Activation of PERK Contributes to Apoptosis and G2/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Materials
2.3. Western Blotting
2.4. 3-(4,5,-Dimethylthiazol)-2-yl-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.5. DNA Fragmentation Assay
2.6. Measurement of the MMP
2.7. Detection of G2/M Arrest and Hypodiploid Cells by TAX and NOC
2.8. Statistical Analysis
3. Results
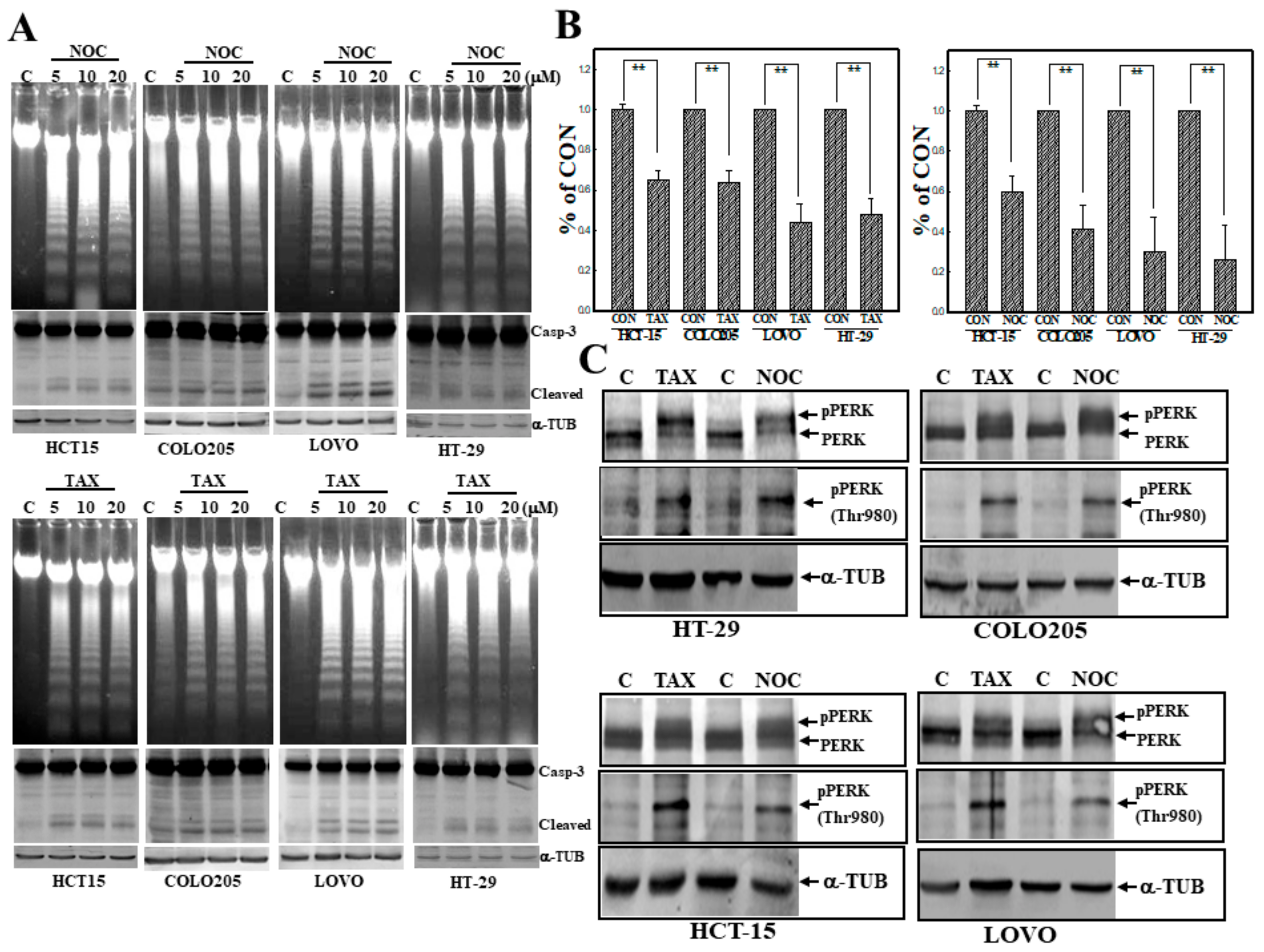
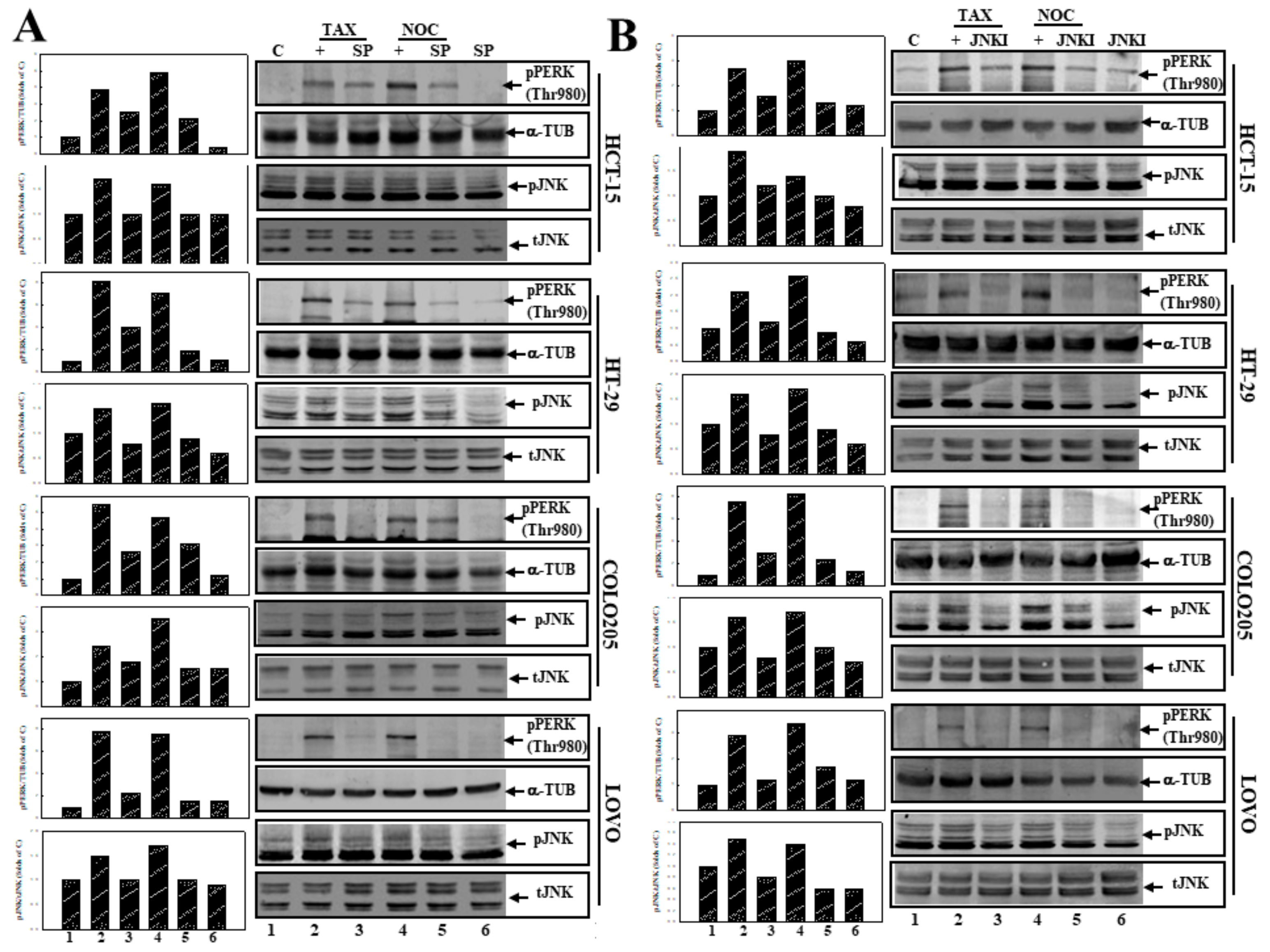
3.1. Induction of Apoptosis by TAX and NOC with Increased Phosphorylation of the PERK Protein in Human CRC Cells
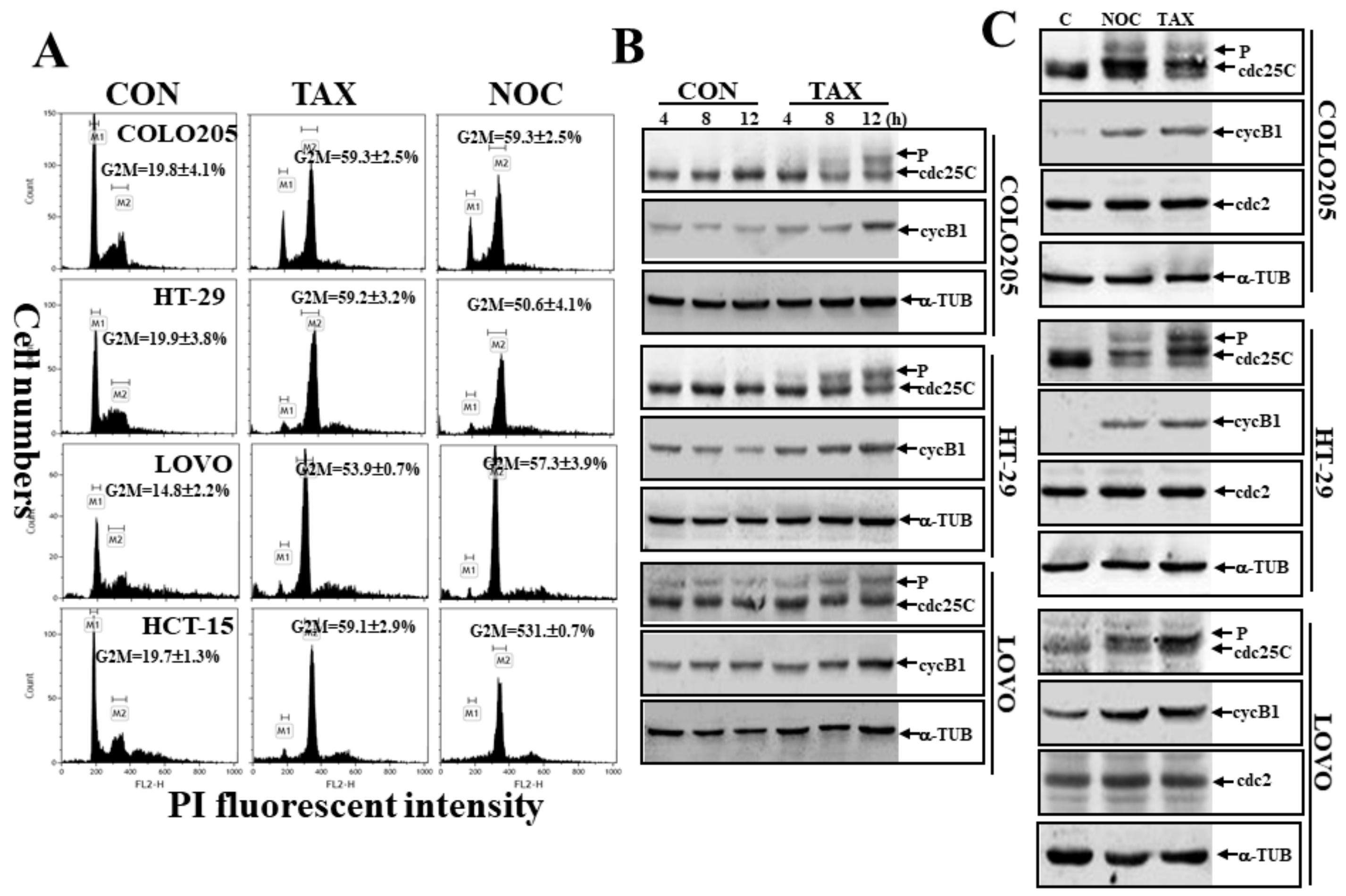
3.2. Induction of G2/M Arrest by TAX and NOC with Increased Phosphorylated Cdc25C and cycB1 in Human CRC Cells
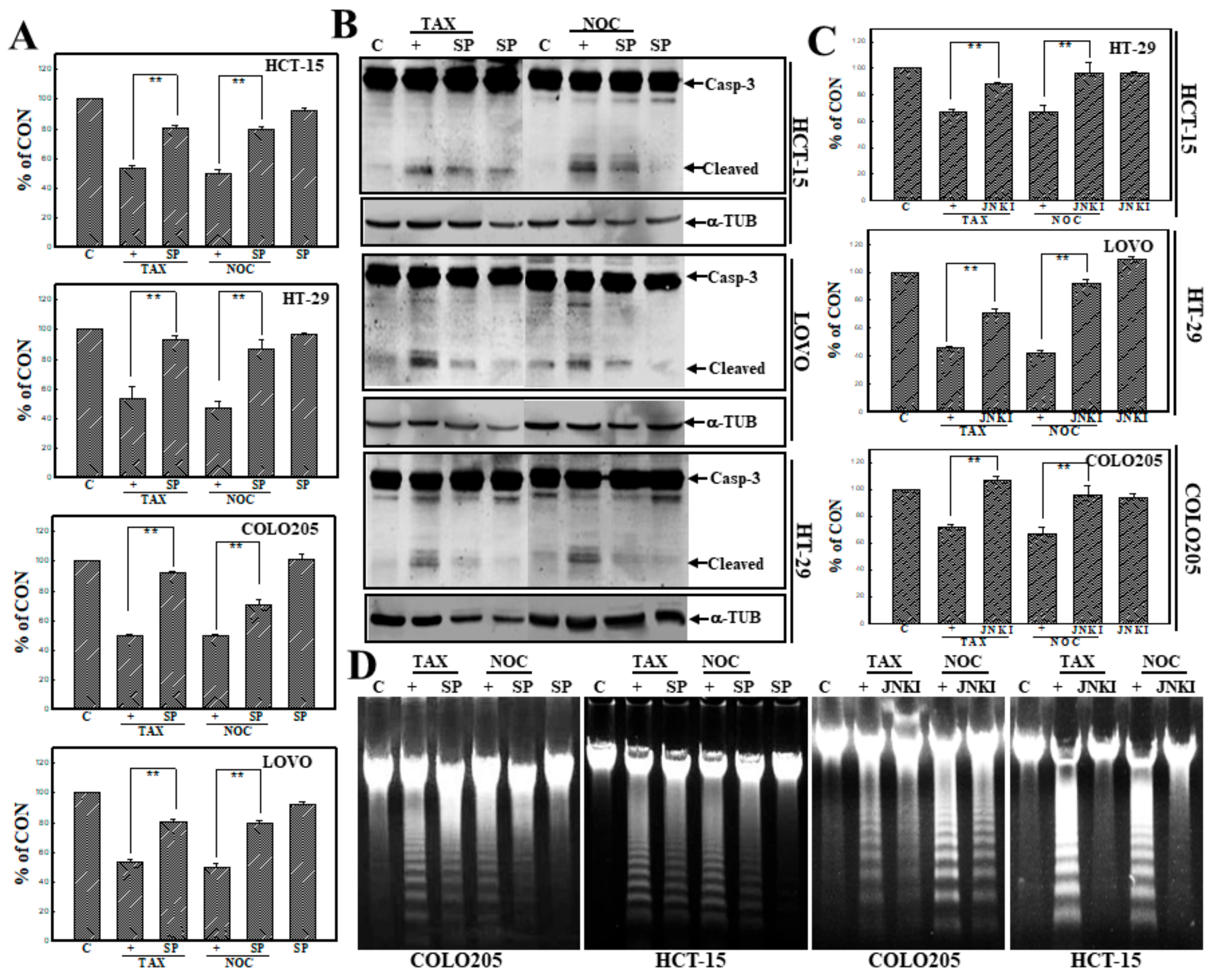
3.3. Activation of JNK Is Involved in TAX- and NOC-Induced Apoptosis of Human CRC Cells
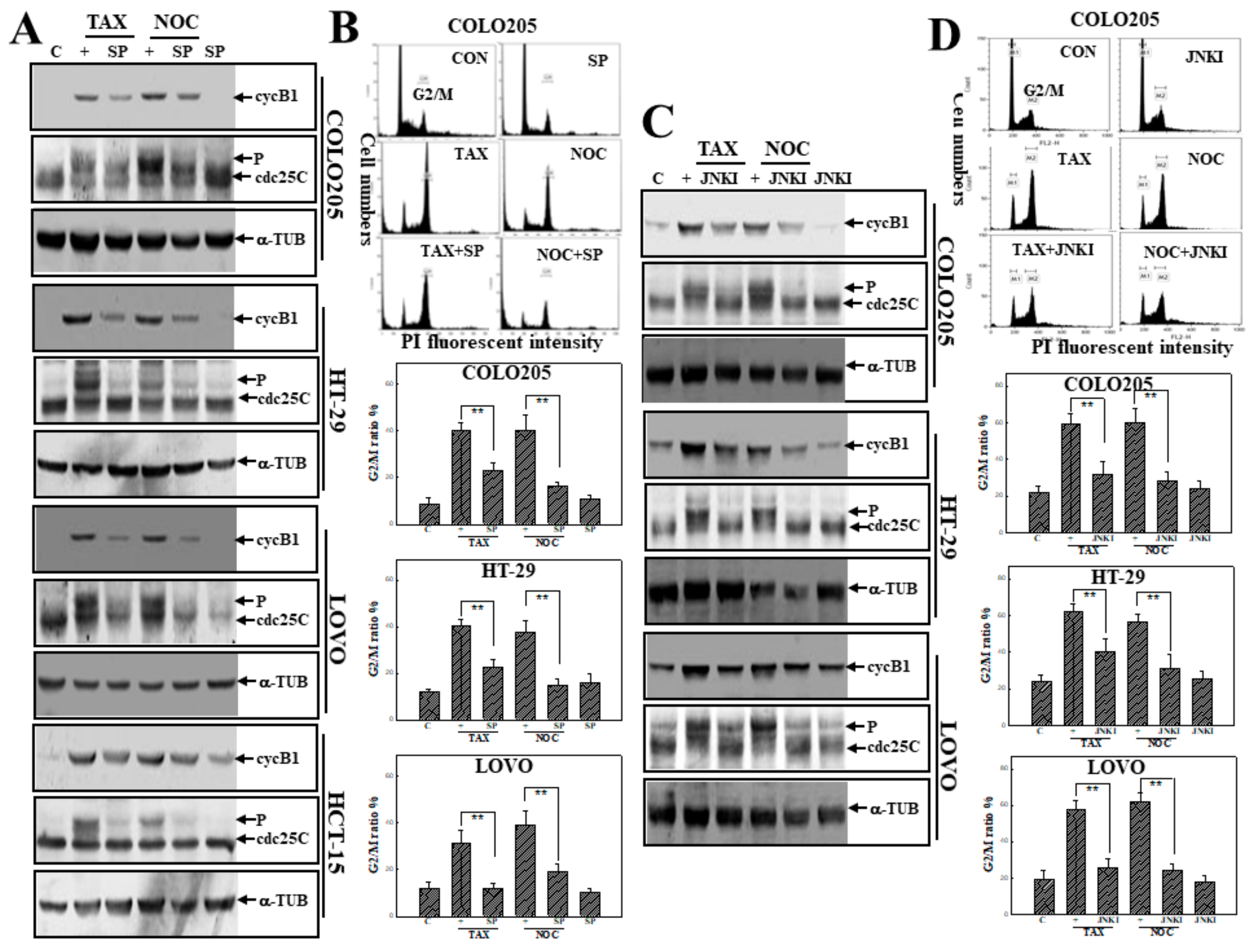
3.4. Activation of JNK in TAX- or NOC-Induced G2/M Arrest of Human CRC Cells
3.5. Suppression of JNK Activation by SP or JNKI Inhibited TAX- and NOC-Induced Phosphorylated PERK Protein Expression in Human CRC Cells
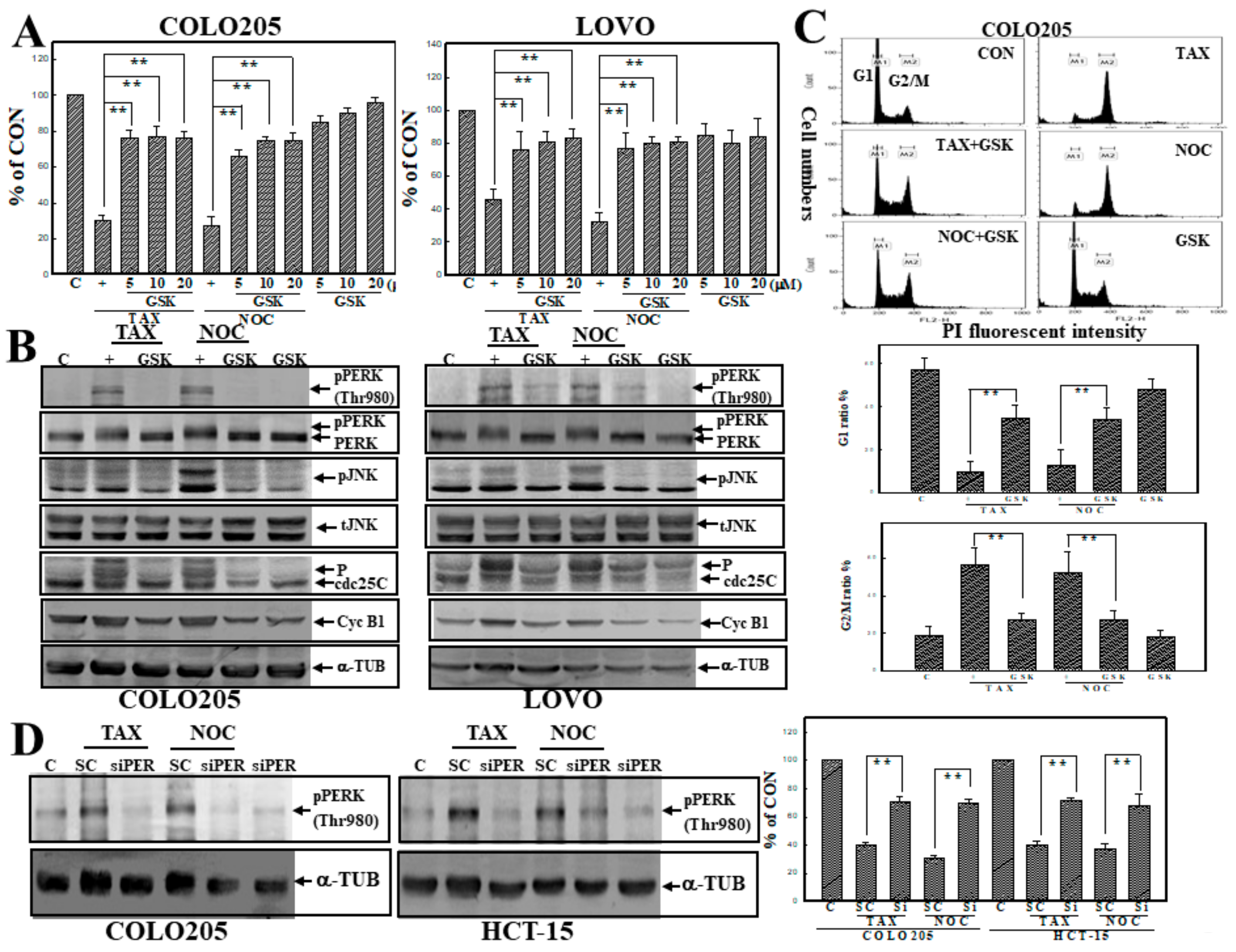
3.6. GSK2606414 (GSK), a PERK Inhibitor, Inhibited TAX- and NOC-Induced Cell Death and G2/M Arrest with Suppression of PERK and JNK Protein Phosphorylation in Human CRC Cells
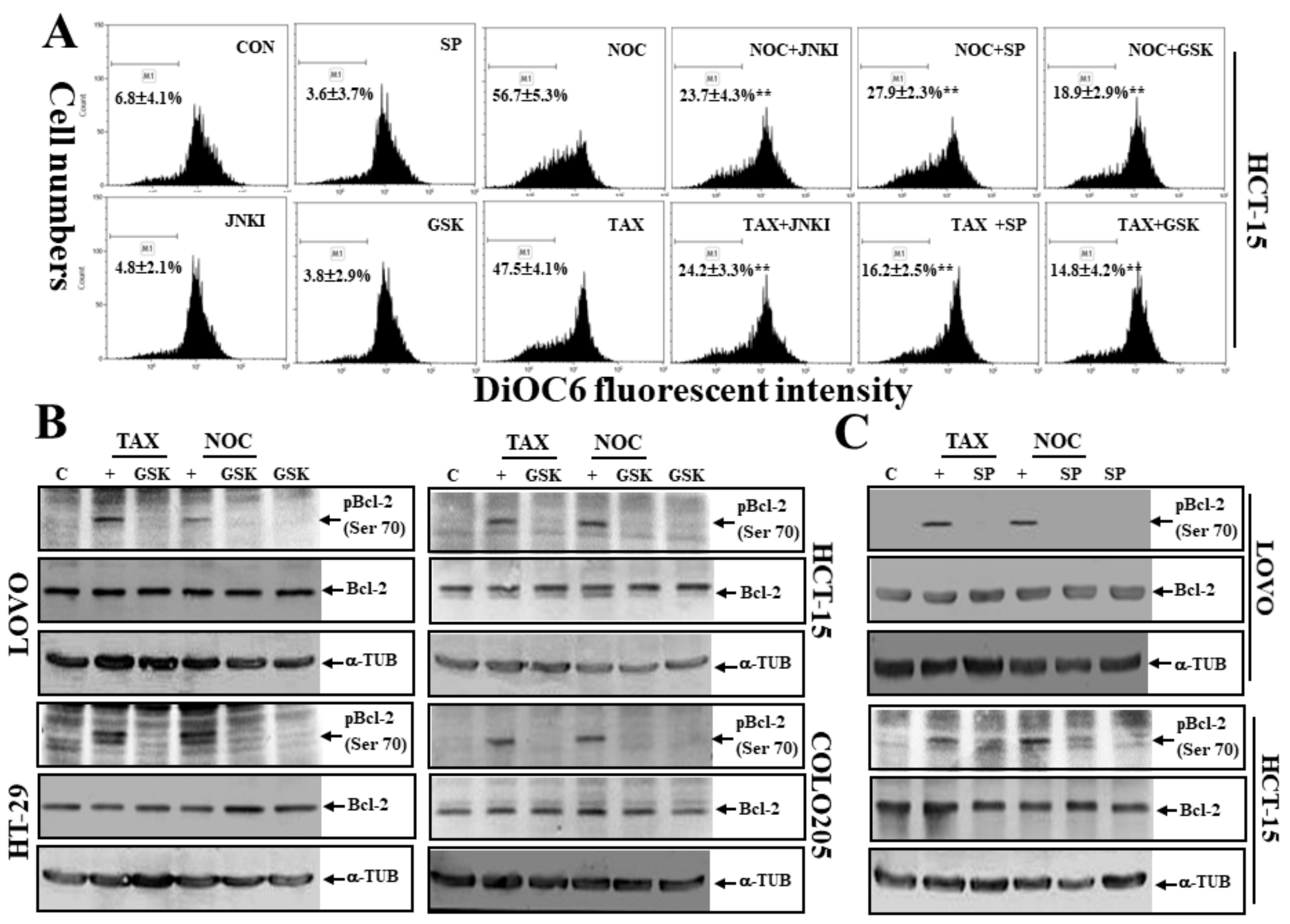
3.7. The PERK Inhibitor GSK and JNK Inhibitors SP and JNKI Decreased TAX- or NOC-Disrupted MMP with Decreased Expression of Phosphorylated Bcl-2 Protein at Ser70
4. Discussion
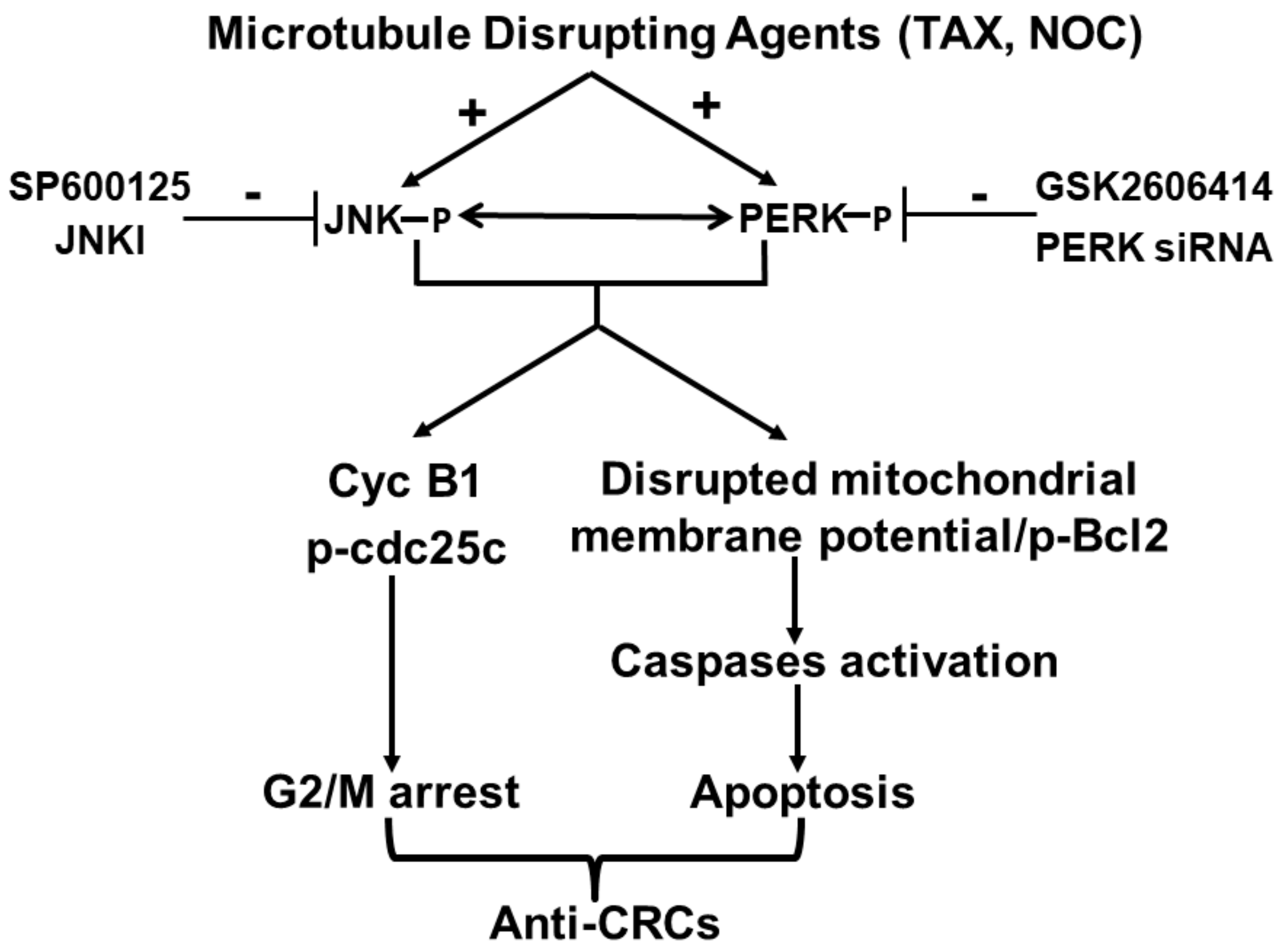
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-TUB | α-tubulin |
| ANOVA | analysis of variance |
| BCIP | 5-bromo-4-chloro-3-indole phosphate |
| Bcl-2 | B-cell lymphoma 2 |
| CRC | colorectal carcinoma |
| Casp | caspase |
| CycB1 | cyclin B1 |
| ELISA | enzyme-linked immunosorbent assay |
| ER | endoplasmic reticulum |
