1. Introduction
Photodynamic therapy (PDT) is a minimally invasive approach for cancer treatment. In this approach, three essential elements are needed to induce local cytotoxicity: a light-activatable photosensitizer (PS), light of a specific wavelength, and oxygen molecules. The activated PS can transfer energy to oxygen and subsequently form cytotoxic reactive oxygen species (ROS), among other reactive molecular species [
1]. The produced reactive species destroy tumor cells, damage tumor vasculature, and induce immune response [
2]. Currently, PDT is used in the clinic for the treatment of cancers such as bladder, skin, head and neck, and prostate [
3,
4,
5]. Due to the hydrophobicity of most of the clinically approved PSs, site-specific light should be applied 2–4 days after the systemic administration of the PS in order to favor the retention of the PS in the tumor. This is mainly driven by the enhanced permeability and retention (EPR) effect [
6] due to the unorganized structure of tumor vasculature, enabling therapeutics (mainly (lipo)protein associated PS) to extravasate into the extravascular space [
7]. In addition, impaired-lymphatic drainage causes the macromolecules to be retained longer in the tumor [
6]. Therefore, longer drug to light intervals are employed in order to preferably damage tumor tissue and, more importantly, reduce damage to normal tissues. Alternatively, applying light shortly after the administration of the PS (0–30 min) preferentially damages tumor-associated vessels. This therapeutic strategy has been named vascular targeted PDT (VTP) [
8]. In this approach, which is driven passively, illumination of the PS mainly confined to the blood vessels causes vessel constriction, blood flow stasis, and thrombus formation. The vascular shutdown after VTP can block nutrients and oxygen supply to the tumor, resulting in necrosis and tumor regression [
9]. The therapeutic benefit of VTP has been recently recognized in the clinic. The PS known as TOOKAD
® (Negma Lerads, Elancourt, Ile-De-France, France; Steba Biotech, Strasbourg, France) was approved in 2017 in Europe and Israel for the treatment of men with low-risk prostate cancer [
10].
Although VTP and conventional PDT are already used in the clinic, in the last decades, efforts have been made to increase specificity and efficacy of the therapy. Next to the local and temporal control of light application, accumulation of the PS specifically and selectivity at the tumor tissue and tumor associated vasculature, can improve the efficacy of the treatment and further decrease side effects, such as photosensitivity and damage to the surrounding nerves and muscles. To this end, certain proteins which only express or are more abundant on tumor cells/vasculature have been targeted using different targeting moieties, such as peptides, antibodies or antibody fragments, and nanocarrier systems, to deliver the PS specifically and selectively to the tumor tissue/vasculature [
11]. Nanobody-targeted PDT is one such approach, which was developed in our group. In this approach, PS molecules are specifically associated with tumor cells by means of nanobodies. Nanobodies (NBs) are the variable domain of heavy chain only antibodies that are naturally found in camelids and considered as the smallest antigen binding fragments [
12]. Nanobodies are ten times smaller than conventional antibodies (15 kDa compared to 150 kDa), which allows them to penetrate the tumor effectively and clear more quickly from the body when not associated with their target [
13,
14]. Moreover, low immunogenicity potential and high solubility make them an ideal targeting moiety for targeted therapies [
15]. In our previous studies, EGFR [
16], c-Met [
17], and US28 [
18] targeted nanobodies conjugated to the photosensitizer IRDye700DX showed specific and potent cytotoxic effects on cells overexpressing these targets. As a proof of principle study, nanobody-targeted PDT was applied on an oral squamous cell carcinoma orthotopic mouse tumor model overexpressing EGFR. Light was applied 1 h post injection of the EGFR targeted nanobody–PS conjugates, leading to approximately 90% of tumor necrosis and importantly minimal damage to the surrounding normal tissues [
19]. In a more recent study, HER2 targeted nanobody–PS conjugates were injected intravenously in HER2-positive breast cancer orthotopic mouse tumor model. Illumination 2 h later induced significant tumor regression after a single nanobody-targeted PDT treatment [
20].
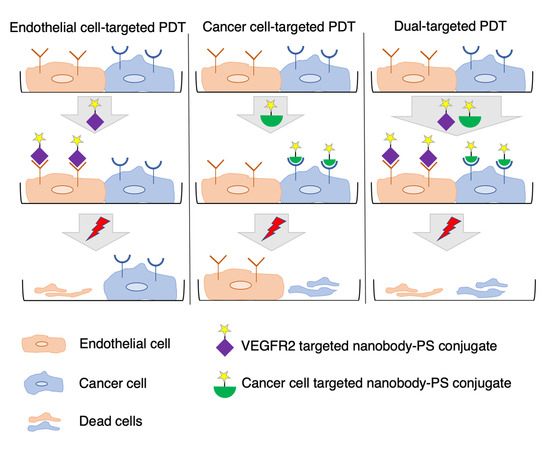
Following up on the promising results we obtained in both in vitro and in vivo studies, we explored the possibility of combined targeting of endothelial and cancer cells in vitro, in order to improve the efficacy of targeted PDT. We hypothesized that dual targeting of receptors on endothelial and cancer cells is likely beneficial in tumors with high heterogeneity of target expression and/or intermediate/low target expression levels [
21,
22]. In line with this, our previous study showed that dual targeting of two cancer cell targets, namely HER2 and CAIX, improved tumor imaging in an orthotopic model of breast cancer [
23]. For VTP, one of the target proteins which is mainly overexpressed on tumor-associated vessels, is vascular endothelial growth factor receptor 2 (VEGFR2). This receptor belongs to the tyrosine kinase family and has been shown to play an essential role in tumor angiogenesis and progression [
24]. VEGFR2 is activated upon binding of its natural ligand (VEGF). This initiates a phosphorylation cascade that ultimately results in the proliferation and migration of endothelial cells [
25]. Under hypoxic conditions, VEGF is upregulated, leading to the subsequent upregulation of VEGFR2 expression in vascular endothelial cells, which ultimately leads to neovascularization [
26,
27]. Higher expression of VEGFR2 on tumor-associated vessels and lower expression in normal blood vessels makes this receptor an ideal target for selective delivery of therapeutics to the tumor neovasculature [
28].
To enable targeting of endothelial cells with nanobody-targeted PDT, we developed and characterized novel nanobodies targeting mouse VEGFR2. Nanobodies were conjugated to IRDye700DX and the phototoxicity induced by the conjugates was assessed in vitro on murine endothelial cell lines with different levels of VEGFR2 expression in monocultures. For simultaneous targeting of endothelial and cancer cells, a previously described EGFR targeted nanobody–PS conjugate was employed [
16,
19] and in co-cultures an oral squamous cell carcinoma cell line was combined with a murine endothelial cell line. Our results confirm the hypothesis that dual targeting of endothelial and cancer cells leads to more potent PDT.
3. Discussion
Efficacy of targeted PDT can be hampered by heterogeneity of target expression and/or moderate/low target expression levels. To circumvent these, we explored the possibility of combined targeting of endothelial and cancer cells in vitro, as a proof of concept. For this, we developed nanobodies targeting mouse VEGFR2, which is overexpressed mainly on tumor vasculature. After thorough characterization of these nanobodies, we showed that the conjugates are potent PDT agents, specifically killing cells expressing VEGFR2. Moreover, dual targeting of endothelial and cancer cells, using nanobody–photosensitizer conjugates targeting VEGFR2 and EGFR, respectively, showed improved efficacy.
