Real-World Data on Cabozantinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma: Focus on Sequences and Prognostic Factors
Abstract
:1. Introduction
2. Results
2.1. Overall Population
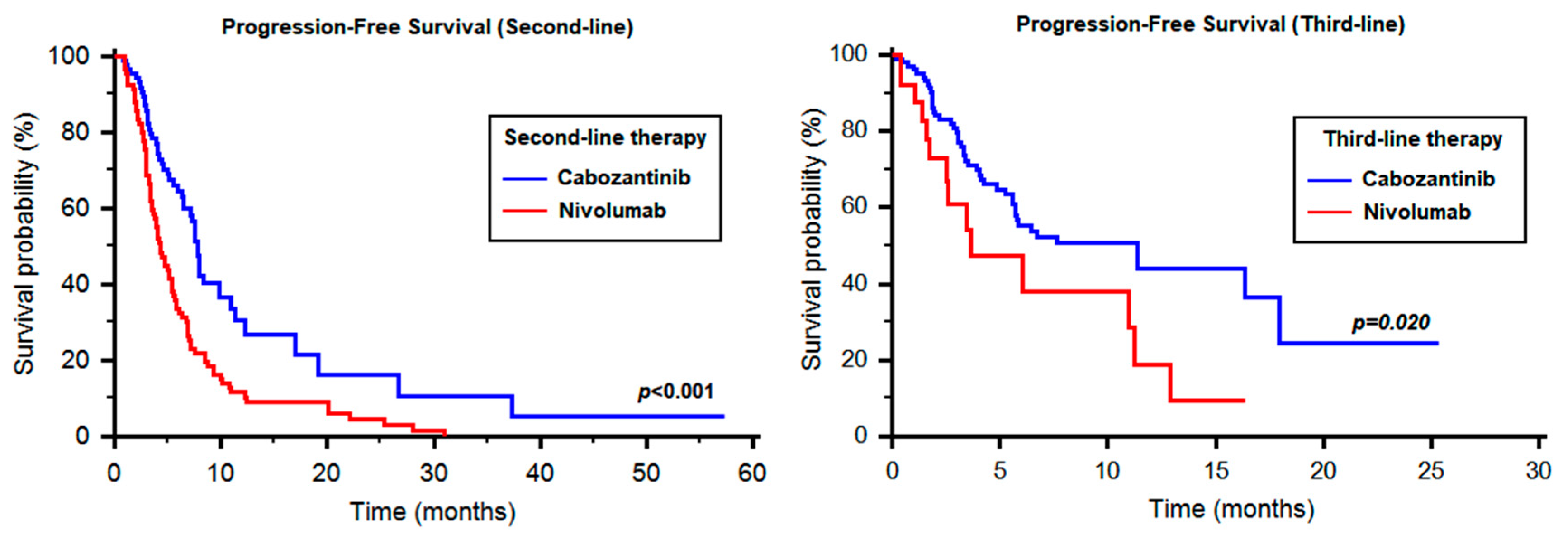
2.2. Progression-Free Survival of Cabozantinib as Second-Line Therapy
2.3. Overall Survival of Cabozantinib as Second-Line Therapy
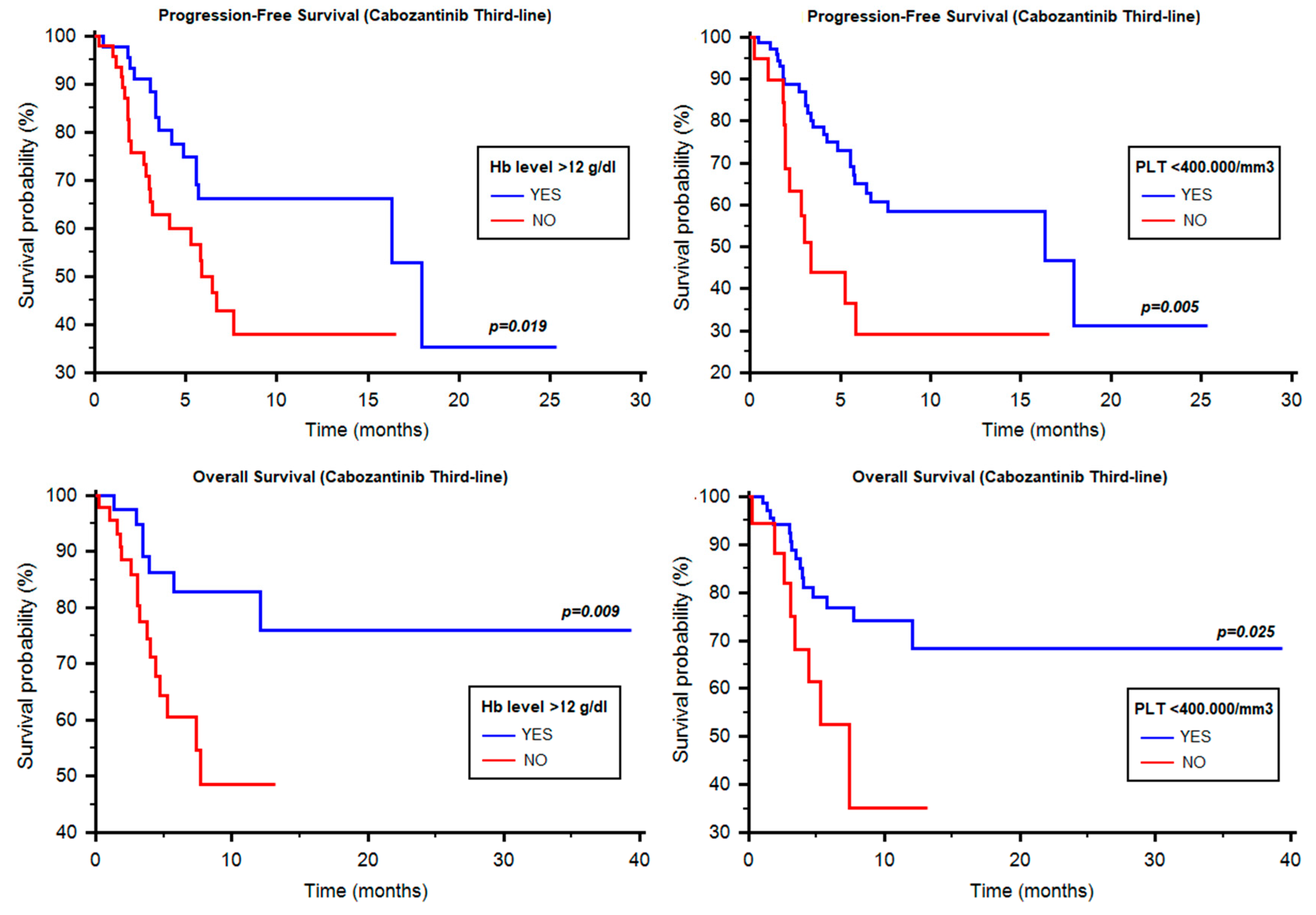
2.4. Progression-Free Survival of Cabozantinib as Third-Line Therapy
2.5. Overall Survival of Cabozantinib as Third-Line Therapy
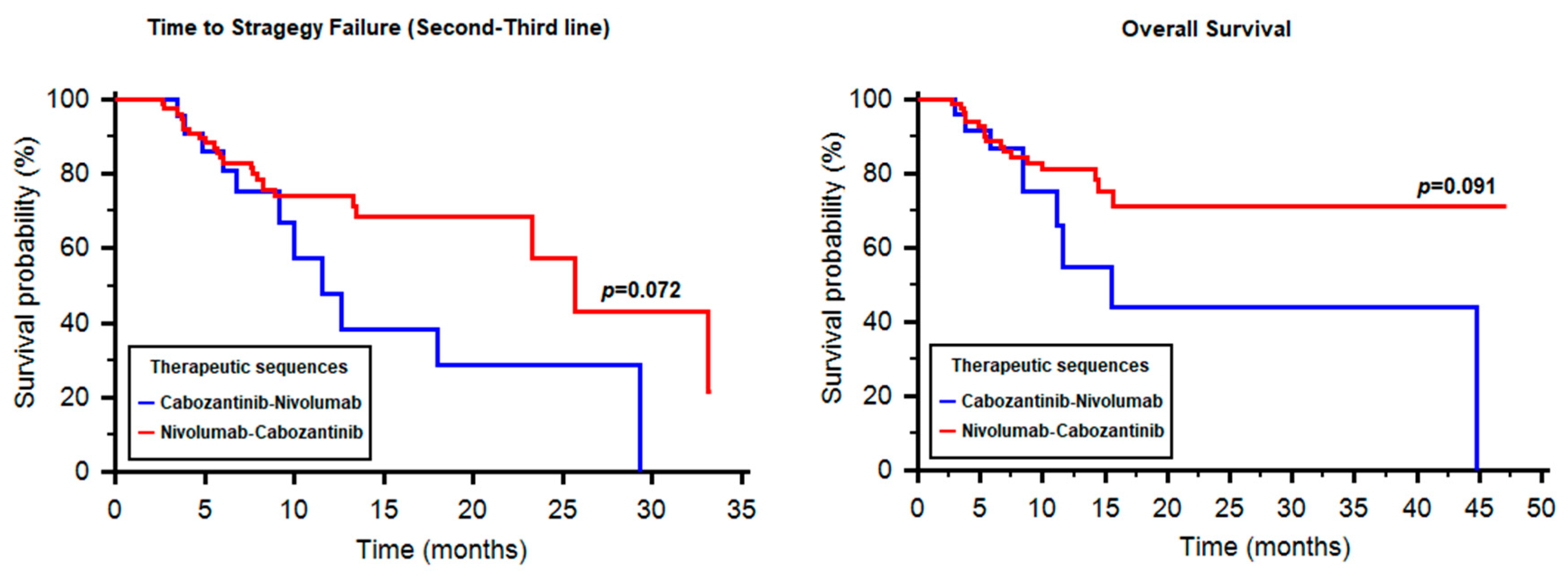
2.6. Time to Strategy Failure and Sequencing: Cabozantinib vs. Nivolumab
3. Discussion
4. Patients and Methods
4.1. Study Population
4.2. Treatment Regimens and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomized phase 3 trial. Lancet Oncol. 2013, 14, 552–562. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Escudier, B.; Pluzanska, A.; Koralewski, P.; Ravaud, A.; Bracarda, S.; Szczylik, C.; Chevreau, C.; Filipek, M.; Melichar, B.; Bajetta, E.; et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: Arandomised, double-blind phase III trial. Lancet 2007, 370, 2103–2111. