Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Cytokine Serum Levels
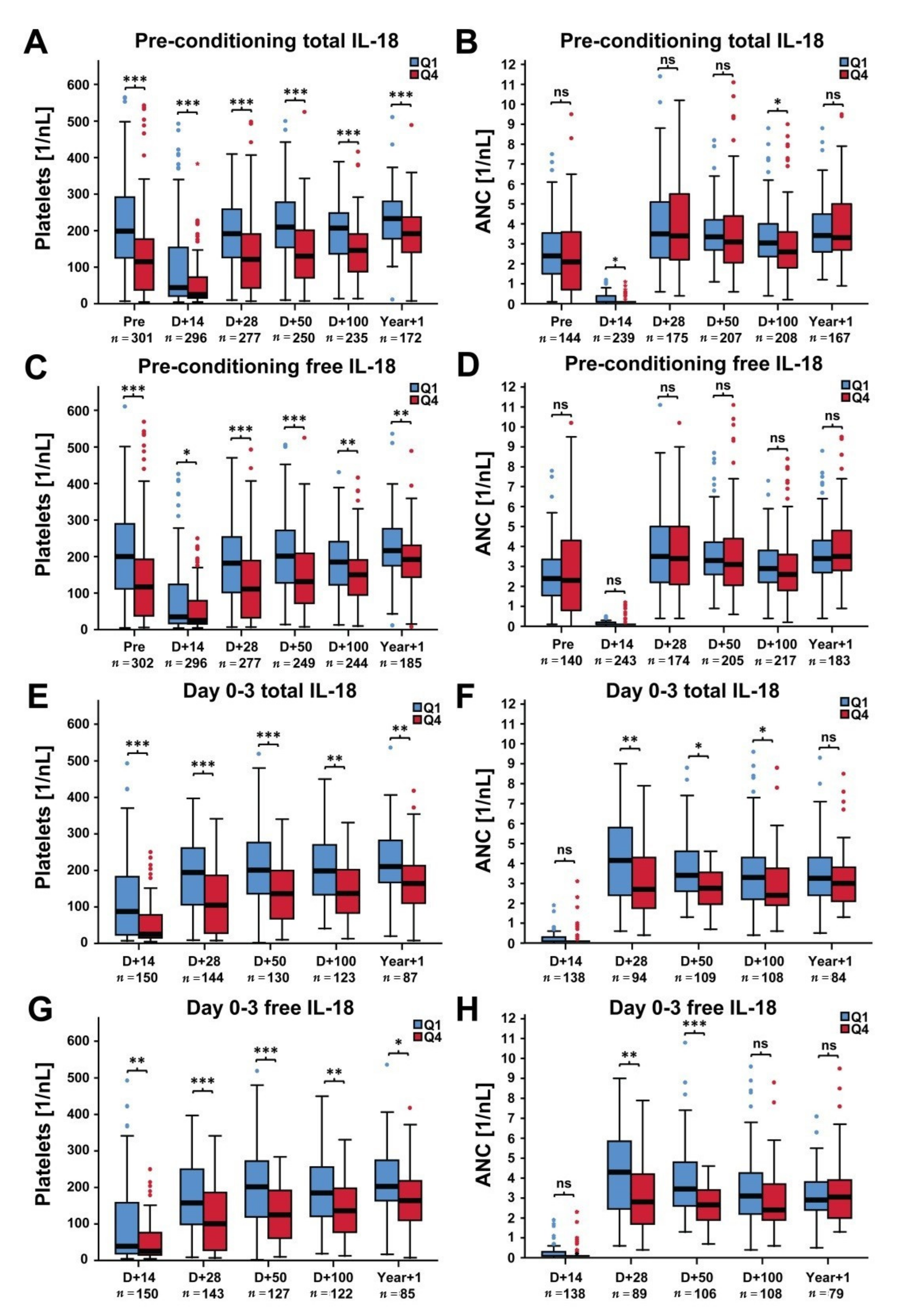
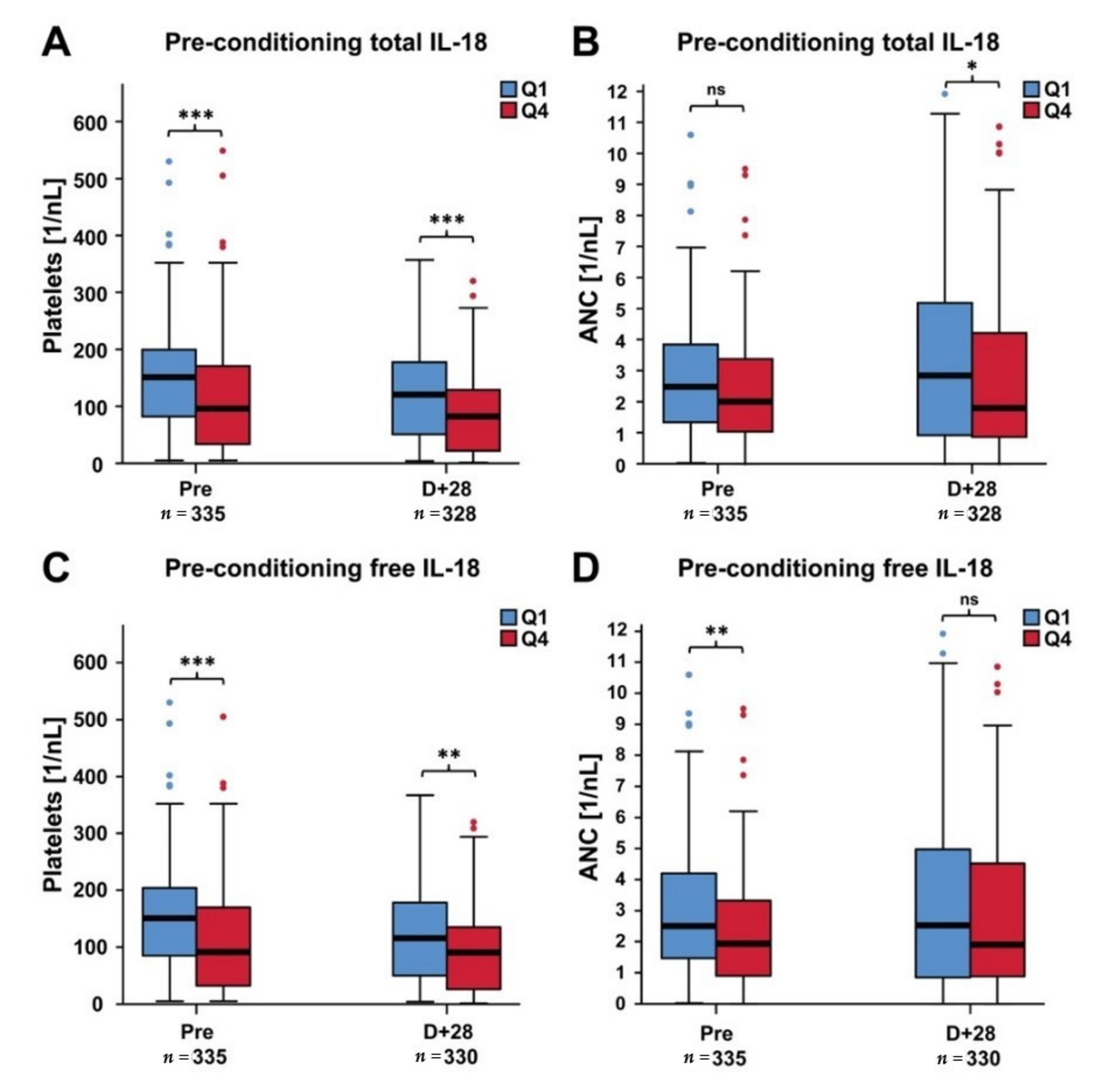
2.2. Platelets and ANC in Patients with Low Versus High IL-18 Levels
2.3. Cytokine Serum Levels and Hematopoietic Recovery
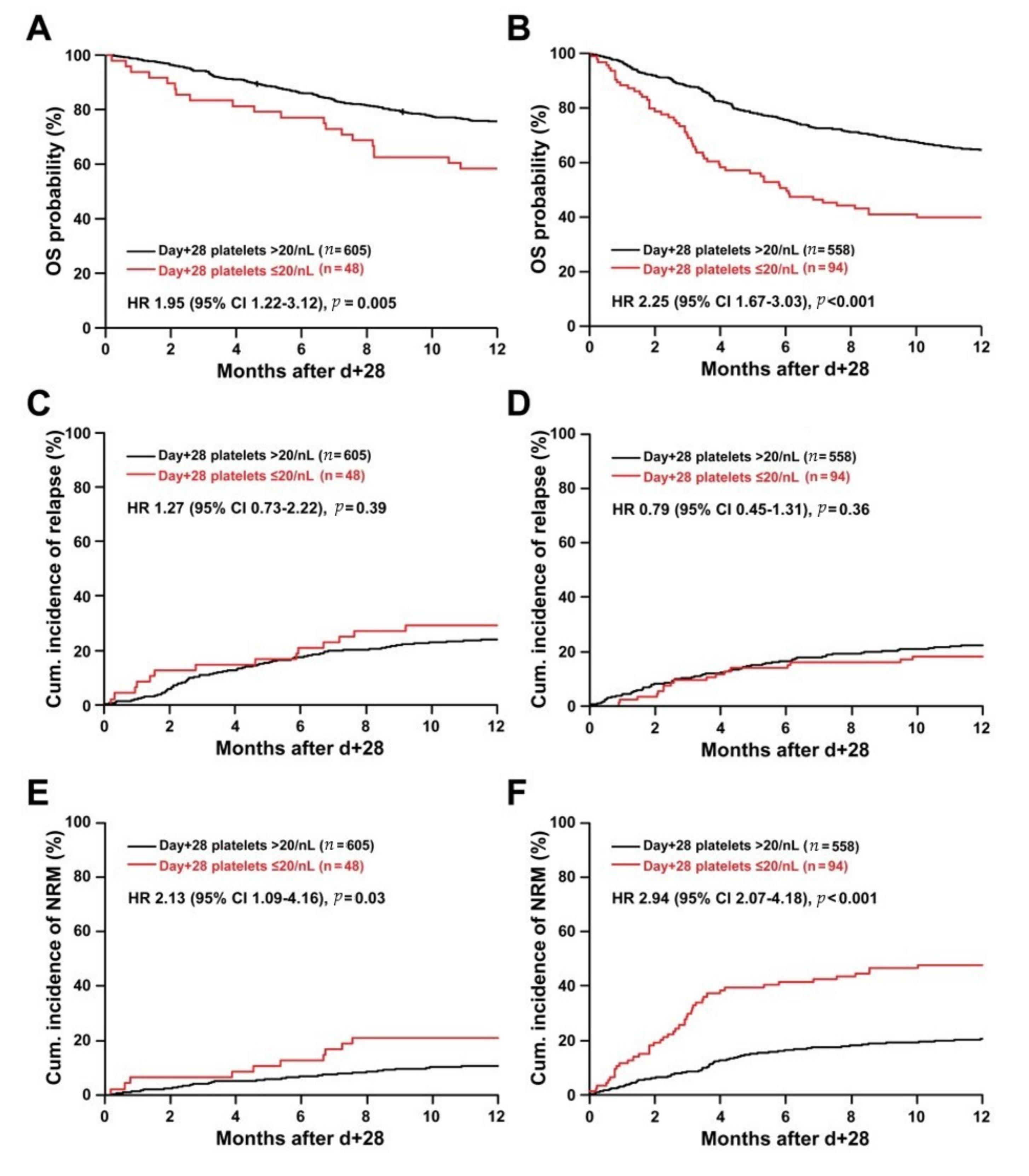
2.4. Day +28 Platelet Recovery and Outcome
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Assessment of Cytokine Serum Levels
4.3. Definitions and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies, S.M.; Kollman, C.; Anasetti, C.; Antin, J.H.; Gajewski, J.; Casper, J.T.; Nademanee, A.; Noreen, H.; King, R.; Confer, D.; et al. Engraftment and survival after unrelated-donor bone marrow transplantation: A report from the national marrow donor program. Blood 2000, 96, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Dominietto, A.; Raiola, A.M.; Van Lint, M.T.; Lamparelli, T.; Gualandi, F.; Berisso, G.; Bregante, S.; Frassoni, F.; Casarino, L.; Verdiani, S.; et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: Graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br. J. Haematol. 2001, 112, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.N. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant. 2002, 29, 545–552. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, A.; Piaggio, G.; Podestà, M.; Pitto, A.; Bruno, B.; Di Grazia, C.; Gualandi, F.; Occhini, D.; Raiola, A.M.; Dominietto, A.; et al. Boost of CD34+-selected peripheral blood cells without further conditioning in patients with poor graft function following allogeneic stem cell transplantation. Haematologica 2006, 91, 935–940. [Google Scholar] [PubMed]
- Wagner, J.; Flowers, M.E.; Longton, G.; Storb, R.; Martin, P. Use of screening studies to predict survival among patients who do not have chronic graft-versus-host disease at day 100 after bone marrow transplantation. Biol. Blood Marrow Transplant. 2001, 7, 239–240. [Google Scholar] [CrossRef][Green Version]
- Bolwell, B.; Pohlman, B.; Sobecks, R.; Andresen, S.; Brown, S.; Rybicki, L.; Wentling, V.; Kalaycio, M. Prognostic importance of the platelet count 100 days post allogeneic bone marrow transplant. Bone Marrow Transplant. 2003, 33, 419–423. [Google Scholar] [CrossRef][Green Version]
- Ramírez, P.; Brunstein, C.G.; Miller, B.S.; DeFor, T.; Weisdorf, D. Delayed platelet recovery after allogeneic transplantation: A predictor of increased treatment-related mortality and poorer survival. Bone Marrow Transplant. 2010, 46, 981–986. [Google Scholar] [CrossRef]