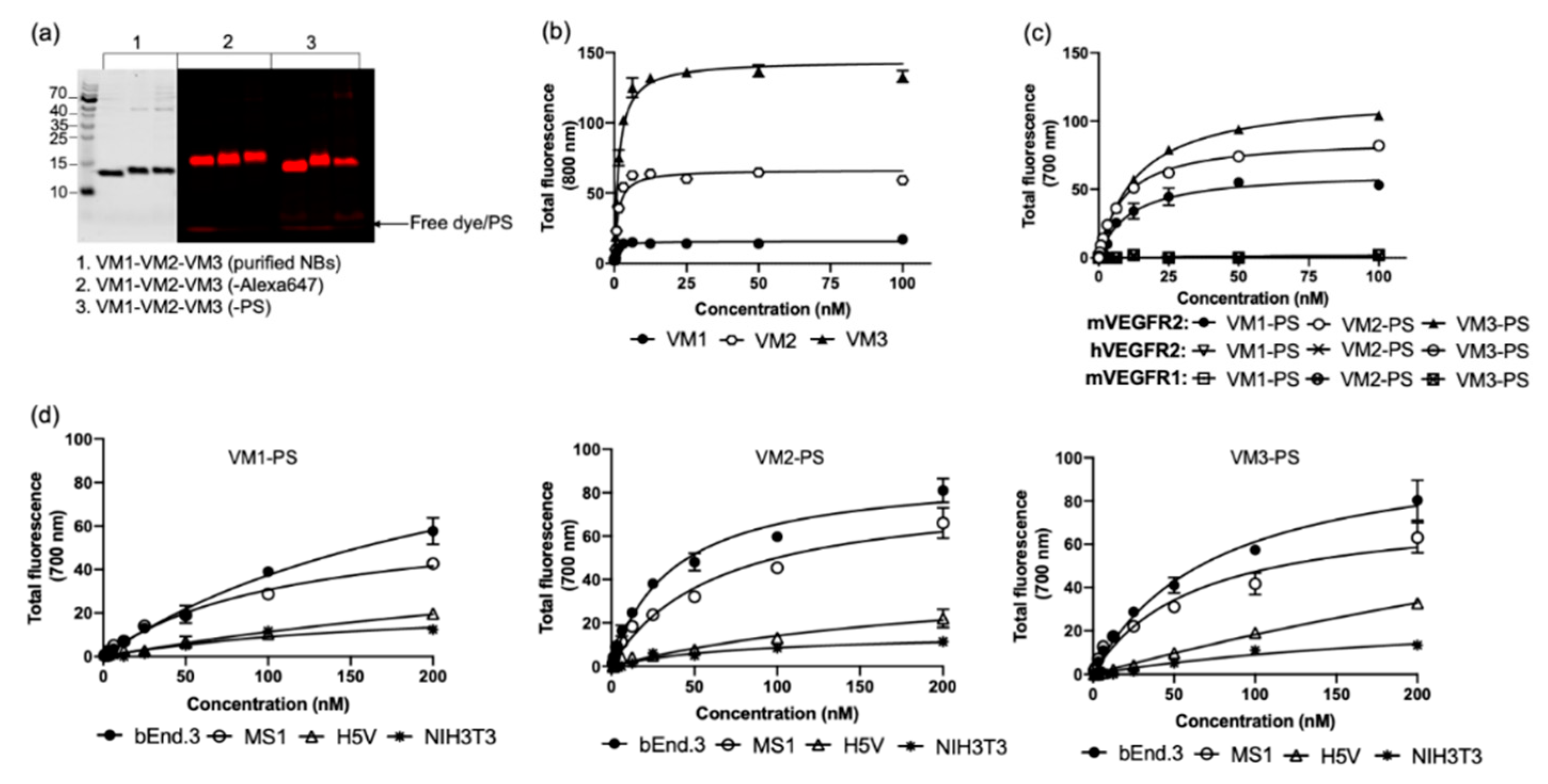
Our mVEGFR2 targeting nanobodies demonstrated high binding affinity to the target protein with single-digit nanomolar dissociation constants (K
D). Interestingly these nanobodies showed different maximum binding values (B
max). This might be caused by: (a) the different epitope accessibility of the nanobodies; and/or (b) the indirect recognition of the binding of the nanobodies with anti-VHH antibody, as these nanobodies are from three different sequence families. With directly labeled nanobodies, which remained high affinity binders, the difference in B
max was smaller among the different nanobodies, supporting the second explanation. To verify the specificity of the nanobodies, different techniques were applied on both protein and cellular levels. On protein level, we showed that all nanobodies only bound to mVEGFR2 and not hVEGFR2 or mVEGFR1. Among the VEGFR family members, mVEGFR1 was employed because its extracellular domain shares the highest sequence homology (43%) with the one of VEGFR2 [
29]. On the cellular level, binding affinity of the nanobodies to the endothelial cells was not determined as endothelial cells detach from the well plates when incubated at 4 °C. However, when conducting the assays at 37 °C, the amount of NB–PS conjugates associated with the murine cell lines correlated well with the expression levels of VEGFR2. This was also verified by flow cytometry analysis, indicating specificity of these nanobodies for mVEGFR2. Other high affinity nanobodies have been described to bind VEGFR2, however only the human variant [
30]. The fact that these nanobodies only bind mouse VEGFR2 will enable dedicated studies on the effects of treatment on tumor vasculature. For translational purposes, cross reactive nanobodies could be obtained through dedicated selections.
