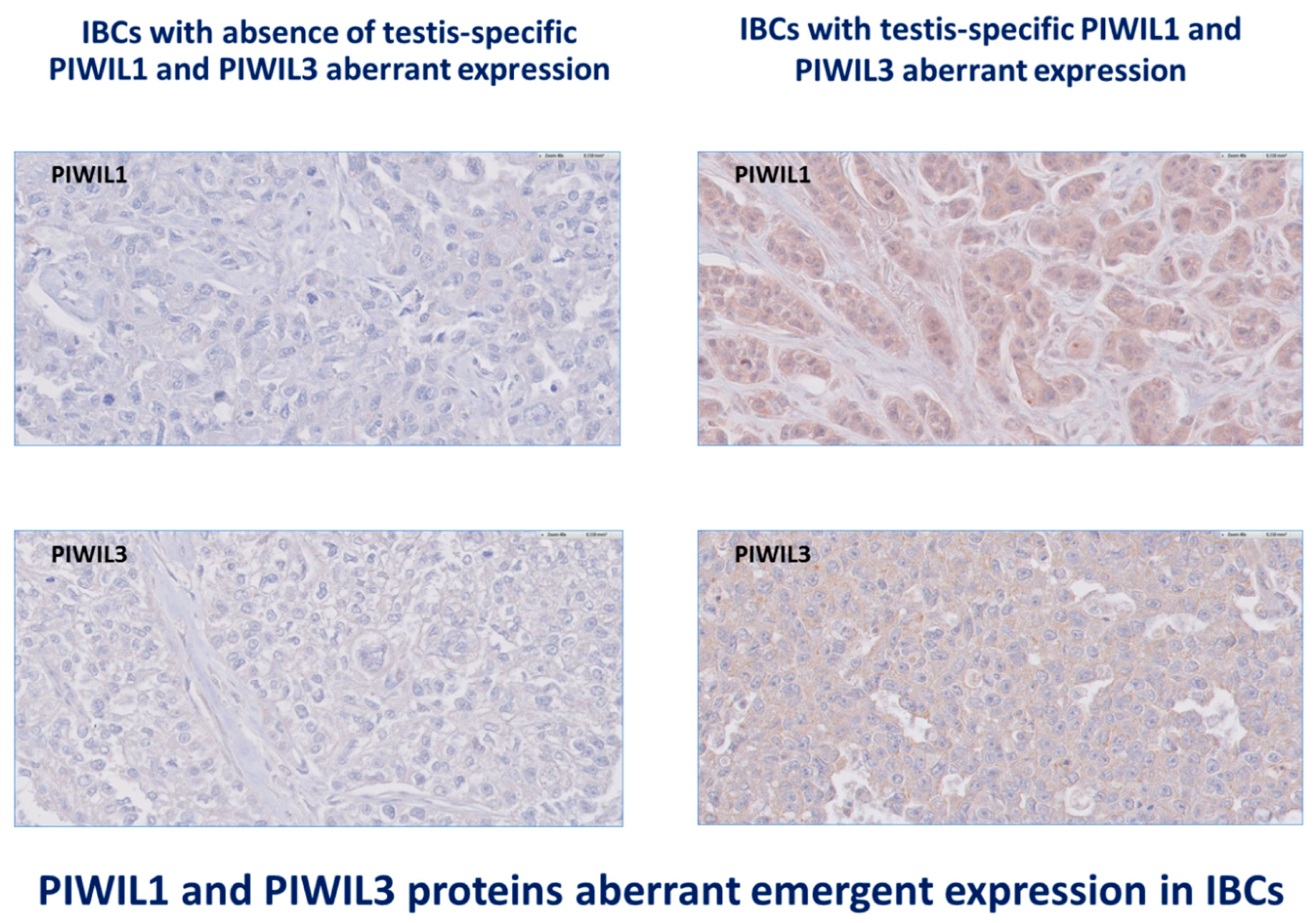
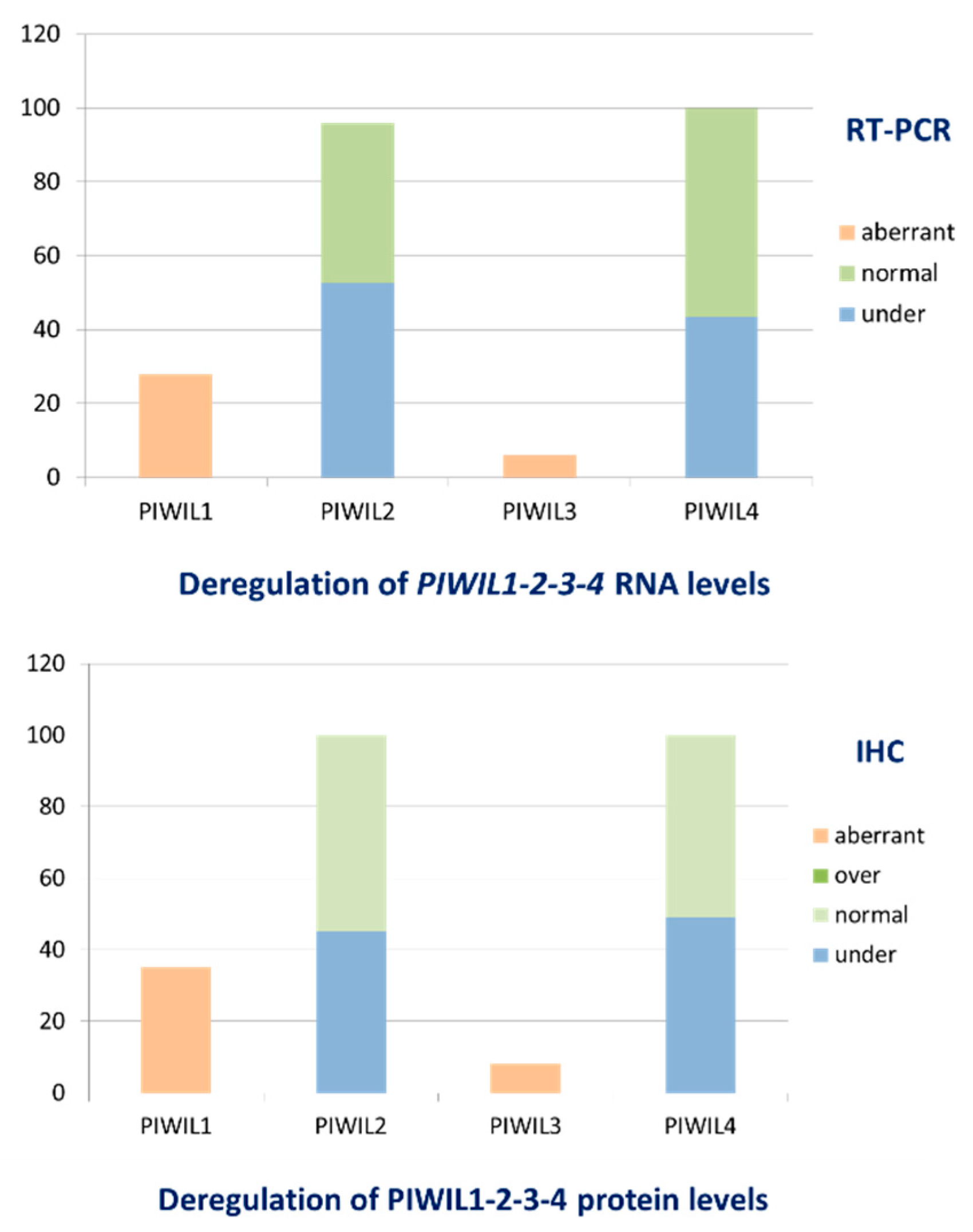
3.3. IBCs Are Characterized by Deregulation of All Proteins PIWIL1-2-3-4 Levels of Expression
3.4. The Oncogenic Variant PL2L60 of the Oncosuppressor PIWIL2 Is Underexpressed in IBCs
3.5. Similar Patterns of PIWIL1-2-3-4 Abnormal Levels of Expression Are Observed in a Multitumoral Panel, Suggesting a Generic Mechanism Initiating PIWI–piRNA Pathway Deregulation in Cancers
3.6. Deregulation of the PIWIL1-2-3-4 Proteins Is Early during Breast Carcinogenesis
3.7. PIWIL1-2-3-4 mRNA Expression Levels Are Correlated with Classical Clinicopathological Factors
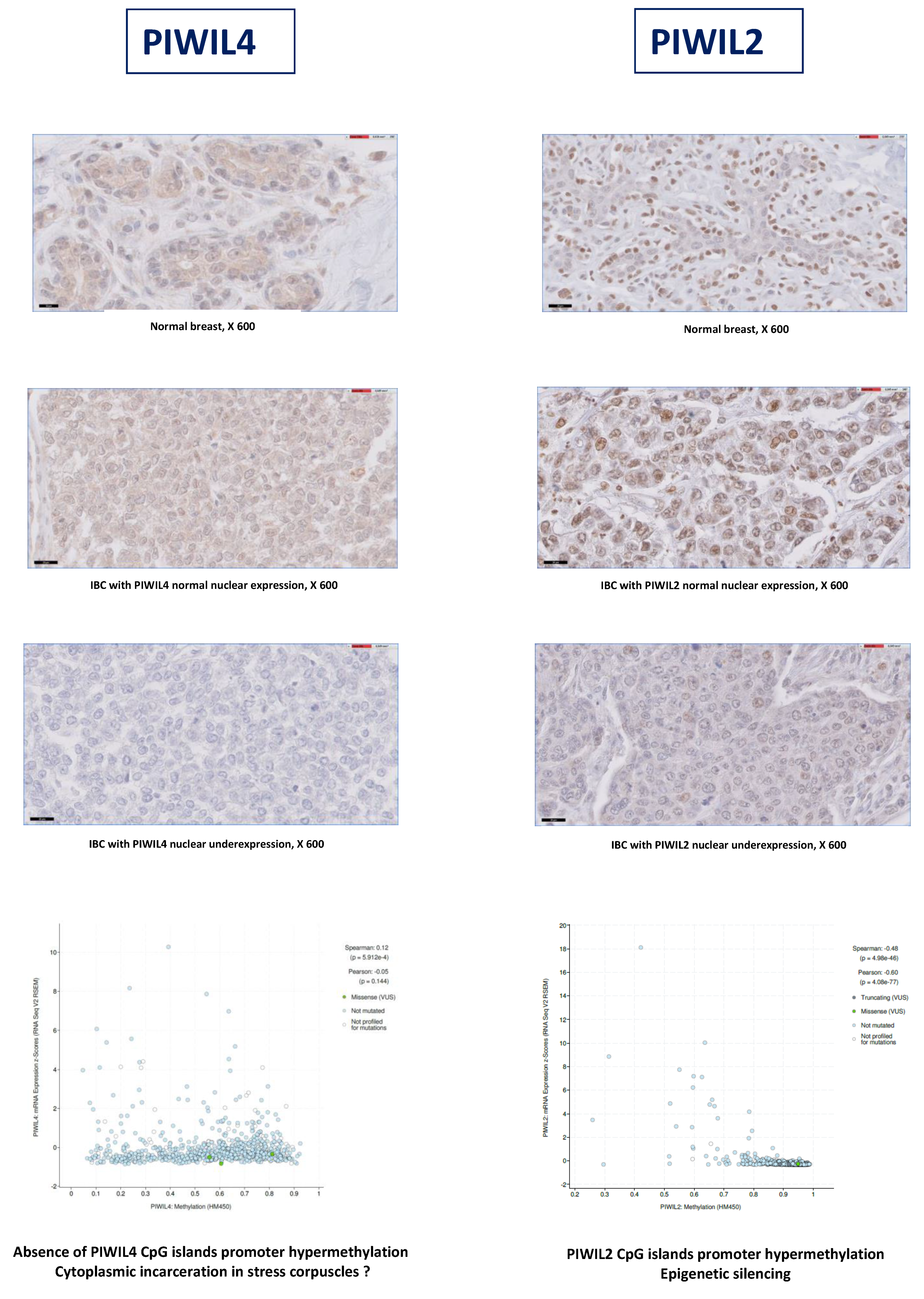
3.8. PIWIL2-4 Underexpression Is Related to Epigenetic or Post-Transcriptional Mechanisms
3.9. PIWIL2 Underexpressing IBCs Are Significantly Correlated at Protein Level with Downregulation of Histone H1, DNMT1, HP1 and SUV39H1 Proteins and Upregulation of CD8 Cytotoxic Immune Reaction
3.10. PIWIL2 and PIWIL4 Genes Are Associated with Hallmarks of Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yong, W.S.; Hsu, F.M.; Chen, P.Y. Profiling genome-wide DNA methylation. Epigenet. Chromatin 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Farazi, T.A.; Juranek, S.A.; Tuschl, T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development 2008, 135, 1201–1214. [Google Scholar] [CrossRef]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef]
- Völler, D.; Linck, L.; Bruckmann, A.; Hauptmann, J.; Deutzmann, R.; Meister, G.; Bosserhoff, A.K. Argonaute Family Protein Expression in Normal Tissue and Cancer Entities. PLoS ONE 2016, 11, e0161165. [Google Scholar] [CrossRef]
- Cox, D.N.; Chao, A.; Baker, J.; Chang, L.; Qiao, D.; Lin, H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998, 12, 3715–3727. [Google Scholar] [CrossRef]
- Klein, J.D.; Qu, C.; Yang, X.; Fan, Y.; Tang, C.; Peng, J.C. C-Fos repression by Piwi regulates drosophila ovarian germline formation and tissue morphogenesis. PLoS Genet. 2016, 12, e1006281. [Google Scholar] [CrossRef]
- Stuwe, E.; Tóth, K.F.; Aravin, A.A. Small but sturdy: Small RNAs in cellular memory and epigenetics. Genes Dev. 2014, 28, 423–431. [Google Scholar] [CrossRef]
- Yin, D.T.; Wang, Q.; Chen, L.; Liu, M.Y.; Han, C.; Yan, Q.; Shen, R.; He, G.; Duan, W.; Li, J.J.; et al. Germline stem cell gene PIWIL2 mediates DNA repair through relaxation of chromatin. PLoS ONE 2011, 6, e27154. [Google Scholar] [CrossRef]
- Rizzo, F.; Hashim, A.; Marchese, G.; Ravo, M.; Tarallo, R.; Nassa, G.; Giurato, G.; Rinaldi, A.; Cordella, A.; Persico, M.; et al. Timed regulation of P-element-induced wimpy testis-interacting RNA expression during rat liver regeneration. Hepatology 2014, 60, 798–806. [Google Scholar] [CrossRef]