| FBS | fetal bovine serum |
| GSK | GSK2606414 |
| JNK | c-Jun N-terminal kinase |
| JNKI | JNK inhibitor |
| MAPK | mitogen-activated protein kinase |
| MEM | minimum essential medium |
| MTA | microtubule-targeting agent |
| MPF | M-phase-promoting factor |
| MTT | 3-(4,5,-dimethylthiazol)-2-yl-2,5-diphenyltetrazolium bromide |
| NBT | nitroblue tetrazolium |
| NOC | nocodazole |
| PARP | poly(ADP ribose) polymerase |
| PERK | protein kinase RNA-like endoplasmic reticular kinase |
| PI3K | phosphoinositide 3-kinase |
| SDS | sodium dodecylsulfate |
| SD | standard deviation |
| SP | SP600125 |
| TAX | taxol |
References
- Emmanuel, A.; Haji, A. Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: Is it worth that extra effort? A review of the literature. Int. J. Colorectal Dis. 2016, 31, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Abal, M.; Andreu, J.M.; Barasoain, I. Taxanes: Microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr. Cancer Drug Targets 2003, 3, 193–203. [Google Scholar] [CrossRef]
- Shi, J.; Mitchison, T.J. Cell death response to anti-mitotic drug treatment in cell culture, mouse tumor model and the clinic. Endocr. Relat. Cancer 2017, 24, T83–T96. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.; Wang, J.; Wang, Z.; Huang, D.; Yan, M.; Kamran, M.; Liu, Q.; Xu, B. Aurora kinase A stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J. Cell Mol. Med. 2019, 23, 6442–6453. [Google Scholar] [CrossRef]
- Florian, S.; Mitchison, T.J. Anti-Microtubule Drugs. Methods Mol. Biol. 2016, 1413, 403–421. [Google Scholar]
- Chang, W.L.; Yu, C.C.; Chen, C.S.; Guh, J.H. Tubulin-binding agents down-regulate matrix metalloproteinase-2 and -9 in human hormone-refractory prostate cancer cells—A critical role of Cdk1 in mitotic entry. Biochem. Pharmacol. 2015, 94, 12–21. [Google Scholar] [CrossRef]