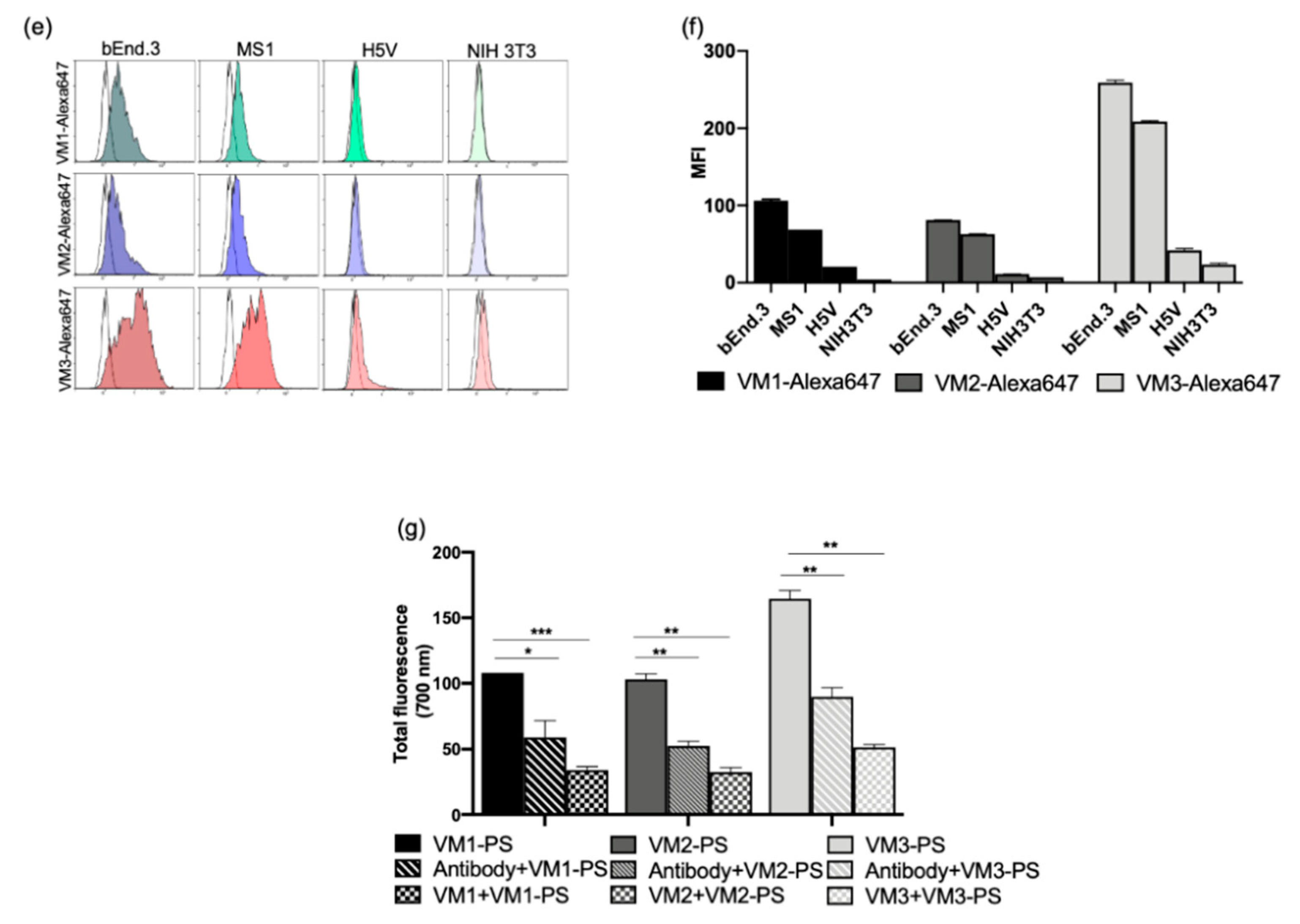
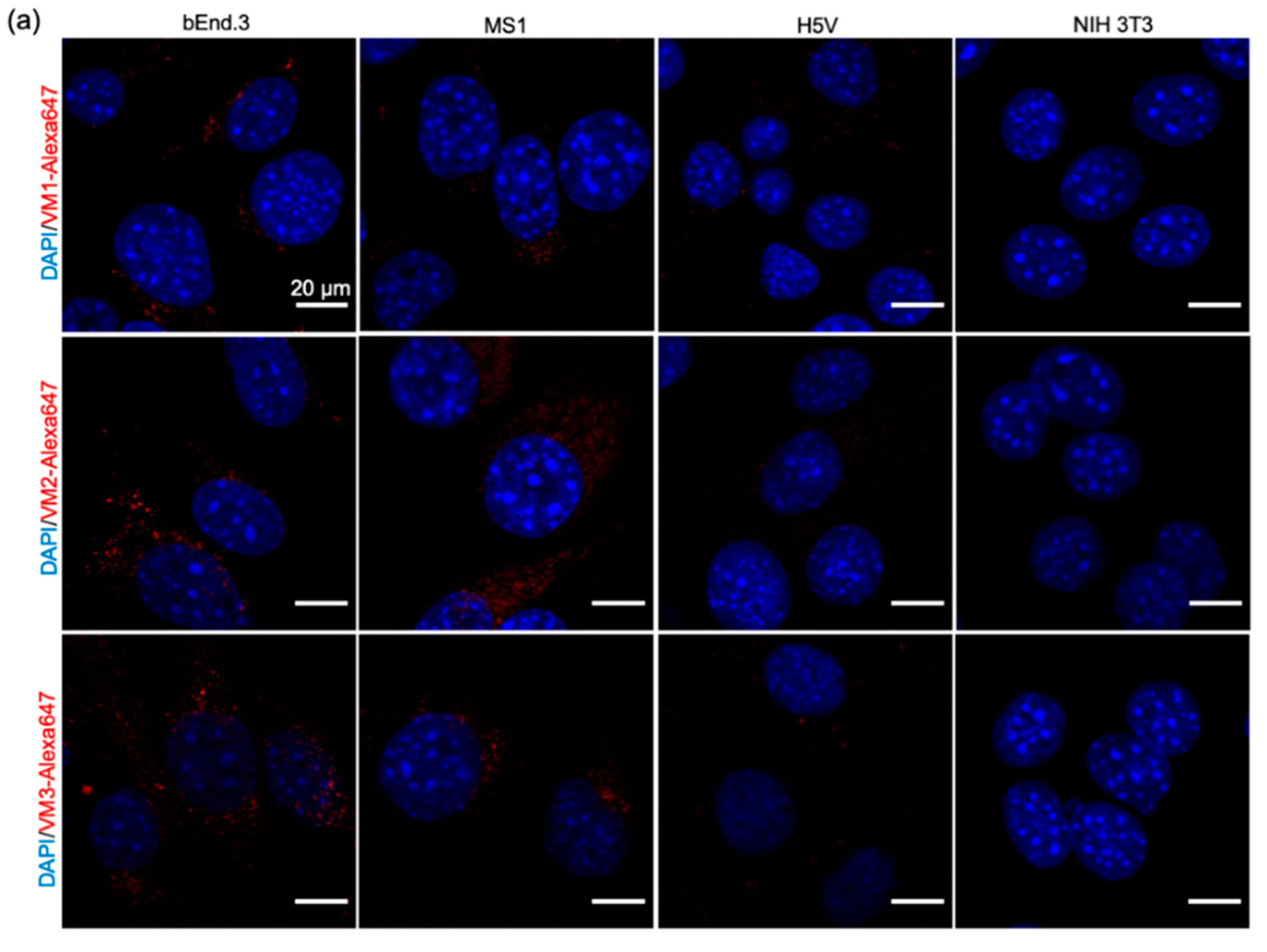
In our microscopic investigations, internalization and perinuclear accumulation of the NB–Alexa647 conjugates in the cells were observed by confocal imaging, which is in agreement with the results obtained with the commercial anti-VEGFR2 antibody. These results are also in agreement with the findings of other groups where perinuclear staining of VEGFR2 was shown after short incubation with anti-VEGFR2 antibody [
31,
32]. This perinuclear localization of monovalent nanobodies or antibody is likely a result of constitutive internalization of the receptor, as Gampel and co-workers showed that a significant portion of VEGFR2 undergoes constitutive endocytosis [
31]. In nanobody-targeted PDT, the damage is inflicted where the nanobody–PS is located, thus here damage will be mostly perinuclear/intracellular. In addition to the static condition, we also explored the association of the NB–Alexa647 conjugates to the cells under flow condition. In in vivo condition, endothelial cells are exposed to the constant blood flow which might change and reduce the interaction of the nanobodies with the cells. Therefore, we decided to investigate whether incubating bEnd.3 cells with NB–Alexa674 conjugates for 1 h under unidirectional flow would still enable cell association and internalization. The setup we used for the experiments under flow is particularly appropriate to study the interaction of the therapeutics with endothelial cells and can, to some extent, predict the in vivo association of the nanobodies targeting endothelial cells when administered systemically [
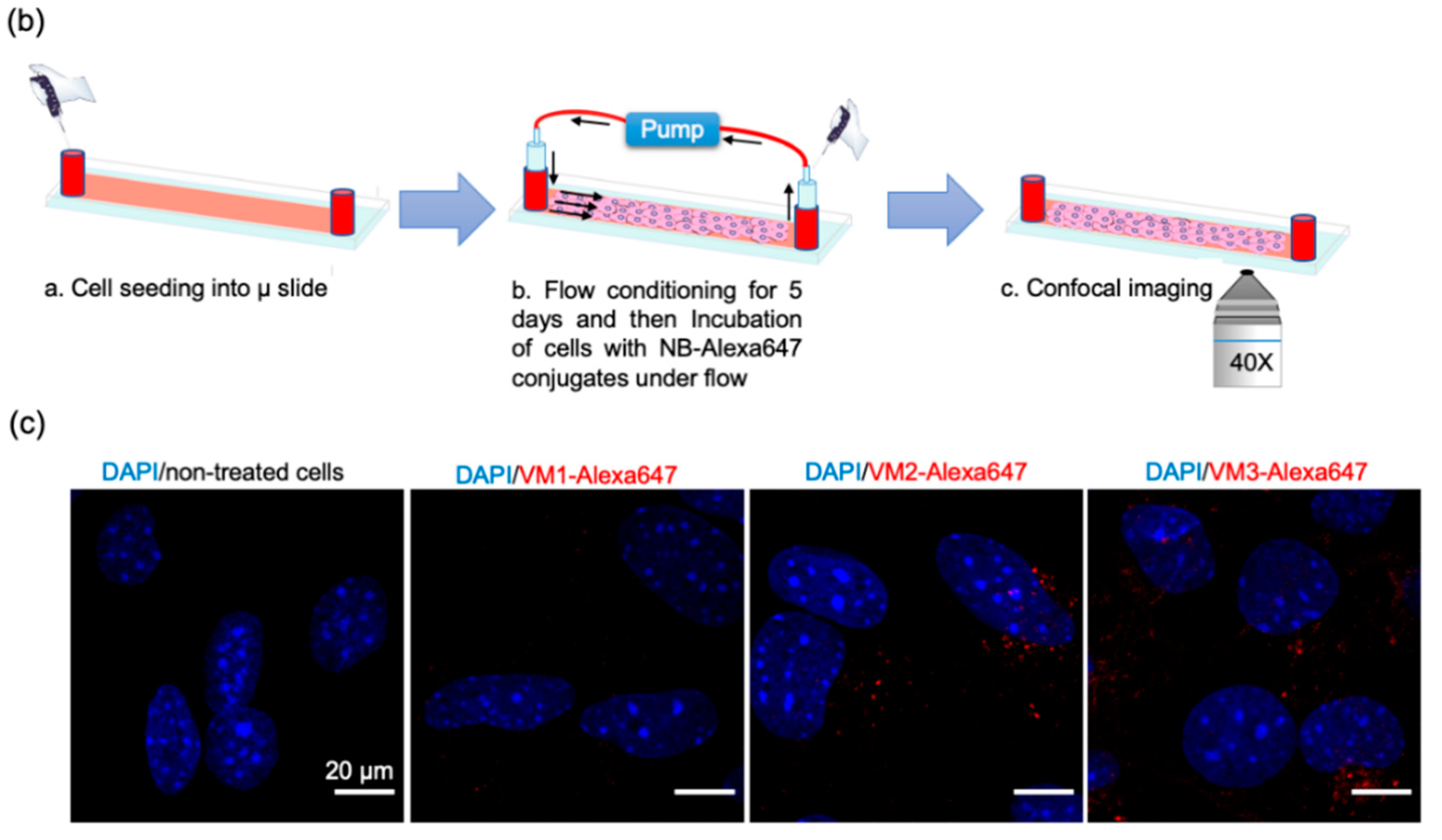
33]. These experiments were conducted at a shear stress of 300/s, which is comparable to the shear stress reported for the human carotid artery. The alignment of F actin cytoskeleton fibers suggests that cells were shear-adapted (