- Akahoshi, Y.; Kimura, S.-I.; Gomyo, A.; Hayakawa, J.; Tamaki, M.; Harada, N.; Kusuda, M.; Kameda, K.; Ugai, T.; Wada, H.; et al. Delayed platelet recovery after allogeneic hematopoietic stem cell transplantation: Association with chronic graft-versus-host disease and survival outcome. Hematol. Oncol. 2017, 36, 276–284. [Google Scholar] [CrossRef]
- Moneib, H.; Hafez, H.; Abdalla, A.; Hassanain, O.; Lehmann, L.; El Haddad, A. Day +100 Platelet Count Predicts Survival After Allogeneic Hematopoietic Stem-Cell Transplantation in Children With Hematologic Malignancies. Clin. Lymphoma Myeloma Leuk. 2019, 19, e221–e227. [Google Scholar] [CrossRef]
- Novick, D.; Kim, S.; Kaplanski, G.; Dinarello, C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013, 25, 439–448. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2017, 281, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Ferrara, J.L. Role of interleukin-18 in acute graft-vs-host disease. J. Lab. Clin. Med. 2003, 141, 365–371. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Lin, E.; Lowry, S.F.; Rivadeneira, D.E.; Potter, S.; Barie, P.S.; Nathan, C.F. Elevation of IL-18 in human sepsis. J. Clin. Immunol. 2000, 20, 212–215. [Google Scholar] [CrossRef]
- Emmanuilidis, K.; Weighardt, H.; Matevossian, E.; Heidecke, C.-D.; Ulm, K.; Bartels, H.; Siewert, J.-R.; Holzmann, B. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: High serum IL-18 AS an early predictive indicator of lethal outcome. Shock 2002, 18, 301–305. [Google Scholar] [CrossRef]
- Radujkovic, A.; Kordelas, L.; Dai, H.; Schult, D.; Majer-Lauterbach, J.; Dietrich, B.; Müller-Tidow, C.; Dreger, P.; Luft, T. Interleukin-18 and outcome after allogeneic stem cell transplantation: A retrospective cohort study. EBioMedicine 2019, 49, 202–212. [Google Scholar] [CrossRef]
- Silberstein, L.; Goncalves, K.A.; Kharchenko, P.V.; Turcotte, R.; Kfoury, Y.; Mercier, F.; Baryawno, N.; Severe, N.; Bachand, J.; Spencer, J.A.; et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell 2016, 19, 530–543. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Kaplanski, G. Indeed, IL-18 is more than an inducer of IFN-γ. J. Leukoc. Biol. 2018, 104, 237–238. [Google Scholar] [CrossRef]
- Gratwohl, A.; Stern, M.; Brand, R.; Apperley, J.; Baldomero, H.; De Witte, T.; Dini, G.; Rocha, V.; Passweg, J.; Sureda, A.; et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation. Cancer 2009, 115, 4715–4726. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef]
- Bornhäuser, M.; Kienast, J.; Trenschel, R.; Burchert, A.; Hegenbart, U.; Stadler, M.; Baurmann, H.; Schäfer-Eckart, K.; Holler, E.; Kröger, N.; et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: A prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012, 13, 1035–1044. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. A goodness-of-fit test for the multiple logistic regression model. Commun. Statist. Theor. Method. 1980, 9, 1043–1069. [Google Scholar] [CrossRef]