- Malone, C.D.; Brennecke, J.; Dus, M.; Stark, A.; Mc Combie, W.R.; Sachidanandam, R.; Hannon, G.J. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 2009, 137, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Roovers, E.F.; Rosenkranz, D.; Mahdipour, M.; Han, C.T.; He, N.; Chuva de Sousa Lopes, S.M.; van der Westerlaken, L.A.; Zischler, H.; Butter, F.; Roelen, B.A.; et al. Piwi proteins and piRNAs in mammalian oocytes and early embryos. Cell Rep. 2015, 10, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.T.; Kang, J.Y.; Dai, P.; Wang, X.; Li, F.; Zhao, S.; Zhang, M.; Hua, M.M.; Lu, Y.; Zhu, Y.; et al. Ubiquitination-Deficient Mutations in Human Piwi Cause Male Infertility by Impairing Histone-to-Protamine Exchange during Spermiogenesis. Cell 2017, 169, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Kamaliyan, Z.; Pouriamanesh, S.; Amin-Beidokhti, M.; Rezagholizadeh, A.; Mirfakhraie, R. HIWI2 rs508485 Polymorphism Is Associated with Non-obstructive Azoospermia in Iranian Patients. Rep. Biochem. Mol. Biol. 2017, 5, 108–111. [Google Scholar]
- Yin, H.; Lin, H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 2007, 450, 304–308. [Google Scholar] [CrossRef]
- Kiuchi, T.; Koga, H.; Kawamoto, M.; Shoji, K.; Sakai, H.; Arai, Y.; Ishihara, G.; Kawaoka, S.; Sugano, S.; Shimada, T.; et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509, 633–636. [Google Scholar] [CrossRef]
- Navarro, A.; Tejero, R.; Vinolas, N.; Cordeiro, A.; Marrades, R.M.; Fuster, D.; Caritg, O.; Moises, J.; Munoz, C.; Molins, L.; et al. The significance of PIWI family expression in human lung embryogenesis and non-small cell lung cancer. Oncotarget 2015, 6, 31544–31556. [Google Scholar] [CrossRef]
- Rajasethupathy, P.; Antonov, I.; Sheridan, R.; Frey, S.; Sander, C.; Tuschl, T.; Kandel, E.R. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 2012, 149, 693–707. [Google Scholar] [CrossRef]
- Buckley, B.A.; Burkhart, K.B.; Gu, S.G.; Spracklin, G.; Kershner, A.; Fritz, H.; Kimble, J.; Fire, A.; Kennedy, S. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 2012, 489, 447–451. [Google Scholar] [CrossRef]
- Gu, S.G.; Pak, J.; Guang, S.; Maniar, J.M.; Kennedy, S.; Fire, A. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat. Genet. 2012, 44, 157–164. [Google Scholar] [CrossRef]