Figure S4), in agreement with other studies [
34]. Among the NB–Alexa647 conjugates, VM2 and VM3 were associated with cells. VM1 on the other hand was barely associated with cells, despite showing cell association under the static condition. These results are in agreement with a recently published study showing that the uptake of nanoparticles into endothelial cells is decreased by increasing the flow rate [
33,
34].
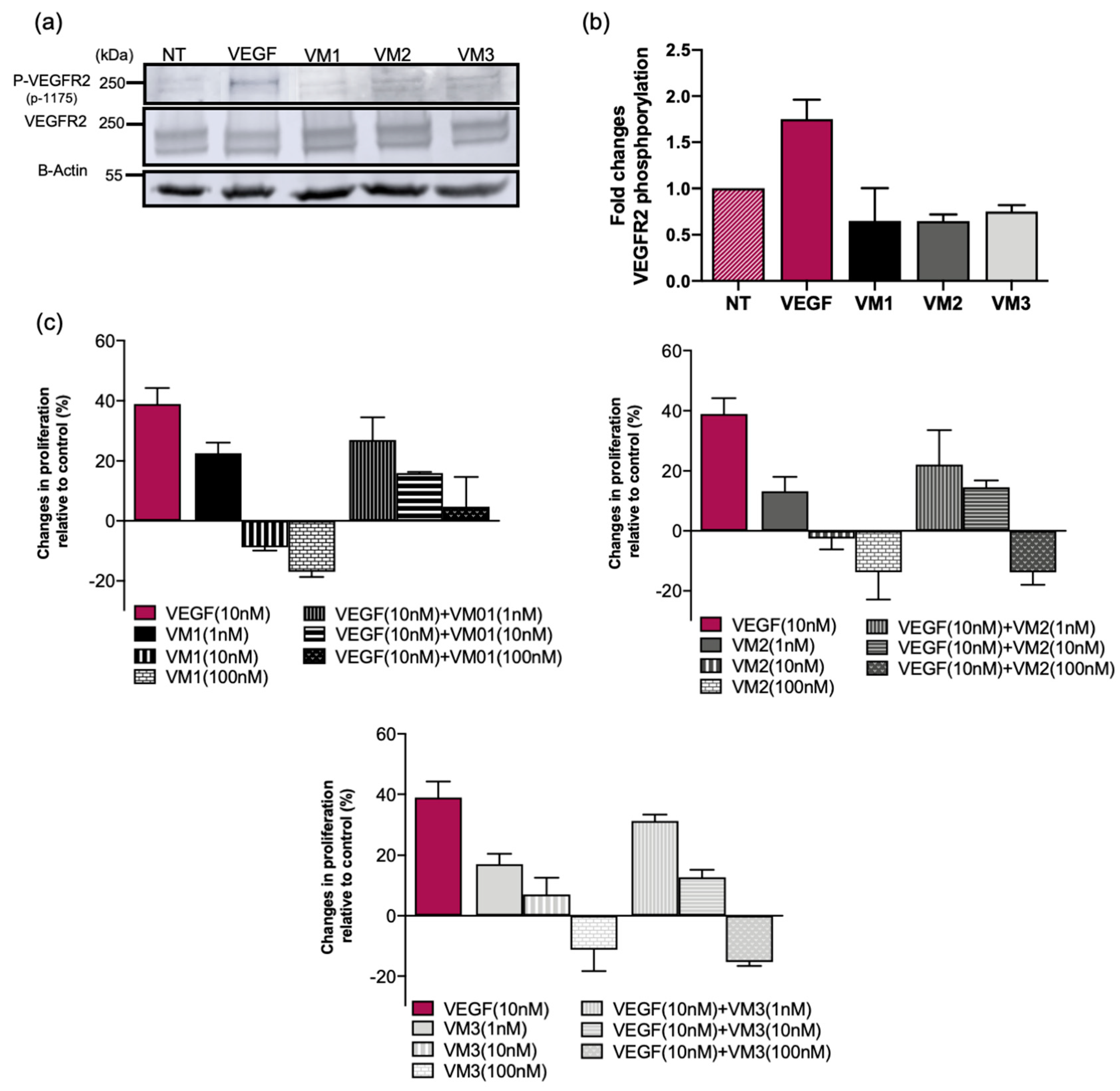
Although nanobody-targeted PDT protocols are conducted in a short period of time, i.e., light is applied 1–2 h post intravenous injection, and involve a single treatment session, we considered it important to assess the agonistic potential of these nanobodies. The phosphorylation assay proved that VEGFR2 is not activated by the nanobodies, ruling out any agonistic activity. The nanobodies did not induce cell proliferation when used at 10 or 100 nM. However, at lower concentration (1 nM), cell proliferation was slightly induced by the nanobodies over the three-day incubation. This likely will not cause any problem in vivo for two reasons: (a) nanobodies have shorter half-life and will be cleared within a day; and (b) the concentration of nanobodies used for in vivo study will likely be higher than 1 nM. Interestingly, the nanobodies also inhibited VEGF-stimulated proliferation in low nanomolar concentrations, suggesting competition of the nanobodies with VEGF (confirmed with a competition assay in
Figure S3) and inhibition of VEGF signaling pathways in vitro. However, this inhibition might not be observed in the tumor microenvironment as the concentration and the affinity of the ligand to the receptor is higher than that of nanobodies. Therefore, the competition of the nanobodies with VEGF should be verified in physiological and more complex conditions.