- Yamazaki, R.; Kuwana, M.; Mori, T.; Okazaki, Y.; Kawakami, Y.; Ikeda, Y.; Okamoto, S. Prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation: Associations with impaired platelet production and increased platelet turnover. Bone Marrow Transplant. 2006, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bielski, M.; Yomtovian, R.; Lazarus, H.M.; Rosenthal, N. Prolonged isolated thrombocytopenia after hematopoietic stem cell transplantation: Morphologic correlation. Bone Marrow Transplant. 1998, 22, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, H.; Xu, L.; Liu, D.; Wang, J.; Liu, K.; Huang, X. Prolonged Thrombocytopenia Following Allogeneic Hematopoietic Stem Cell Transplantation and Its Association with a Reduction in Ploidy and an Immaturation of Megakaryocytes. Biol. Blood Marrow Transplant. 2011, 17, 274–280. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Wu, D.; Zhang, W.; Wang, H.; Su, P.; Yao, J.; Liang, C.; Feng, S.; Han, M.; et al. Inflammation-Associated Cytokines IGFBP1 and RANTES Impair the Megakaryocytic Potential of HSCs in PT Patients after Allo-HSCT. Biol. Blood Marrow Transplant. 2018, 24, 1142–1151. [Google Scholar] [CrossRef]
- Shaiegan, M.; Iravani, M.; Babaee, G.R.; Ghavamzadeh, A. Effect of IL-18 and sIL2R on aGVHD occurrence after hematopoietic stem cell transplantation in some Iranian patients. Transpl. Immunol. 2006, 15, 223–227. [Google Scholar] [CrossRef]
- Fujimori, Y.; Takatsuka, H.; Takemoto, Y.; Hara, H.; Okamura, H.; Nakanishi, K.; Kakishita, E. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Br. J. Haematol. 2000, 109, 652–657. [Google Scholar] [CrossRef]
- Scholl, S.; Sayer, H.G.; Kasper, C.; Pietraszczyk, M.; Kliche, K.-O.; Clement, J.H. Increase of interleukin-18 serum levels after engraftment correlates with acute graft-versus-host disease in allogeneic peripheral blood stem cell transplantation. J. Cancer Res. Clin. Oncol. 2004, 130, 704–710. [Google Scholar] [CrossRef]
- Nakamura, H.; Komatsu, K.; Ayaki, M.; Kawamoto, S.; Murakami, M.; Uoshima, N.; Yagi, T.; Hasegawa, T.; Yasumi, M.; Karasuno, T.; et al. Serum levels of soluble IL-2 receptor, IL-12, IL-18, and IFN-gamma in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Allergy Clin. Immunol. 2000, 106, 45–50. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Thacker, S.G.; Berthier, C.C.; Cohen, C.D.; Kretzler, M.; Kaplan, M. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J. Immunol. 2011, 187, 6143–6156. [Google Scholar] [CrossRef] [PubMed]