- Shirayama, M.; Seth, M.; Lee, H.C.; Gu, W.; Ishidate, T.; Conte, D. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 2012, 150, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Banerjee, S.; Zhou, H.; Jammalamadaka, A.; Arcila, M.; Manjunath, B.S.; Kosik, K.S. Identification of piRNAs in the central nervous system. RNA 2011, 17, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.P.; Yao, M.J.; Chang, S.Y.; Gou, L.T.; Liu, M.F.; Qiu, Z.L.; Yuan, X.B. Novel function of PIWIL1 in neuronal polarization and migration via regulation of microtubule-associated proteins. Mol. Brain 2015, 8, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, S.; Palanisamy, K.; Arunachalam, J.P.; Chidambaram, S. Possible role of HIWI2 in modulating tight junction proteins in retinal pigment epithelial cells through Akt signaling pathway. Mol. Cell. Biochem. 2017, 427, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Henaoui, I.S.; Jacovetti, C.; Guerra Mollet, I.; Guay, C.; Sobel, J.; Eliasson, L.; Regazzi, R. PIWI-interacting RNAs as novel regulators of pancreatic beta cell function. Diabetologia 2017, 17, 4368–4376. [Google Scholar] [CrossRef]
- Jones, B.C.; Wood, J.G.; Chang, C.; Tam, A.D.; Franklin, M.J.; Siegel, E.R.; Helfand, S.L. A somatic piRNA pathway in the Drosophila fat body ensures metabolic homeostasis and normal lifespan. Nat. Commun. 2016, 7, 13856. [Google Scholar] [CrossRef]
- Ng, K.W.; Anderson, C.; Marshall, E.A.; Minatel, B.C.; Enfield, K.S.; Saprunoff, H.L.; Lam, W.L.; Martinez, V.D. Piwi-interacting RNAs in cancer: Emerging functions and clinical utility. Mol. Cancer 2016, 15, 5–19. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Suzuki, R.; Honda, S.; Kirino, Y. PIWI Expression and Function in Cancer. Front. Genet. 2012, 3, 204–212. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Guo, J.; Ma, H.; Li, J.; Dong, B.; Jin, G.; Zhang, J.; Wu, J.; Meng, L.; et al. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int. J. Cancer 2006, 118, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Law, P.T.; Qin, H.; Ching, A.K.; Lai, K.P.; Co, N.N.; He, M.; Lung, R.W.; Chan, A.W.; Chan, T.F.; Wong, N. Deep sequencing of small RNA transcriptome reveals novel non-coding RNAs in hepatocellular carcinoma. J. Hepatol. 2013, 58, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Gao, H.; Jin, J.M.; Li, A.X.; Kim, Y.S.; Pal, S.K.; Nelson, R.A.; Lau, C.M.; Guo, C.; et al. Piwi-Interacting RNAs (piRNAs) Are Dysregulated in Renal Cell Carcinoma and Associated with Tumor Metastasis and Cancer-Specific Survival. Mol. Med. 2015, 21, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.I.; Qin, Q.; Lerro, M.C.; Fu, A.; Dubrow, R.; Claus, E.B.; DeWan, A.T.; Wang, G.; Lin, H.; Zhu, Y. PIWI-interacting RNAs in Gliomagenesis: Evidence from Post-GWAS and Functional Analyses. Cancer Epidemiol. Prev. Biomark. 2016, 25, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Grochola, L.F.; Greither, T.; Taubert, H.; Möller, P.; Knippschild, U.; Udelnow, A.; Henne-Bruns, D.; Würl, P. The stem cell-associatedHiwi gene in human adenocarcinoma of the pancreas: Expressionand risk of tumour-related death. Br. J. Cancer 2008, 99, 1083–1088. [Google Scholar] [CrossRef]