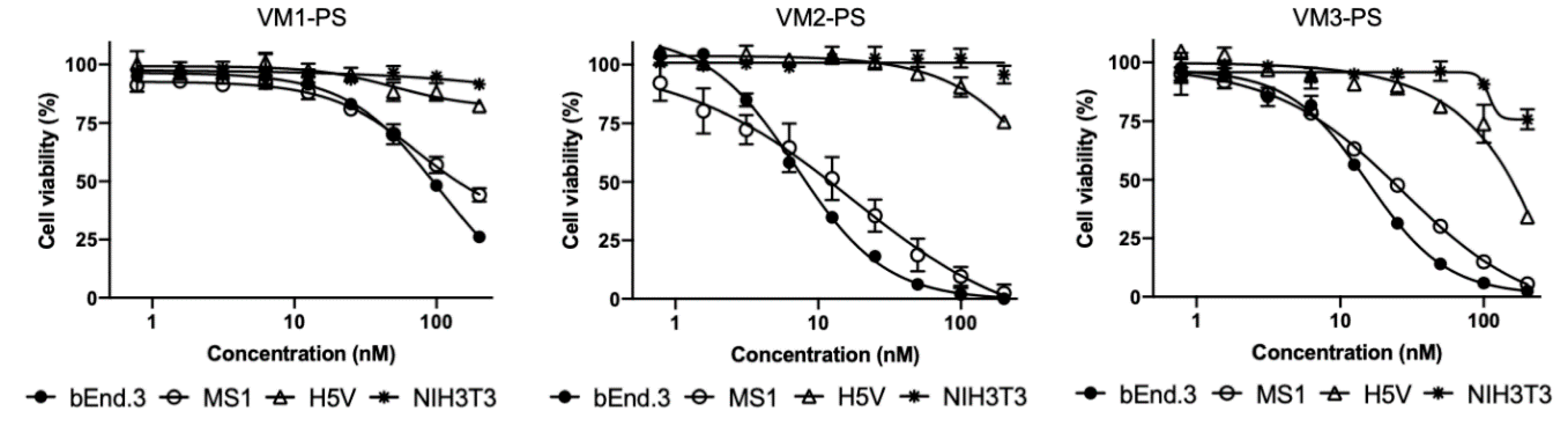
Among the NB–PS conjugates with similar DOC, VM2–PS and VM3–PS induced nearly 100% cell death in bEnd.3 and MS1 cells when tested at 100 nM. EC
50 values were calculated in nanomolar ranges, indicating that the NB–PS conjugates are potent PDT agents. Importantly, non-VEGFR2 expressing cells were not affected after PDT, providing support for the specificity of nanobody-targeted PDT approach. Besides VEGFR2, there are other receptors on tumor vasculature which can be used for targeted VTP, as recently reviewed [
35]. Frochot and colleagues reported integrin αvβ3 targeted peptides for VTP. Phototoxicity induced by cyclic RGD peptide–PS conjugate at 1 μM was assessed after 24-h incubation with HUVEC overexpressing αvβ3 cells and 50% growth inhibition (LD
50) value was reported 3.1 J/cm
2 [
36]. In another study, Neuropilin-1 targeted heptapeptide was conjugated to tetraphenylchlorin (TPC) and PDT efficacy was determined in HUVEC 24 h after incubation with 100 nM conjugate or free PS. Treatment with free PS showed little cytotoxicity, whereas cells treated with the peptide–PS conjugate had 10.4-fold lower viability. LD
50 value was reported as 0.47 ± 0.23 and 4.9 ± 0.64 J/cm
2 for the conjugate and free PS, respectively [
37]. Comparison between these studies is difficult due to different conditions employed, however the low nM EC
50 values presented here certainly encourage further exploration of anti-VEGFR2 conjugates in a preclinical setting.