- Stone, A.; Cowley, M.J.; Valdes-Mora, F.; McCloy, R.A.; Sergio, C.M.; Gallego-Ortega, D.; Caldon, C.E.; Ormandy, C.J.; Biankin, A.V.; Gee, J.M.; et al. BCL-2 hypermethylation is a potential biomarker of sensitivity to antimitotic chemotherapy in endocrine-resistant breast cancer. Mol. Cancer Ther. 2013, 12, 1874–1885. [Google Scholar] [CrossRef]
- Bonfoco, E.; Ceccatelli, S.; Manzo, L.; Nicotera, P. Colchicine induces apoptosis in cerebellar granule cells. Exp. Cell Res. 1995, 218, 189–200. [Google Scholar] [CrossRef]
- Bumbasirević, V.; Skaro-Milić, A.; Mircić, A.; Djuricić, B. Apoptosis induced by microtubule disrupting drugs in normal murine thymocytes in vitro. Scanning Microsc. 1995, 9, 509–516. [Google Scholar] [PubMed]
- Lindenboim, L.; Haviv, R.; Stein, R. Inhibition of drug-induced apoptosis by survival factors in PC12 cells. J. Neurochem. 1995, 64, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Parekh, H.; Simpkins, H. The transport and binding of taxol. Gen. Pharmacol. 1997, 29, 167–172. [Google Scholar] [CrossRef]
- Trudeau, M.E. First-line treatment of metastatic breast cancer. Anticancer Drugs 1996, 7 (Suppl. 2), 9–12. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Fallis, A.G.; Brown, D.L. Differential effects of taxol on two human cancer cell lines. Oncol. Res. 1997, 9, 237–248. [Google Scholar] [PubMed]
- Eckert, K.; Fuhrmann-Selter, T.; Maurer, H.R. Docetaxel enhances the expression of E-cadherin and carcinoembryonic antigen (CEA) on human colon cancer cell lines in vitro. Anticancer Res. 1997, 17, 7–12. [Google Scholar]
- Hui, A.; Kulkarni, G.V.; Hunter, W.L.; McCulloch, C.A.; Cruz, T.F. Paclitaxel selectively induces mitotic arrest and apoptosis in proliferating bovine synoviocytes. Arthritis Rheum. 1997, 40, 1073–1084. [Google Scholar] [CrossRef]
- Olah, E.; Csokay, B.; Prajda, N.; Kote-Jarai, Z.; Yeh, Y.A.; Weber, G. Molecular mechanisms in the antiproliferative action of taxol and tiazofurin. Anticancer Res. 1996, 16, 2469–2477. [Google Scholar]