- Zeng, Y.; Qu, L.K.; Meng, L.; Liu, C.Y.; Dong, B.; Xing, X.F.; Wu, J.; Shou, C.C. HIWI expression profile in cancer cells and its prognostic value for patients with colorectal cancer. Chin. Med. J. 2011, 124, 2144–2149. [Google Scholar]
- Zhang, H.; Ren, Y.; Xu, H.; Pang, D.; Duan, C.; Liu, C. The expression of stem cell protein Piwil2 and piR-932 in breast cancer. Surg. Oncol. 2013, 22, 217–223. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhou, J.M.; Wang, L.R.; He, H.W.; Wang, X.L.; Tao, Z.H.; Sun, H.C.; Wu, W.Z.; Fan, J.; Tang, Z.Y.; et al. HIWI is associated with prognosis in patients with hepatocellular carcinoma after curative resection. Cancer 2012, 118, 2708–2717. [Google Scholar] [CrossRef]
- Liu, S.S.; Liu, N.; Liu, M.Y.; Sun, L.; Xia, W.Y.; Lu, H.M.; Fu, Y.J.; Yang, G.L.; Bo, J.J.; Liu, X.X.; et al. An unusual intragenic promoter of PIWIL2 contributes to aberrant activation of oncogenic PL2L60. Oncotarget 2017, 8, 46104–46120. [Google Scholar] [CrossRef]
- Ye, Y.; Yin, D.T.; Chen, L.; Zhou, Q.; Shen, R.; He, G.; Yan, Q.; Tong, Z.; Issekutz, A.C.; Shapiro, C.L.; et al. Identification of Piwil2-like (PL2L) proteins that promote tumorigenesis. PLoS ONE 2010, 5, e13406. [Google Scholar] [CrossRef]
- Sasaki, T.; Shiohama, A.; Minoshima, S.; Shimizu, N. Identification of eight members of the Argonaute family in the human genome. Genomics 2003, 82, 323–330. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidiniumthiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401. [Google Scholar] [CrossRef] [PubMed]
- Bieche, I.; Parfait, B.; le Doussal, V.; Olivi, M.; Rio, M.C.; Lidereau, R.; Vidaud, M. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: An outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 2001, 61, 1652–1658. [Google Scholar] [PubMed]
- Didier, G.; Brezellec, P.; Remy, E.; Henaut, A. GeneANOVA-gene expression analysis of variance. Bioinformatics 2002, 18, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Skanderup, A.J.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Meseure, D.; Vacher, S.; Lallemand, F.; Alsibai, K.D.; Hatem, R.; Chemlali, W.; Nicolas, A.; De Koning, L.; Pasmant, E.; Callens, C.; et al. Prognostic value of a newly identified MALAT1 alternatively spliced transcript in breast cancer. Br. J. Cancer 2016, 114, 1395–1404. [Google Scholar] [CrossRef]