For the co-culture setup, MS1 cells with moderate level of VEGFR2 expression and OSC cells with moderate level of EGFR expression were selected. In particular for EGFR, our previous research has shown that organoids grown from tumors of patients with head and neck cancers possess low/moderate EGFR levels, compared to cell lines traditionally used in EGFR research (e.g., A431 cell line) [
38]. When EGFR and VEGFR2 targeting nanobodies were applied in combination, noting the particular set-up used here with human cancer cells and murine endothelial cells, no interference was observed of either nanobody–PS (VM2–PS or 7D12–PS) on the association of the other nanobody–PS (7D12–PS or VM2–PS) with cells (
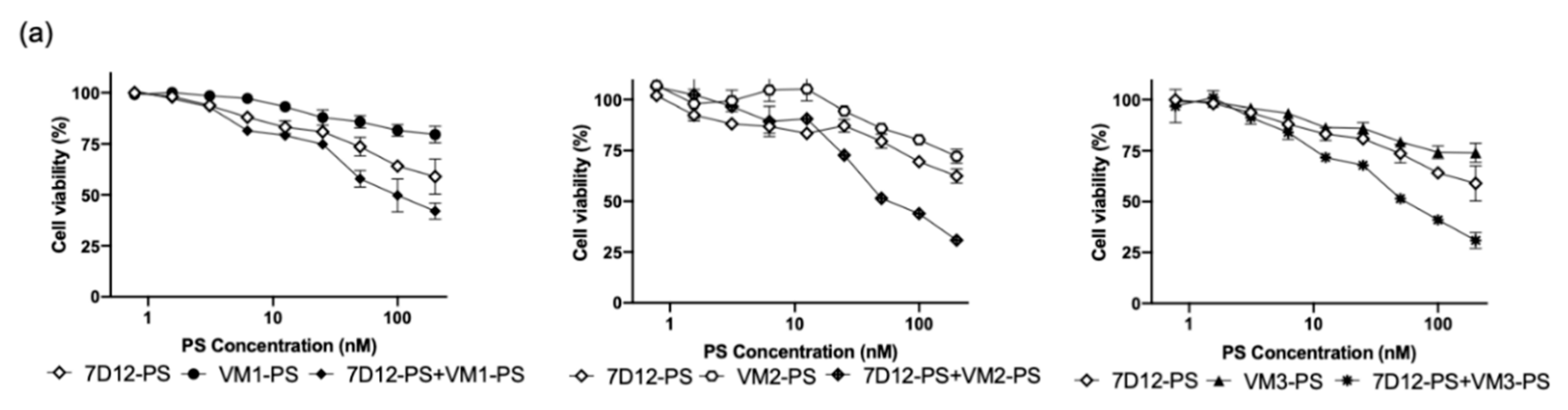
Figure S5). After illumination, the combined treatment was significantly more effective than the individual treatments Importantly, EC
50 values were still in nanomolar level when VEGFR2 and EGFR were simultaneously targeted. For the individual treatments, when endothelial and cancer cells were co-seeded, obviously part of cells were not killed due to the lack of target expression, thus the higher EC
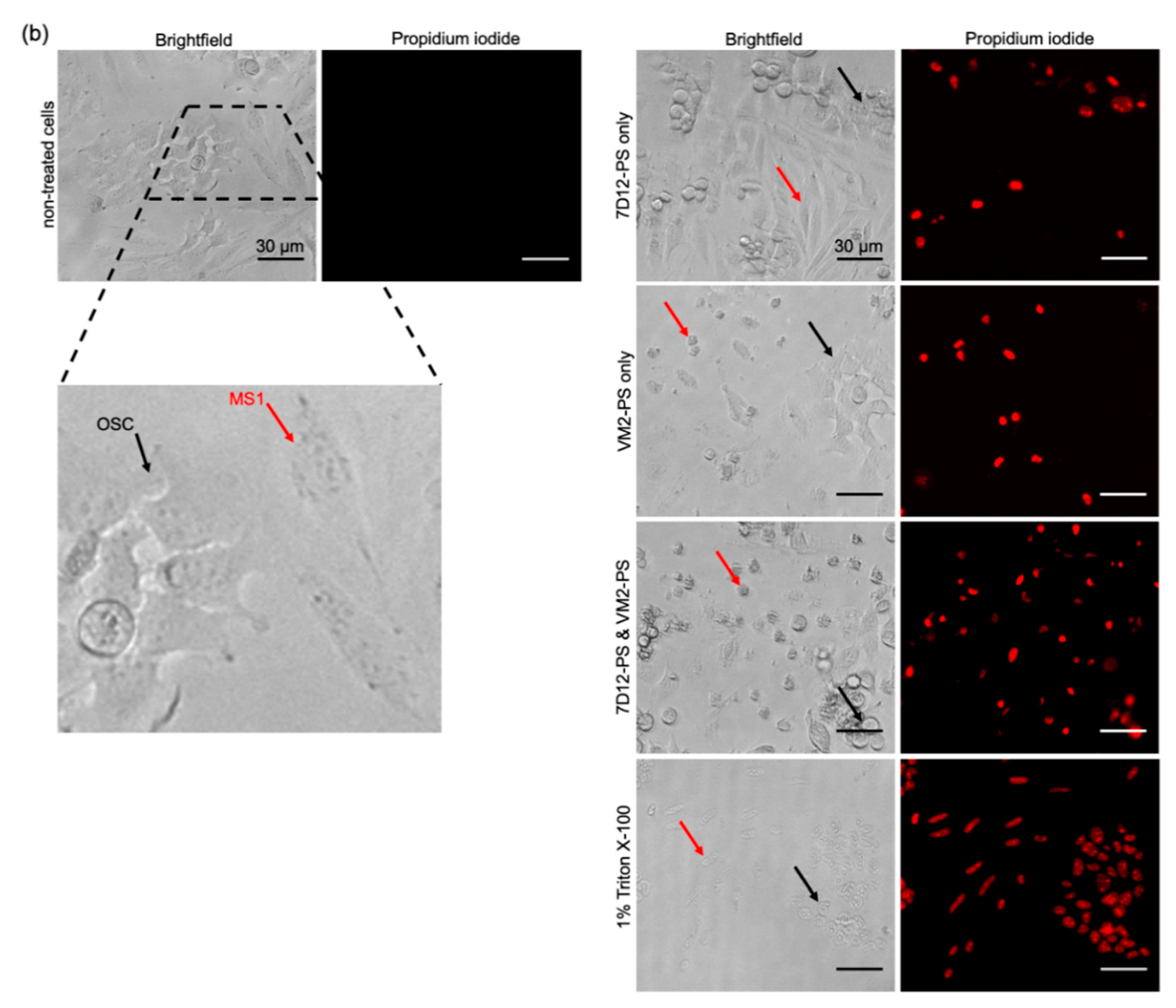
50 values when compared to those obtained in the monoculture setup. Importantly, when nanobodies were applied separately, one cell population was specifically killed (
Figure 5b), whereas, when the combination of the nanobodies was used, cytotoxicity was significantly higher. Interestingly, the cytotoxicity is even higher than the sum of the separate effects, which suggests synergism. The higher cytotoxicity induced by combined nanobodies could be due to the overall higher amount of reactive oxygen/nitrogen species produced during illumination, which can affect adjacent cells [
1,
39]. These results support our hypothesis that simultaneous targeting of endothelial and cancer cells can improve nanobody-targeted efficacy. Follow-up studies will aim at the efficacy evaluation of the conjugates in mice with human tumors. In this context, the cancer cell targeted nanobody–PS conjugates are expected to efficiently distribute through tumors and allow light application 1–2 h after intravenous injection [
19,
20], while these VEGFR2 nanobody–PS conjugates are expected to interact and be taken up by the (mouse) tumor vasculature. Of note, the time point post injection at which light will be applied will be carefully determined, aiming at the most practical and effective protocol.
Intratumoral heterogeneity is one of the leading causes of therapeutic resistance and treatment failure, and one of the main reasons for poor overall survival in cancer patients. Importantly, it has been shown that targets against which therapeutics are available are not expressed in a homogeneous manner in tumor tissues [
21,
22,
38]. Therefore, combination approaches that target cancer cells and tumor vasculature can potentiate the therapeutic response [
40]. Further preclinical studies are needed to investigate the vascular effects of this conjugates and the feasibility for clinical application, to improve selectivity and efficacy of cancer treatment.
4. Materials and Methods
4.1. Cell Lines
The following murine cell lines were used in this study: brain endothelial cells (bEnd.3), pancreatic islet endothelial cells (MS1), heart endothelial cells (H5V), and fibroblast cells (NIH 3T3). bEnd.3 and H5V cell lines were generous gifts from prof. Enrico Mastrobattista (Department of Pharmaceutical Sciences, Utrecht, The Netherlands) and prof. Ingrid Molema (Department of Pharmacology, Groningen, The Netherlands), respectively. MS1 (ATCC
® CRL-2279TM) and NIH 3T3 were (ATCC
® CRL-1658™) purchased from ATCC. All cell lines except MS1 were cultured in Dulbecco’s modified eagle’s medium (Lonza, Basel, Switzerland) containing 4.5 g/L glucose and glutamine supplemented with 1% penicillin/streptomycin (Sigma-Aldrich, Zwijndrecht, The Netherlands) and 10% fetal bovine serum (FBS, Sigma-Aldrich). MS1 cells were grown in DMEM from ATCC (30-2002™). Oral squamous cell carcinoma cell line OSC-19-luc2-cGFP (OSC, Radiology and Molecular Imaging department, Leiden, The Netherlands) were cultured as described previously [
19].