- Valenti, A.M.; Niero, A.; Monti, G.; Marangolo, F.; Marangolo, M. Paclitaxel and N-methylformamide: In vitro interactions in human colon cancer cell line. Anticancer Res. 1997, 17, 2491–2497. [Google Scholar]
- Han, C.R.; Lee, J.Y.; Kim, Y.H. Prometaphase arrest-dependent phosphorylation of Bcl-2 and Bim reduces the association of Bcl-2 with Bak or Bim, provoking Bak activation and mitochondrial apoptosis in nocodazole-treated Jurkat T cells. Apoptosis 2014, 19, 224–240. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Li, Y.; Guo, Y.; Wang, J.; Li, Y.; Shen, B.; Sun, D.; Zhang, J. Nocodazole increases the ERK activity to enhance MKP-1expression which inhibits p38 activation induced by TNF-α. Mol. Cell Biochem. 2012, 364, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.M.; Schröder, M. Sensing endoplasmic reticulum stress. Adv. Exp. Med. Biol. 2012, 738, 153–168. [Google Scholar] [PubMed]
- Tian, H.; Li, Y.; Kang, P.; Wang, Z.; Yue, F.; Jiao, P.; Yang, N.; Qin, S.; Yao, S. Endoplasmic reticulum stress-dependent autophagy inhibits glycated high-density lipoprotein-induced macrophage apoptosis by inhibiting CHOP pathway. J. Cell Mol. Med. 2019, 23, 2954–2969. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Chien, C.C.; Chang, J.; Chen, Y.C. Pro-apoptotic effect of haem oxygenase-1 in human colorectal carcinoma cells via endoplasmic reticular stress. J. Cell Mol. Med. 2019, 23, 5692–5704. [Google Scholar] [CrossRef]
- Doyle, K.M.; Kennedy, D.; Gorman, A.M.; Gupta, S.; Healy, S.J.; Samali, A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell Mol. Med. 2011, 15, 2025–2039. [Google Scholar] [CrossRef]
- Wu, W.S.; Chien, C.C.; Chen, Y.C.; Chiu, W.T. Protein Kinase RNA-Like Endoplasmic Reticulum Kinase-Mediated Bcl-2 Protein Phosphorylation Contributes to Evodiamine-Induced Apoptosis of Human Renal Cell Carcinoma Cells. PLoS ONE 2016, 11, e0160484. [Google Scholar] [CrossRef]