- Czech, B.; Munafò, M.; Ciabrelli, F.; Eastwood, E.; Fabry, M.; Kneuss, E.; Hannon, G.J. piRNA-Guided Genome Defense: From Biogenesis to Silencing. Annu. Rev. Genet. 2018, 52, 131–157. [Google Scholar] [CrossRef]
- Luteijn, M.J.; Ketting, R.F. PIWI-interacting RNAs: From generation to transgenerational epigenetics. Nat. Rev. Genet. 2013, 14, 523–534. [Google Scholar] [CrossRef]
- He, G.; Chen, L.; Ye, Y.; Xiao, Y.; Hua, K.; Jarjoura, D.; Nakano, T.; Barsky, S.H.; Shen, R.; Gao, J.-X. Piwil2 expressed in various stages of cervical neoplasia is a potential complementary marker for p16INK4a. Am. J. Transl. Res. 2010, 2, 156–169. [Google Scholar]
- Qu, X.; Liu, J.; Zhong, X.; Li, X.; Zhang, Q. PIWIL2 promotes progression of non-small cell lung cancer by inducing CDK2 and Cyclin A expression. J. Transl. Med. 2015, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Ren, Z.-J.; Wang, F.; Liu, M.; Li, X.; Tang, H. PIWIL4 regulates cervical cancer cell line growth and is involved in down-regulating the expression of p14ARF and p53. FEBS Lett. 2012, 586, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, K.E.; Goswami, M.; Hourigan, C.S.; Oetjen, K.A. Immunological effects of hypomethylating agents. Expert Rev. Hematol. 2017, 10, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Shen, J.; Zhang, L.; Li, M.; Hu, W.; Cho, C. Therapeutic targeting of noncoding RNAs in hepatocellular carcinoma: Recent progress and future prospects (Review). Oncol. Lett. 2018, 15, 3395–3402. [Google Scholar] [CrossRef]
- Al-Janabi, O.; Wach, S.; Nolte, E.; Weigelt, K.; Rau, T.T.; Stoehr, C.G.; Legal, W.; Schick, S.; Greither, T.; Hartmann, A.; et al. Piwi-like 1 and 4 gene transcript levels are associated with clinicopathological parameters in renal cell carcinomas. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 686–690. [Google Scholar] [CrossRef]
- Li, D.; Sun, X.; Yan, D.; Huang, J.; Luo, Q.; Tang, H.; Peng, Z. Piwil2 modulates the proliferation and metastasis of colon cancer via regulation of matrix metallopeptidase 9 transcriptional activity. Exp. Boil. Med. 2012, 237, 1231–1240. [Google Scholar] [CrossRef]
- Krishnan, P.; Ghosh, S.; Graham, K.; Mackey, J.R.; Kovalchuk, O.; Damaraju, S. Piwi-interacting RNAs and PIWI genes as novel prognostic markers for breast cancer. Oncotarget 2016, 7, 37944–37956. [Google Scholar] [CrossRef]
- Gebert, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Moszyńska, A.; Cabaj, A.; Króliczewski, J.; Madanecki, P.; Ochocka, R.J.; Crossman, D.K.; Collawn, J.F.; et al. PIWI proteins contribute to apoptosis during the UPR in human airway epithelial cells. Sci. Rep. 2018, 8, 16431. [Google Scholar] [CrossRef]