- Durpès, M.-C.; Morin, C.; Paquin-Veillet, J.; Beland, R.; Paré, M.; Guimond, M.-O.; Rekhter, M.; King, G.L.; Geraldes, P. PKC-β activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovasc. Res. 2015, 106, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wu, J.; Li, Y.; Xia, Y.; Chu, P.; Qi, K.; Yan, Z.; Yao, H.; Liu, Y.; Xu, K.; et al. Blockage of caspase-1 activation ameliorates bone marrow inflammation in mice after hematopoietic stem cell transplantation. Clin. Immunol. 2016, 162, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Viola, D.; Sacchi, A.; Pinnetti, C.; Casetti, R.; Cimini, E.; Tumino, N.; Antinori, A.; Ammassari, A.; Agrati, C. IL-18 and Stem Cell Factor affect hematopoietic progenitor cells in HIV-infected patients treated during primary HIV infection. Cytokine 2018, 103, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Fantuzzi, G.; Giamila, F. Interleukin-18 and Host Defense against Infection. J. Infect. Dis. 2003, 187, S370–S384. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.-N.; Zhu, X.-J.; Wang, Q.; Wang, C.-Y.; Qin, P.; Peng, J.; Hou, M. High-dose dexamethasone regulates interleukin-18 and interleukin-18 binding protein in idiopathic thrombocytopenic purpura. Haematologica 2009, 94, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.-N.; Zhu, X.-J.; Peng, J.; Qin, P.; Zhuang, X.-W.; Wang, H.-C.; Hou, M. Interleukin 18 and interleukin 18 binding protein in patients with idiopathic thrombocytopenic purpura. Br. J. Haematol. 2009, 144, 755–761. [Google Scholar] [CrossRef]
- Zhu, M.; Rong, X.; Li, M.; Wang, S. IL-18 and IL-35 in the serum of patients with sepsis thrombocytopenia and the clinical significance. Exp. Ther. Med. 2019, 19, 1251–1258. [Google Scholar] [CrossRef]
- Korpelainen, S.; Hämäläinen, S.; Vänskä, M.; Koivula, I.; Pulkki, K.; Jantunen, E.; Juutilainen, A.; Purhonen, A.-K. Plasma level of interleukin-18 and complicated course of febrile neutropenia in hematological patients after intensive chemotherapy. Cytokine 2020, 129, 155021. [Google Scholar] [CrossRef]
- Gabay, C.; Fautrel, B.; Rech, J.; Spertini, F.; Feist, E.; Kötter, I.; Hachulla, E.; Morel, J.; Schaeverbeke, T.; Hamidou, M.A.; et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann. Rheum. Dis. 2018, 77, 840–847. [Google Scholar] [CrossRef]
- Kiltz, U.; Kiefer, D.; Braun, J.M.; Schiffrin, E.J.; Girard-Guyonvarc’, H.C.; Gabay, C. Prolonged treatment with Tadekinig alfa in adult-onset Still’s disease. Ann. Rheum. Dis. 2018, 79, e10. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Gaertner, F.; Massberg, S. Platelets in Host Defense: Experimental and Clinical Insights. Trends Immunol. 2019, 40, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Bounes, F.V.; Ruiz, S.; Gratacap, M.-P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Gray, R.J. A Class of KK-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Scrucca, L.; Santucci, A.; Aversa, F. Regression modeling of competing risk using R: An in depth guide for clinicians. Bone Marrow Transplant. 2010, 45, 1388–1395. [Google Scholar] [CrossRef]