- Chen, T.C.; Chien, C.C.; Wu, M.S.; Chen, Y.C. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 2016, 23, 68–78. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, W.; Zhang, D.; Pang, Y.; Shi, J.; Wan, S.; Cheng, K.; Wang, J.; Cheng, S. Circulating tumor cell detection in hepatocellular carcinoma based on karyoplasmic ratios using imaging flow cytometry. Sci. Rep. 2016, 6, 39808. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Saqcena, M.; Chatterjee, A.; Garcia, A.; Frias, M.A.; Foster, D.A. Reciprocal regulation of AMP-activated protein kinase and phospholipase D. J. Biol. Chem. 2015, 290, 6986–6993. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Donehower, R.C. Paclitaxel (taxol). N. Engl. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yoo, Y.H. Taxol-induced growth arrest and apoptosis is associated with the upregulation of the Cdk inhibitor, p21WAF1/CIP1, in human breast cancer cells. Oncol. Rep. 2012, 28, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Wagenknecht, B.; Grimmel, C.; Dichgans, J.; Weller, M. Taxol-mediated augmentation of CD95 ligand-induced apoptosis of human malignant glioma cells: Association with bcl-2 phosphorylation but neither activation of p53 nor G2/M cell cycle arrest. Br. J. Cancer. 1998, 77, 404–411. [Google Scholar] [CrossRef][Green Version]
- Doree, M.; Labbe, J.C.; Picard, A. M phase-promoting factor: Its identification as the M phase-specific H1 histone kinase and its activation by dephosphorylation. J. Cell Sci. 1989, 1989, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Duesbery, N.S.; Vande Woude, G.F. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. Biol. Cell. 1998, 90, 461–466. [Google Scholar] [PubMed]
- Dalal, S.N.; Schweitzer, C.M.; Gan, J.; De Caprio, J.A. Cytoplasmic localization of human cdc25C during interphase requires an intact 14-3-3 binding site. Mol. Cell Biol. 1999, 19, 4465–4479. [Google Scholar] [CrossRef]