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Gore, M.E.; Szczylik, C.; Porta, C.; Bracarda, S.; Bjarnason, G.A.; Oudard, S.; Lee, S.H.; Haanen, J.; Castellano, D.; Vrdoljak, E.; et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br. J. Cancer 2015, 113, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Nosov, D.; Eisen, T.; Bondarenko, I.; Lesovoy, V.; Lipatov, O.; Tomczak, P.; Lyulko, O.; Alyasova, A.; Harza, M.; et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J. Clin. Oncol. 2013, 31, 3791–3799. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Piva, F.; Carrozza, F.; Di Nunno, V.; Cimadamore, A.; Martignetti, A.; Montironi, R.; Battelli, N. Tivozanib for the treatment of renal cell carcinoma. Expert Opin. Pharmacother. 2018, 19, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Di Nunno, V.; Cubelli, M.; Massari, F. The role of the MET/AXL pathway as a new target for multikinase inhibitors in renal cell carcinoma. Expert Rev. Precis. Med. Drug Dev. 2017, 2, 169–175. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Mainwaring, P.N.; Rini, B.I.; Donskov, F.; Hammers, H.; Hutson, T.E.; Lee, J.L.; Peltola, K.; et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1814–1823. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib as initial targeted therapy for patients with metastatic renal cell Carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Hessel, C.; Halabi, S.; Sanford, B.; Michaelson, M.D.; Hahn, O.; Walsh, M.