| Parameter | Training Cohort (n = 714) | Confirmation Cohort (n = 673) | p |
|---|---|---|---|
| Age (years) at alloSCT (median, IQR) | 54 (45–61) | 54 (44–61) | 0.71 |
| Patient sex, n (%) Female Male | 274 (38) 440 (62) | 314 (47) 359 (53) | 0.002 |
| Disease stage before alloSCTa, n (%) Early Intermediate Late | 251 (35) 204 (29) 259 (36) | 304 (45) 235 (35) 134 (20) | <0.001 |
| Diagnosis, n (%) AML MDS/MPN Lymphoma ALL MM | 261 (37) 121 (17) 222 (31) 28 (4) 82 (11) | 344 (51) 134 (20) 99 (15) 70 (10) 26 (4) | <0.001 |
| Conditioningb, n (%) RIC MAC | 649 (91) 65 (9) | 531 (79) 142 (21) | <0.001 |
| Donor, n (%) RD MUD MMUD | 207 (29) 359 (50) 148 (21) | 192 (29) 319 (47) 162 (24) | 0.31 |
| Donor sex, n (%) Female Male | 229 (32) 485 (68) | 232 (34) 441 (66) | 0.34 |
| ATG treatment, n (%) No Yes | 222 (31) 492 (69) | 284 (42) 389 (58) | <0.001 |
| GVHD prophylaxis, n (%) CNI + MTX CNI + MMF | 216 (30) 498 (70) | 632 (94) 41 (6) | <0.001 |
| Stem cell source, n (%) Peripheral blood Bone marrow | 673 (94) 41 (6) | 603 (90) 70 (10) | 0.001 |
| Pre-conditioning total IL-18 (pg/mL) (median, IQR) | 627 (437–930) | 692 (462–1026) | 0.04 |
| Pre-conditioning IL-18BP (pg/mL) (median, IQR) | 5200 (4313–5987) | 11,404 (9282–12,101) | <0.001 |
| Pre-conditioning free IL-18 (pg/mL) (median, IQR) | 414 (266–658) | 414 (283–608) | 0.96 |
| Pre-conditioning CXCL9 (pg/mL) (median, IQR) | 199 (85–679) | 211 (114–382) | 0.92 |
| Pre-conditioning CXCL10 (pg/mL) (median, IQR) | 85 (45–186) | – | – |
| Pre-conditioning IFNγ (pg/mL) (median, IQR) | 7.5 (2.0–18.7) | – | – |
| Odds Ratio (OR) and Area under the ROC Curve (AUC) per log2 Increase* in Pre-Conditioning Cytokine | ||||
|---|---|---|---|---|
| Cytokine | Platelets Day +28 ≤20/nL (n = 41) vs. >20/nL (n = 512) | Platelets Day +50 ≤100/nL (n = 117) vs. >100/nL (n = 391) | Platelets Year +1 ≤150/nL (n = 84) vs. >150/nL (n = 274) | ANC Day +28 ≤1/nL (n = 43) vs. >1/nL (n = 331) |
| Total IL-18 OR (95% CI), p AUC (95% CI), p | 1.56 (1.20–2.03), 0.001 0.68 (0.60–0.76), <0.001 | 1.52 (1.24–1.86), <0.001 0.63 (0.58–0.69), <0.001 | 1.28 (1.01–1.61), 0.04 0.57 (0.50-0.64), 0.04 | 1.61 (1.23–2.13), 0.001 0.65 (0.56–0.74), 0.002 |
| IL-18BP OR (95% CI), p AUC (95% CI), p | 0.78 (0.60–1.02), 0.07 0.46 (0.38–0.54), 0.41 | 0.98 (0.86–1.11), 0.72 0.50 (0.45–0.56), 0.94 | 0.96 (0.82–1.10), 0.55 0.51 (0.44–0.58), 0.75 | 0.86 (0.68–1.09), 0.20 0.50 (0.41–0.58), 0.91 |