- Kristjansdottir, K.; Rudolph, J. Cdc25 phosphatases and cancer. Chem. Biol. 2004, 11, 1043–1051. [Google Scholar] [CrossRef]
- Cho, Y.C.; Park, J.E.; Park, B.C.; Kim, J.H.; Jeong, D.G.; Park, S.G.; Cho, S. Cell cycle-dependent Cdc25C phosphatase determines cell survival by regulating apoptosis signal-regulating kinase 1. Cell Death Differ. 2015, 22, 1605–1617. [Google Scholar] [CrossRef]
- Ruvolo, P.P.; Deng, X.; May, W.S. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001, 15, 515–522. [Google Scholar] [CrossRef]
- Brichese, L.; Cazettes, G.; Valette, A. JNK is associated with Bcl-2 and PP1 in mitochondria: Paclitaxel induces its activation and its association with the phosphorylated form of Bcl-2. Cell Cycle 2004, 3, 1312–1319. [Google Scholar] [CrossRef][Green Version]
- Arlt, A.; Müerköster, S.S.; Schäfer, H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett. 2013, 332, 346–358. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.P.; Huang, H.M.; Shen, S.C.; Lee, W.R.; Chen, Y.C. Nilotinib induction of melanogenesis via reactive oxygen species-dependent JNK activation in B16F0 mouse melanoma cells. Exp. Dermatol. 2018, 27, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Yang, L.Y.; Shen, S.C.; Chen, Y.C. IGF-I plus E2 induces proliferation via activation of ROS-dependent ERKs and JNKs in human breast carcinoma cells. J. Cell Physiol. 2007, 212, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S.; Chien, C.C.; Liu, K.H.; Chen, Y.C.; Chiu, W.T. Evodiamine Prevents Glioma Growth, Induces Glioblastoma Cell Apoptosis and Cell Cycle Arrest through JNK Activation. Am. J. Chin. Med. 2017, 45, 879–899. [Google Scholar] [CrossRef]
- Sheikh, A.; Takatori, A.; Hossain, M.S.; Hasan, M.K.; Tagawa, M.; Nagase, H.; Nakagawara, A. Unfavorable neuroblastoma prognostic factor NLRR2 inhibits cell differentiation by transcriptional induction through JNK pathway. Cancer Sci. 2016, 107, 1223–1232. [Google Scholar] [CrossRef]