- Fu, A.; I Jacobs, D.; Zhu, Y. Epigenome-wide analysis of piRNAs in gene-specific DNA methylation. RNA Boil. 2014, 11, 1301–1312. [Google Scholar] [CrossRef]
- Iwasaki, Y.W.; Murano, K.; Ishizu, H.; Shibuya, A.; Iyoda, Y.; Siomi, M.C.; Siomi, H.; Saito, K. Piwi Modulates Chromatin Accessibility by Regulating Multiple Factors Including Histone H1 to Repress Transposons. Mol. Cell 2016, 63, 408–419. [Google Scholar] [CrossRef]
- Kong, Y.; Rose, C.M.; Cass, A.A.; Williams, A.; Darwish, M.; Lianoglou, S.; Haverty, P.M.; Tong, A.-J.; Blanchette, C.; Albert, M.L.; et al. Transposable element expression in tumors is associated with immune infiltration and increased antigenicity. Nat. Commun. 2019, 10, 5228. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Kage, H.; Aki, N.; Sano, A.; Kitagawa, H.; Nagase, T.; Yatomi, Y.; Ohishi, N.; Takai, D. The induction of H3K9 methylation by PIWIL4 at the p16Ink4a locus. Biochem. Biophys. Res. Commun. 2007, 359, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Sugiura, A.; Balko, J.M. Therapeutic potential of DNA methyltransferase inhibitors with immune checkpoint inhibitor therapy in breast cancer. Cell Stress 2018, 2, 69–71. [Google Scholar] [CrossRef]
- Egli, M.; Manoharan, M. Re-Engineering RNA Molecules into Therapeutic Agents. Accounts Chem. Res. 2019, 52, 1036–1047. [Google Scholar] [CrossRef] [PubMed]

| Normal Tissue RNA Level | Malignant Tumors RNA Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Number | PIWIL1 | PIWIL2 | PIWIL3 | PIWIL4 | Number | PIWIL1 | PIWIL2 | PIWIL3 | PIWIL4 |
| Anal canal | 17 | 0 (0–9.33) | 17.9 (4.77–64.0) | 0 (0–1.44) | 319 (30.0–426) | 48 | 0.92 (0–96.2) | 7.82 (1.25–717) | 0 (0–0) | 48.6 (10.8–390) |
| Head and neck | 27 | 0 (0–2.42) | 51.2 (1.98–160) | 0 (0–0.54) | 68.8 (35.7–251) | 50 | 0.50 (0–187) | 12.2 (0.37–409) | 0 (0–0) | 42.4 (7.45–282) |
| Prostate | 8 | 0 (0–2.25) | 23.0 (8.98–60.7) | 0 (0–0) | 72.7 (34.2–124) | 48 | 0 (0–107) | 13.5 (1.82–47.2) | 0 (0–1.55) | 40.8 (14.9–84.8) |
| Ovary | 27 | 1.32 (0–36.5) | 54.5 (12.9–119) | 0 (0–0.94) | 160 (84.3–277) | 52 | 11.9 (0–229.6) | 10.6 (0.96–148) | 0 (0–23.7) | 100 (13.2–1442) |
| Thyroid | 9 | 0.50 (0–6.17) | 13.7 (8.05–146) | 0 (0–0) | 57.9 (40.4–95.6) | 31 | 8.93 (0–455) | 13.3 (2.41–40.3) | 0 (0–0.51) | 48.0 (13.1–130) |
| Cervix | 14 | 0 (0–2.98) | 47.2 (9.12–108) | 0 (0–0) | 124 (36.9–332) | 37 | 2.14 (0–785) | 7.94 (1.14–549) | 0 (0–0) | 68.9 (7.56–825) |
| Skin | 9 | 0 (0–14.0) | 40.7 (22.1–110) | 0 (0–0) | 67.5 (25.7–134) | 27 | 0 (0–5.64) | 11.6 (0–58.2) | 0 (0–49.1) | 47.9 (0–223) |