| Free IL-18 OR (95% CI), p AUC (95% CI), p | 1.72 (1.32–2.23), <0.001 0.72 (0.65–0.79), <0.001 | 1.45 (1.20–1.75), <0.001 0.64 (0.58–0.69), <0.001 | 1.23 (1.01–1.50), 0.04 0.56 (0.49–0.63), 0.12 | 1.81 (1.36–2.41), <0.001 0.67 (0.58–0.76), <0.001 |
| CXCL9 OR (95% CI), p AUC (95% CI), p | 0.93 (0.81–1.06), 0.27 0.43 (0.34–0.52), 0.13 | 0.99 (0.91–1.09), 0.91 0.50 (0.44–0.56), 0.96 | 0.98 (0.88–1.08), 0.62 0.47 (0.41–0.54), 0.42 | 1.02 (0.88–1.17), 0.80 0.50 (0.42–0.58), 0.98 |
| CXCL10 OR (95% CI), p AUC (95% CI), p | 1.01 (0.87–1.18), 0.90 0.50 (0.42–0.59), 0.93 | 0.98 (0.88–1.08), 0.63 0.47 (0.41–0.53), 0.39 | 0.95 (0.85–1.06), 0.38 0.47 (0.40–0.53), 0.36 | 1.02 (0.87–1.18), 0.83 0.50 (0.41–0.59), 0.96 |
| IFNγ OR (95% CI), p AUC (95% CI), p | 1.06 (0.91–1.25), 0.44 0.54 (0.44–0.65), 0.39 | 1.01 (0.91–1.12), 0.86 0.50 (0.44–0.57), 0.94 | 0.90 (0.79–1.01), 0.08 0.43 (0.36–0.50), 0.05 | 1.07 (0.91–1.27), 0.39 0.48 (0.39–0.57), 0.55 |
| Odds Ratio (OR) and Area under the ROC Curve (AUC) per log2 Increase* in Day 0–3 Cytokine | ||||
|---|---|---|---|---|
| Cytokine | Platelets Day +28 ≤20/nL (n = 28) vs. >20/nL (n = 257) | Platelets Day +50 ≤100/nL (n = 67) vs. >100/nL (n = 196) | Platelets Year +1 ≤150/nL (n = 55) vs. >150/nL (n = 125) | ANC Day +28 ≤1/nL (n = 31) vs. >1/nL (n = 175) |
| Total IL-18 OR (95% CI), p AUC (95% CI), p | 2.27 (1.52–3.39), <0.001 0.70 (0.59–0.81), 0.001 | 1.95 (1.40–2.72), <0.001 0.68 (0.61–0.75), <0.001 | 1.72 (1.19–2.48), 0.004 0.63 (0.55–0.72), 0.005 | 2.07 (1.34–3.21), 0.001 0.67 (0.56–0.79), 0.002 |
| IL-18BP OR (95% CI), p AUC (95% CI), p | 1.50 (0.76–2.96), 0.29 0.55 (0.45-0.66), 0.35 | 1.70 (1.02–2.84), 0.04 0.59 (0.52–0.66), 0.03 | 1.03 (0.62–1.68), 0.92 0.49 (0.40–0.58), 0.82 | 1.45 (0.75–2.78), 0.27 0.58 (0.48–0.68), 0.15 |
| Free IL-18 OR (95% CI), p AUC (95% CI), p | 2.12 (1.45–3.10), <0.001 0.70 (0.58–0.81), 0.001 | 1.86 (1.34–2.59), <0.001 0.68 (0.61–0.75), <0.001 | 1.74 (1.21–2.48), 0.003 0.64 (0.56–0.73), 0.002 | 1.95 (1.28–2.96), 0.002 0.66 (0.55–0.78), 0.004 |
| CXCL9 OR (95% CI), p AUC (95% CI), p | 1.21 (1.01–1.46), 0.04 0.61 (0.49–0.72), 0.06 | 1.09 (0.97–1.21), 0.14 0.56 (0.47–0.64), 0.18 | 0.98 (0.86–1.12), 0.77 0.47 (0.38–0.57), 0.56 | 1.05 (0.89–1.23), 0.58 0.54 (0.42–0.66), 0.49 |
| IFNγ OR (95% CI), p AUC (95% CI), p | 1.20 (0.99–1.44), 0.06 0.61 (0.50–0.71), 0.07 | 1.29 (1.11–1.51), 0.001 0.66 (0.58–0.74), <0.001 | 1.01 (0.85–1.19), 0.93 0.52 (0.42–0.62), 0.72 | 1.20 (0.99–1.46), 0.06 0.64 (0.53–0.75), 0.02 |
| Covariate, Effect | Platelets Day +28 ≤20/nL (n = 41) vs. >20/nL (n = 512) | Platelets Day +50 ≤100/nL (n = 117) vs. >100/nL (n = 391) | Platelets Year +1 ≤150/nL (n = 84) vs. >150/nL (n = 274) | ANC Day +28 ≤1/nL (n = 43) vs. >1/nL (n = 331) | ||||