4.2. Llama Immunization and Library Construction
Immunizations of two llamas were performed at Kaneka Eurogentec S.A (Liege, Belgium). Animals received four injections of murine VEGFR2/Fc purified protein (25 μg per injection; R&D Systems, Abingdon, UK) with interval of 2 weeks. As a boost injection, membrane preparations derived from 10
8 bEnd.3 cells were injected 2 weeks after the final injection [
41]. Specific immune response was confirmed by testing pre-immune serum and serum after the second and the final boost. The total mRNA isolated from PBLs was transcribed to cDNA, and specific primers were used to amplify the VhH regions, which were eventually ligated into the phagemid vector pUR8100-Myc-His as described previously [
42]. Transformation of electrocompetent TG1 cells resulted in the generation of two libraries of approximately 0.7 × 10
7 transformants each, which were further used for phage display.
4.3. Phase Display Selections and Phage ELISA
Two rounds of phage selections on purified protein were performed in order to identify the specific VEGFR2 binding nanobodies. Phage selection was performed as described previously [
42] with the only difference being the amount of coated protein which was 1 μg of VEGFR2/Fc protein or equimolar of an irrelevant protein with Fc tail. For phage ELISA, the protocol described previously was followed [
42] except that the amount of coated protein was 300 ng/well VEGFR2/Fc or equimolar of an irrelevant protein with Fc tail.
4.4. Nanobody Production and Purification
The nanobody sequences were re-cloned in a modified pET21 to introduce a N-terminal pelB signal sequence and a C-terminal EPEA tag. The resulting plasmids were transformed in heat-shock competent E. coli BL21. Single colonies were grown overnight in YT-2x medium supplemented with 100 μg/mL ampicillin and 2% glucose. Nanobodies were produced in a 5-L bioreactor (Eppendorf BioFlo 115 benchtop fermentor) in Terrific Broth (TB) medium containing 0.1% glucose and 100 μg/mL ampicillin and overnight pre-culture (1 mL/100 mL TB). Once bacterial growth was in the log phase, isopropyl β-D-1-thiogalactopyranoside (IPTG) with final concentration of 1 mM was added to induce nanobody expression overnight at 25 °C. The cells were then centrifuged at 4800× g for 20 min at 4 °C and the resulting pellet was resuspended in 300 mL PBS. After 2 freeze–thaw cycles, periplasmic fraction was separated by centrifugation at 10,000× g for 20 min at 4 °C. The nanobodies were purified by Äkta Xpress chromatography system (GE healthcare, Hoevelaken, The Netherlands) using 5-mL C-tag column (ThermoFisher Scientific, Etten-Leur, The Netherlands) and 5-mL HiTrap Desalting column (GE healthcare). The purity of the nanobodies was verified by SDS-PAGE under reducing condition.
4.5. Conjugation of the Nanobodies to Photosensitizer IRDye700DX NHS and Alexa Fluor™ 647 NHS Ester
The nanobodies were conjugated to PS/fluorophore via random NHS-mediated coupling to lysine amino acids, adapted from [
16]. Briefly, nanobodies (2 mg/mL) were incubated with 4 molar equivalents of the PS (LI-COR, Bad Homburg, Germany) for 3 h at room temperature (RT). The NB–PS conjugates were purified using four PD-10 desalting columns pre-equilibrated with PBS, as recommended by the manufacturer (GE healthcare). To conjugate nanobodies to Alexa647 NHS Ester (ThermoFisher Scientific), 2 mg/mL nanobodies were incubated with 3 molar equivalents of fluorophore for 2 h at RT. The conjugates were purified from free fluorophore using three Zeba spin desalting columns (2 mL, ThermoFisher Scientific) which were pre-equilibrated with PBS. The purity of the conjugates was determined by SDS-PAGE. Immediately after running the gel, the fluorescence of PS/Alexa647 was detected with an Odyssey infrared scanner at 700 nm. Total protein was subsequently visualized with PageBlue
TM staining. The degree of conjugation (DOC) was determined following the manufacturer’s protocol by measuring the absorbance at 280 and 689 nm for PS or 280 and 650 nm for Alexa647 using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). For the co-culture experiment, 7D12–PS conjugate with DOC of 0.5 was prepared as described previously [
19].
4.6. Determination of Binding Affinity
Mouse VEGFR2 (His tag, Sino Biological, 300 ng/well), equimolar of human VEGFR2 (His tag, Sino Biological, Vienna, Austria) and mouse VEGFR1 (R&D systems) proteins were coated on MaxiSorpTM plate (ThermoFisher Scientific) overnight at 4 °C. Next day, wells were washed twice with PBS and blocked with 4% milk in PBS for 30 min at RT. A dilution range of the nanobodies and NB–PS and NB–Alexa647 conjugates (0.1–100 nM) in 2% mPBS was added to the wells in triplicate and incubated for 2 h at RT. For the labeled nanobodies, after 3 times washing with PBS, fluorescence was detected with an Odyssey infrared scanner at 700 nm. For the unlabeled nanobodies, wells were washed 3 times with 2% mPBS, followed by the addition of rabbit anti-VHH antibody (QVQ B.V.). After 1-h incubation at RT, wells were washed with 2% mPBS and then incubated with IRDye800CW goat anti-rabbit secondary antibody (LI-COR). The plates were scanned the same way as mentioned above. Data were analyzed with GraphPad Prism 8.0 software. The experiments were performed at least three times and data shown as mean ± SD.
4.7. Flow Cytometry Analysis
First, four murine cell lines were seeded in 48 well plates (25,000 cells/well, Nunc, Roskilde, Denmark). The next day, cells were incubated with monoclonal anti-VEGFR2-PE antibody (50 nM, ThermoFisher Scientific, catalog n 12-5821-82) for 1 h at 37 °C. Afterwards, cells washed three times with PBS and then trypsinized. The cells were washed once with PBS and then resuspended in 1% BSA-PBS. The same protocol was used for the NB–Alexa647 conjugates (50 nM). The unstained controls for each cell line were taken along. Measurement was carried out on the BD FACSCanto II (BD Biosciences, San Jose, CA, USA) with standard filter sets. Analysis was performed using FlowLogicTM software version 7.3 (Inivai Technologies, Mentone Victoria, Australia).
4.8. In Vitro Association without and with Pre-Blocking
To determine association of the NB–PS conjugates with cells, four murine cell lines were seeded (8000 cells/well) in 96-well plates one day before the experiment. Cells were incubated with VEGFR2 targeted NB–PS conjugates (0.78–200 nM) in DMEM medium without phenol red supplemented with 10% FBS, 1% penicillin/streptomycin for 1 h at 37 °C. After 2 times washing with DMEM medium, the total fluorescence of the associated conjugates was measured using an Odyssey infrared scanner at 700 nm.
To explore association of the NB–PS conjugates with cells in the presence of anti-VEGFR2 antibody or unconjugated nanobodies, bEnd3 cells were pre-incubated with 250 nM anti-VEGFR2 antibody (ThermoFisher Scientific) or unconjugated nanobodies for 10 min at RT, followed by the addition of the NB–PS conjugates (25 nM) and incubation for 1 h at 37 °C. NB–PS conjugates were added alone to the cells in order to obtain 100% fluorescent intensity. Cells were washed twice with PBS and total fluorescence was detected at 700 nm. Data were analyzed with GraphPad Prism 8.0 software and shown as mean ± SD. Analysis of significance was performed for each nanobody through unpaired t-test. p < 0.05 was considered significant.