- Yu, T.; Liu, K.; Wu, Y.; Fan, J.; Chen, J.; Li, C.; Zhu, G.; Wang, Z.; Li, L. High interstitial fluid pressure promotes tumor cell proliferation and invasion in oral squamous cell carcinoma. Int. J. Mol. Med. 2013, 32, 1093–1100. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Y.; Peng, W. Colchicine induces apoptosis in HT-29 human colon cancer cells via the AKT and c-Jun N-terminal kinase signaling pathways. Mol. Med. Rep. 2015, 12, 5939–5944. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-induced cell death: Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Sánchez-Pérez, T.; Ortiz-Ferrón, G.; López-Rivas, A. Mitotic arrest and JNK-induced proteasomal degradation of FLIP and Mcl-1 are key events in the sensitization of breast tumor cells to TRAIL by antimicrotubule agents. Cell Death Differ. 2010, 17, 883–894. [Google Scholar] [CrossRef]
- Cellurale, C.; Sabio, G.; Kennedy, N.J.; Das, M.; Barlow, M.; Sandy, P.; Jacks, T.; Davis, R.J. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol. Cell Biol. 2011, 31, 1565–1576. [Google Scholar] [CrossRef]
- Saqcena, M.; Mukhopadhyay, S.; Hosny, C.; Alhamed, A.; Chatterjee, A.; Foster, D.A. Blocking anaplerotic entry of glutamine into the TCA cycle sensitizes K-Ras mutant cancer cells to cytotoxic drugs. Oncogene 2015, 34, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Saqcena, M.; Foster, D.A. Synthetic lethality in KRas-driven cancer cells created by glutamine deprivation. Oncoscience 2015, 2, 807–808. [Google Scholar] [PubMed]
- Fan, Y.; Simmen, T. Mechanistic Connections between Endoplasmic Reticulum (ER) Redox Control and Mitochondrial Metabolism. Cells 2019, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