|---|---|---|---|---|---|---|---|---|
| Model with total IL-18, aOR (95% CI), p | Model with free IL-18, aOR (95% CI), p | Model with total IL-18, aOR (95% CI), p | Model with free IL-18, aOR (95% CI), p | Model with total IL-18, aOR (95% CI), p | Model with free IL-18, aOR (95% CI), p | Model with total IL-18, aOR (95% CI), p | Model with free IL-18, aOR (95% CI), p | |
| Total IL-18, per log2 increase * | 1.61 (1.21–2.15), 0.001 | – | 1.52 (1.22–1.88), <0.001 | – | 1.28 (1.01–1.63), 0.04 | – | 1.63 (1.20–2.21), 0.002 | – |
| Free IL-18, per log2 increase * | – | 1.78 (1.34–2.36), <0.001 | – | 1.43 (1.18–1.74), 0.002 | – | 1.22 (0.99–1.50), 0.06 | – | 1.76 (1.30–2.39), <0.001 |
| Donor, mismatched vs. matched | 3.43 (1.70–6.92), 0.001 | 3.45 (1.72–7.04), 0.001 | 2.69 (1.66–4.37), <0.001 | 2.68 (1.65–4.35), <0.001 | 1.21 (0.64–2.29), 0.56 | 1.22 (0.65–2.30), 0.54 | 1.84 (0.86–3.93), 0.11 | 1.89 (0.88–4.05), 0.10 |
| Stem cell source, PB vs. BM | 0.34 (0.12–0.98), 0.05 | 0.32 (0.11–0.94), 0.04 | 0.74 (0.30–1.86), 0.53 | 0.75 (0.30–1.88), 0.54 | 0.19 (0.07–0.54), 0.002 | 0.19 (0.07–0.54), 0.002 | 0.13 (0.04–0.41), 0.001 | 0.13 (0.04–0.42), 0.001 |
| ATG, yes vs. no | 1.42 (0.59–3.41), 0.43 | 1.45 (0.60–3.52), 0.41 | 1.64 (0.95–2.84), 0.08 | 1.72 (1.00–2.97), 0.05 | 0.96 (0.54–1.70), 0.88 | 0.97 (0.55–1.73), 0.93 | 4.15 (1.45–11.89), 0.008 | 4.12 (1.42–11.92), 0.009 |
| Conditioning, MAC vs. RIC | 1.50 (0.53–4.23), 0.45 | 1.43 (0.51–4.07), 0.50 | 1.36 (0.64–2.91), 0.42 | 1.27 (0.60–2.70), 0.54 | 1.33 (0.55–3.24), 0.53 | 1.27 (0.52–3.08), 0.60 | 2.78 (1.00–7.75), 0.05 | 2.86 (1.01–8.07), 0.05 |
| Disease stage, high vs intermediate/low | 0.73 (0.36–1.47), 0.38 | 0.69 (0.34–1.40), 0.30 | 1.38 (0.88–2.15), 0.16 | 1.34 (0.86–2.09), 0.20 | 1.52 (0.89–2.60), 0.12 | 1.50 (0.88–2.55), 0.14 | 1.51 (0.76–3.03), 0.24 | 1.44 (0.72–2.91), 0.30 |
| Goodness-of- fit test† | Χ2 = 6.43 (8 df), p = 0.60 | Χ2 = 6.83 (8 df), p = 0.56 | Χ2 = 10.10 (8 df), p = 0.29 | Χ2 = 11.26 (8 df), p = 0.19 | Χ2 = 6.16 (8 df), p = 0.63 | Χ2 = 8.24 (8 df), p = 0.41 | Χ2 = 7.47 (8 df), p = 0.49 | Χ2 = 10.08 (8 df), p = 0.26 |
| Cytokine | Odds Ratio (OR) and Area under the ROC Curve (AUC) per log2 Increase* in Pre-Conditioning Cytokine | |
|---|---|---|
| Platelets Day +28 ≤20/nL (n = 100) vs. >20/nL (n = 561) | ANC Day +28 ≤1/nL (n = 161) vs. >1/nL (n = 500) | |
| Total IL-18 OR (95% CI), p AUC (95% CI), p | 1.42 (1.07–1.90), 0.02 0.58 (0.52–0.65), 0.008 | 1.15 (0.91–1.45), 0.26 0.54 (0.49–0.59), 0.13 |