4.9. Immunofluorescence Microscopy
4.9.1. Microscopic Evaluation of the NB–Alexa647 Accumulation in Murine Cell Lines under Static Conditions
Ten thousand cells were seeded a day before the experiment in 16-well Lab-Tek chamber slides (ThermoFisher Scientific). Fifty nanomolar concentration of the NB–Alexa647 conjugates was incubated with the cells for 1 h at 37 °C. Then, cells were washed three times with PBS and fixed with 4% solution of formaldehyde in PBS for 30 min at RT. Formalin-induced autofluorescence was quenched after incubation with 100 mM glycine in PBS for 10 min at RT. Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min at RT followed by washing with PBS. As control, anti-VEGFR2 monoclonal antibody (55B11, Cell Signaling, Leiden, The Netherlands) was used, following the protocol recommended by the manufacturer. After 1-h incubation with the antibody, wells were washed three times with 0.1% BSA-PBS, and, subsequently, Alexa Fluor™488 Phalloidin (final concentration 33 nM, ThermoFisher Scientific) in 0.1% BSA-PBS was added to the wells and incubated for 30 min. After two times washing with PBS, cells were incubated with 4′,6-diamidino-2-phenylindole (DAPI, 0.25 μg/mL, ThermoFisher Scientific) for 5 min. The slides were mounted with SlowFade (Invitrogen, Leiden, The Netherlands) and imaged with LSM700 confocal laser scanning microscope using 40× oil immersion objective (Carl Zeiss Microscopy, Jena, Germany). The images were analyzed with ImageJ.
4.9.2. Microscopic Evaluation of the NB–Alexa647 Accumulation in bEnd.3 Cells under Flow Conditions
The experiments under flow were performed using ibidi pump system®. bEnd.3 cells were seeded in a 0.6-mm μ-Slide I Luer in DMEM medium containing 10% FBS and 1% penicillin/streptomycin at a density of 1.6 × 106 cells per slide. The slides were incubated for 2 h at 37 °C and then connected to a pump-controlled perfusion set under unidirectional and continuous flow (shear rate 300/s, shear stress 0.3 N/m2, viscosity 1 mPa·s) at 37 °C in 5% CO2 for 5 days before addition of the conjugates. The medium was changed every 2 days. The conjugates diluted in complete medium were added to the system (final concentration 50 nM) while flow was shortly stopped. Cells were incubated with the conjugates for 1 h at 37 °C under flow (shear rate 300/s, shear stress 0.3 N/m2, viscosity 1 mPa·s). Afterwards, the flow stopped, the slide was disconnected from the system, and cells were carefully washed three times with PBS. The rest of the cell preparation for confocal microscopy was performed as mentioned above expect for the last step, in which ibidi mounting medium was used before imaging.
4.10. Western Blot Analysis of VEGFR2 Phosphorylation
MS1 cells (250,000 cells/well) were seeded in 6-well plates in DMEM medium containing 10% FBS and 1% penicillin/streptomycin and allowed to adhere overnight. The next day, the cells were rinsed with DMEM containing 0.1% FBS and serum-starved overnight in the same medium. The day of the assay, 50 nM VEGF-A (PEPROTECH®, Cranbury, NJ, USA) or 1 µM nanobodies were added to the cells in the starvation medium and incubated for 15 min at 37 °C. Afterwards, cells were immediately washed twice with ice-cold PBS and total cell lysates were prepared with scraping cells in 100 µL of 1× Laemmli sample buffer and boiled for 10 min at 100 °C. Proteins were separated on 8% SDS-PAGE and blotted onto PVDF membrane (Roche, Mannheim, Germany). The blots were then blocked with 2% BSA in TBS-T (0.05% Tween-20 in 20 mM Tris-buffered saline pH 7.2, TBS-T) buffer for 1 h at RT, followed by staining overnight at 4 °C for phosphorylated receptor using a rabbit monoclonal anti-VEGFR2 phospho-tyrosine 1175 antibody (19A10, Cell Signaling) diluted in 2% BSA in TBS-T. As a loading control, the lower part of the blot was stained for actin overnight with a mouse monoclonal anti-actin antibody (ICN Biomedicals, Irvine, CA, USA). Afterwards, the blots were incubated with IRDye800CW goat anti-rabbit secondary antibody (LI-COR) and IRDye800CW donkey anti-mouse secondary antibody (LI-COR) for 1 h at RT. Bound antibody was visualized with an Odyssey infrared scanner at 800 nm. Afterwards, blots were stripped using 1% SDS and 25 mM glycine pH 2, blocked with 2% BSA and then incubated with rabbit anti-VEGFR2 monoclonal antibody (55B11, Cell Signaling) overnight at 4 °C. After incubation with IRDye800CW goat anti-rabbit secondary antibody, the total VEGFR2 was visualized at 800 nm.
4.11. Proliferation Assay
For the proliferation assay, MS1 cells were used as they showed better compatibility with the assay conditions among the different endothelial cell lines. Two thousand MS1 cells were seeded in 96-well plates in complete medium containing 10% FBS and 1% penicillin/streptomycin. The next day, 10 nM VEGF-A, different concentrations of the nanobodies (1, 10, and 100 nM), or the mixture of both in DMEM containing 0.2% FBS were added to the cells and incubated for 72 h at 37 °C. Cell viability was determined using AlamarBlue® reagent following the manufacturer’s protocol (Bio-Rad, Lunteren, The Netherlands). The percentage of changes in proliferation was calculated relative to the non-treated cells. The experiment was performed at least three times. The assay results are shown as mean ± SD.
4.12. In Vitro PDT
4.12.1. Monoculture
Total fluorescence of the associated NB–PS conjugates with the murine cell lines was determined 1 h after incubation at 37 °C, as described in
Section 4.8. Immediately after, cells were illuminated with 7 mW/cm
2 fluence rate for a total light dose of 20 J/cm
2 using a 690-nm diode laser through a 600-μm optic fiber (Modulight, Tampere, Finland). After overnight incubation at 37 °C, AlamarBlue
® reagent was added to the wells following the manufacturer’s protocol (Bio-Rad) to assess cell viability. Cells with no light/no treatment were used as 100% viability and the percentage of viability calculated relative to the 100% viable cells. Data were analyzed with GraphPad Prism 8.0 software and presented as mean ± SD.
4.12.2. Co-Culture of Endothelial and Cancer Cells
MS1 cells were co-seeded with OSC cells in 96-well plates (1:3 ratio and total number of 10,000 cells/well) one day before the experiment. The next day, cells were incubated with different concentrations of 7D12–PS (targeting EGFR on OSC cells), or VEGFR2 targeting conjugates, or the mixture of both keeping the total concentrations of PS the same (e.g., 100 nM 7D12, 100 nM VM, or 50 nM 7D12 + 50 nM VM). After 1 h of incubation at 37 °C, cells were illuminated, and the percentage of cell viability was determined the next day, as described above.
4.13. Specificity of the Nanobody-Targeted PDT in Co-Culture Setup
MS1/OSC cells were treated with the NB–PS conjugates (100 nM 7D12–PS, VM2–PS, or 50 nM+ 50 nM PS of mixture), and, 24 h after illumination, the medium was slowly removed, and PI (1 μg/mL, Invitrogen) in PBS was added to the cells and incubated for 10 min at 37 °C. Triton (1%) was used as a positive control to induce 100% cell death. The cells were imaged with an EVOS microscope and analyzed with ImageJ.