- Gagliostro, V.; Casas, J.; Caretti, A.; Abad, J.L.; Tagliavacca, L.; Ghidoni, R.; Fabrias, G.; Signorelli, P. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 2012, 44, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Y.; Hua, H.; Li, M.; Luo, T.; Xu, L.; Wang, R.; Liu, D.; Zhang, Y.; Jiang, Y. Blockade of GRP78 sensitizes breast cancer cells to microtubules-interfering agents that induce the unfolded protein response. J. Cell Mol. Med. 2009, 13, 3888–3897. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Leng, A.M.; Zhang, Y.Q.; Wen, Z.; He, J.; Ye, G.N. CUEDC2: Multifunctional roles in carcinogenesis. Front. Biosci. (Landmark Ed.) 2019, 24, 935–946. [Google Scholar]
- Koliarakis, I.; Psaroulaki, A.; Nikolouzakis, T.K.; Kokkinakis, M.; Sgantzos, M.N.; Goulielmos, G.; Androutsopoulos, V.P.; Tsatsakis, A.; Tsiaoussis, J. Intestinal microbiota and colorectal cancer: A new aspect of research. J. BUON. 2018, 23, 1216–1234. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-S.; Chien, C.-C.; Jargalsaikhan, G.; Ilsan, N.A.; Chen, Y.-C. Activation of PERK Contributes to Apoptosis and G2/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells. Cancers 2020, 12, 97. https://doi.org/10.3390/cancers12010097
Wu M-S, Chien C-C, Jargalsaikhan G, Ilsan NA, Chen Y-C. Activation of PERK Contributes to Apoptosis and G2/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells. Cancers. 2020; 12(1):97. https://doi.org/10.3390/cancers12010097
Chicago/Turabian StyleWu, Ming-Shun, Chih-Chiang Chien, Ganbolor Jargalsaikhan, Noor Andryan Ilsan, and Yen-Chou Chen. 2020. "Activation of PERK Contributes to Apoptosis and G2/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells" Cancers 12, no. 1: 97. https://doi.org/10.3390/cancers12010097
APA StyleWu, M.-S., Chien, C.-C., Jargalsaikhan, G., Ilsan, N. A., & Chen, Y.-C. (2020). Activation of PERK Contributes to Apoptosis and G2/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells. Cancers, 12(1), 97. https://doi.org/10.3390/cancers12010097