| Endometrium | 8 | 0.29 (0–5.11) | 69.9 (47.1–99.8) | 0 (0–0) | 177 (111–232) | 29 | 5.87 (0–478) | 7.57 (1.24–59.5) | 0 (0–5.29) | 46.5 (9.39–222) |
| Colon | 30 | 1.37 (0–76.4) | 14.0 (6.24–57.9) | 0 (0–0) | 313 (98.3–467) | 49 | 20.4 (0–1569) | 7.28 (1.37–36.1) | 0 (0–1.29) | 250 (32.0–984) |
| Lung | 16 | 0 (0–2.24) | 9.61 (1.54–32.0) | 0 (0–0) | 90.5 (55.3–151) | 54 | 0 (0–428) | 6.85 (0–236) | 0 (0–89.0) | 73.6 (0–360) |
| Kidney | 18 | 7.24 (0–24.8) | 20.5 (4.30–99.4) | 0 (0–0) | 97.5 (53.3–182) | 22 | 3.44 (0–20.2) | 16.5 (1.79–72.0) | 0 (0–0) | 118 (25.4–753) |
| Pancreas | 10 | 0 (0–5.22) | 24.4 (17.6–70.2) | 0 (0–0) | 530 (380–831) | 22 | 6.34 (0–166) | 37.1 (5.79–80.0) | 0 (0–0) | 414 (118–1536) |
| Liver | 9 | 0 (0–3.92) | 14.2 (7.01–36.3) | 0 (0–0) | 65.2 (25.4–252) | 31 | 0 (0–6.59) | 8.36 (0–102) | 0 (0–17.5) | 33.9 (5.46–354) |
| Bladder | 14 | 0 (0–16.9) | 49.7 (0–129) | 0 (0–0) | 121 (80.7–151) | 49 | 0 (0–47.7) | 4.65 (0–604) | 0 (0–19.3) | 25.0 (2.05–532) |
| Stomach | 11 | 0 (0–174) | 10.1 (0–45.7) | 0 (0–0) | 156 (116–252) | 29 | 4.44 (0–961) | 8.28 (1.35–62.1) | 0 (0–1.77) | 241 (107–522) |
| Brain | 19 | 1.44 (0–18.8) | 26.4 (7.08–67.6) | 0 (0–0) | 103 (46.5–192) | 50 | 0 (0–3.14) | 29.3 (3.84–356) | 0 (0–0.34) | 56.2 (3.39–176) |
| Breast | 11 | 0 (0–7.87) | 66.8 (28.5–118) | 0 (0–0) | 138 (60.2–190) | 96 | 0 (0–6.03) | 11.2 (0–758) | 0 (0–39.2) | 34.9 (7.43–176) |
| Normal Tissues Protein Level | PIWIL1 | PIWIL2 | PIWIL3 | PIWIL4 | Malignant Tumors Protein Level | PIWIL1 | PIWIL2 | PIWIL3 | PIWIL4 |
|---|---|---|---|---|---|---|---|---|---|
| Testis (n = 10) | 2 | 2 | 1.5 | 2 | Testis (n = 10) | 1 | 0–1 | 0 | 0–1 |
| Anal canal (n = 10) | 0 | 1 | 0 | 1 | Anal canal (n = 10) | 1 | 0–1 | 0 | 0–1 |
| Head and neck (n = 10) | 0 | 1 | 0 | 1 | Head and neck (n = 10) | 0 | 0–1 | 0 | 0–1 |
| Prostate (n = 10) | 0 | 1 | 0 | 1 | Prostate (n = 10) | 0 | 0–1 | 0 | 0–1 |
| Ovary (n = 10) | 1 | 1 | 0 | 1 | Ovary (n = 10) | 1–2 | 0–1 | 0 | 0–1 |
| Thyroid (n = 10) | 1 | 1 | 0 | 1 | Thyroid (n =10) | 1–2 | 0–1 | 0 | 0–1 |
| Cervix (n = 10) | 0 | 1 | 0 | 1 | Cervix (n = 10) | 0–1 | 0–1 | 0 | 0–1 |
| Skin (n = 10) | 0 | 1 | 0 | 1 | Skin (n = 10) | 0 | 0–1 | 0 | 0–1 |
| Endometrium (n = 10) | 1 | 1 | 0 | 1 | Endometrium (n = 10) | 1–2 | 0–1 | 0 | 0–1 |
| Colon (n = 10) | 1 | 1 | 0 | 1 | Colon (n = 10) | 1–2 | 0–1 | 0 | 0–1 |
| Lung (n = 10) | 0 | 1 | 0 | 1 | Lung (n = 10) | 0 | 0–1 | 0 | 0–1 |
| Kidney (n = 10) | 1 | 1 | 0 | 1 | Kidney (n = 10) | 1–2 | 0–1 | 0 | 0–1 |
| Pancreas (n = 10) | 0 | 1 | 0 | 1 | Pancreas (n = 10) | 1–2 | 0–1 | 0 | 0–1 |
| Liver (n = 10) | 0 | 1 | 0 | 1 | Liver (n = 10) | 0 | 0–1 | 0 | 0–1 |