| IL-18BP OR (95% CI), p AUC (95% CI), p | 2.60 (1.45–4.66), 0.001 0.60 (0.54–0.67), 0.001 | 1.20 (0.79–1.82), 0.39 0.56 (0.51–0.61), 0.02 |
| Free IL-18 OR (95% CI), p AUC (95% CI), p | 1.31 (0.98–1.76), 0.07 0.56 (0.50–0.63), 0.04 | 1.10 (0.86–1.40), 0.46 0.53 (0.48–0.58), 0.28 |
| CXCL9 OR (95% CI), p AUC (95% CI), p | 0.90 (0.71–1.14), 0.38 0.45 (0.35–0.56), 0.34 | 0.97 (0.81–1.16), 0.70 0.48 (0.40–0.55), 0.53 |
| Covariate, Effect | Platelets Day +28 ≤20/nL (n = 100) Vs. >20/nL (n = 561) | ANC Day +28 ≤1/nL (n = 161) Vs. >1/nL (n = 500) | ||
|---|---|---|---|---|
| Model with total IL-18, aOR (95% CI), p | Model with free IL-18, aOR (95% CI), p | Model with total IL-18, aOR (95% CI), p | Model with free IL-18, aOR (95% CI), p | |
| Total IL-18, per log2 increase * | 1.80 (1.29–2.50), <0.001 | – | 1.28 (0.99–1.65), 0.06 | – |
| Free IL-18, per log2 increase * | – | 1.66 (1.19–2.31), 0.003 | – | 1.22 (0.94–1.59), 0.14 |
| Donor, mismatched vs matched | 1.56 (0.94–2.57), 0.08 | 1.56 (0.95–2.58), 0.08 | 0.84 (0.54–1.31), 0.44 | 0.84 (0.54–1.32), 0.45 |
| Stem cell source, PB vs BM | 0.51 (0.24–1.10), 0.09 | 0.52 (0.25–1.11), 0.09 | 0.19 (0.11–0.35), <0.001 | 0.20 (0.11–0.35), <0.001 |
| ATG, yes vs. no | 3.59 (2.04–6.32), <0.001 | 3.60 (2.05–6.33), <0.001 | 2.96 (1.91–4.60), <0.001 | 2.96 (1.90–4.59), <0.001 |
| Conditioning, MAC vs. RIC | 2.17 (1.26–3.76), 0.006 | 2.18 (1.26–3.76), 0.005 | 1.35 (0.85–2.16), 0.20 | 1.35 (0.85–2.15), 0.21 |
| Disease stage, high vs. intermediate/low | 2.92 (1.70–5.04), <0.001 | 2.98 (1.73–5.13), <0.001 | 2.06 (1.29–3.29), 0.002 | 2.08 (1.30–3.32), 0.002 |
| Goodness-of- fit test† | Χ2 = 4.84 (8 df), p = 0.78 | Χ2 = 5.31 (8 df), p = 0.72 | Χ2 = 7.91 (8 df), p = 0.44 | Χ2 = 12.35 (8 df), p = 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radujkovic, A.; Kordelas, L.; Bogdanov, R.; Müller-Tidow, C.; Beelen, D.W.; Dreger, P.; Luft, T. Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation. Cancers 2020, 12, 2789. https://doi.org/10.3390/cancers12102789
Radujkovic A, Kordelas L, Bogdanov R, Müller-Tidow C, Beelen DW, Dreger P, Luft T. Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation. Cancers. 2020; 12(10):2789. https://doi.org/10.3390/cancers12102789
Chicago/Turabian StyleRadujkovic, Aleksandar, Lambros Kordelas, Rashit Bogdanov, Carsten Müller-Tidow, Dietrich W. Beelen, Peter Dreger, and Thomas Luft. 2020. "Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation" Cancers 12, no. 10: 2789. https://doi.org/10.3390/cancers12102789
APA StyleRadujkovic, A., Kordelas, L., Bogdanov, R., Müller-Tidow, C., Beelen, D. W., Dreger, P., & Luft, T. (2020). Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation. Cancers, 12(10), 2789. https://doi.org/10.3390/cancers12102789