| Bladder (n = 10) | 0 | 1 | 0 | 1 | Bladder (n = 10) | 0 | 0–1 | 0 | 0–1 |
| Stomach (n = 10) | 0 | 1 | 0 | 1 | Stomach (n = 10) | 0–1 | 0–1 | 0 | 0–1 |
| Brain (n = 10) | 1 | 1 | 0 | 1 | Brain (n = 10) | 0–1 | 0–1 | 0 | 0–1 |
| RNA Level | PIWIL1 | PIWIL2 | PIWIL3 | PIWIL4 |
|---|---|---|---|---|
| Normal Tissues (n = 14) | 0 | 1 (0.27–2.52) | 0 | 1 (0.39–3.10) |
| IBCs (n = 526) | 0 (0–15.53) N: 367 (69.8%) A: 159 (30.2%) | 0.34 (0–19.38) U: 254 (48.3%) N: 272 (51.7%) | 0 (0–151.97) N: 495 (94.1%) A: 31 (5.9%) | 0.37 (0–2.96) U: 228 (43.3%) N: 298 (56.7%) |
| NNN (n = 101) | 0 (0–15.53) N: 76 (75.2%) A: 25 (24.8%) | 0.32 (0–16) U: 53 (52.5%) N: 48 (47.5%) | 0 (0–15.97) N: 98 (97%) A: 3 (3%) | 0.61 (0.05–2.96) U: 29 (28.7%) N: 72 (71.3%) |
| RH-HER2+ (n = 73) | 0 (0–4.08) N: 51 (69.9%) A: 22 (30.1%) | 0.31 (0–4.35) U: 38 (52.1%) N: 35 (47.9%) | 0 (0–53.36) N: 62 (84.9%) A: 11 (15.1%) | 0.37 (0–1.75) U: 29 (39.7%) N: 44 (60.3%) |
| RH+HER2- (n = 294) | 0 (0–14.45) N: 203 (69.0%) A: 91 (31.0%) | 0.33 (0–18.39) U: 148 (50.3%) N: 146 (49.7%) | 0 (0–26.49) N: 282 (95.9%) A: 12 (4.1%) | 0.35 (0–2.82) U: 139 (47.3%) N: 155 (52.7%) |
| RH+HER2+ (n = 58) | 0 (0–2.96) N: 37 (63.8%) A: 21 (36.2%) | 0.59 (0.07–19.38) U: 15 (25.9%) N: 43 (74.1%) | 0 (0–9.21) N: 53 (91.4%) A: 5 (8.6%) | 0.32 (0.06–1.45) U: 31 (53.4%) N: 27 (46.6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meseure, D.; Vacher, S.; Boudjemaa, S.; Laé, M.; Nicolas, A.; Leclere, R.; Chemlali, W.; Champenois, G.; Schnitzler, A.; Lesage, L.; et al. Biopathological Significance of PIWI–piRNA Pathway Deregulation in Invasive Breast Carcinomas. Cancers 2020, 12, 2833. https://doi.org/10.3390/cancers12102833
Meseure D, Vacher S, Boudjemaa S, Laé M, Nicolas A, Leclere R, Chemlali W, Champenois G, Schnitzler A, Lesage L, et al. Biopathological Significance of PIWI–piRNA Pathway Deregulation in Invasive Breast Carcinomas. Cancers. 2020; 12(10):2833. https://doi.org/10.3390/cancers12102833
Chicago/Turabian StyleMeseure, Didier, Sophie Vacher, Sabah Boudjemaa, Marick Laé, André Nicolas, Renaud Leclere, Walid Chemlali, Gabriel Champenois, Anne Schnitzler, Laetitia Lesage, and et al. 2020. "Biopathological Significance of PIWI–piRNA Pathway Deregulation in Invasive Breast Carcinomas" Cancers 12, no. 10: 2833. https://doi.org/10.3390/cancers12102833
APA StyleMeseure, D., Vacher, S., Boudjemaa, S., Laé, M., Nicolas, A., Leclere, R., Chemlali, W., Champenois, G., Schnitzler, A., Lesage, L., Dubois, T., & Bieche, I. (2020). Biopathological Significance of PIWI–piRNA Pathway Deregulation in Invasive Breast Carcinomas. Cancers, 12(10), 2833. https://doi.org/10.3390/cancers12102833