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer 2018, 94, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Buti, S.; Bersanelli, M. Is Cabozantinib Really Better Than Sunitinib As First-Line Treatment of Metastatic Renal Cell Carcinoma? J. Clin. Oncol. 2017, 35, 1858–1859. [Google Scholar] [CrossRef]
- Debeljak, N.; Solár, P.; Sytkowski, A.J. Erythropoietin and Cancer: The Unintended Consequences of Anemia Correction. Front. Immunol. 2014, 5, 563. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Nolley, R.; Chan, A.M.W.; Rankin, E.B.; Peehl, D.M. Cabozantinib inhibits tumor growth and metastasis of a patient-derived xenograft model of papillary renal cell carcinoma with MET mutation. Cancer Biol. Ther. 2017, 18, 863–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]


| Clinicopathological Features | N. of Patients (%) |
|---|---|
| Age | |
| Median | 62.56y |
| Range | 24.55–85.76y |
| Gender | |
| Male | 174 (73.42) |
| Female | 63 (26.58) |
| T-Stage at Diagnosis | |
| T1 | 37 (15.61) |
| T2 | 35 (14.77) |
| T3 | 97 (40.93) |
| T4 | 26 (10.97) |
| Unknown | 42 (17.72) |
| Histology | |
| Clear-cell RCC | 182 (76.79) |
| Non-clear-cell RCC | 55 (23.21) |
| Fuhrman or WHO/ISUP Grade | |
| Grade 1 | 4 (1.69) |
| Grade 2 | 62 (26.16) |
| Grade 3 | 86 (36.39) |
| Grade 4 | 32 (13.50) |
| Unknown | 59 (22.36) |
| N. of Metastatic Sites at Recurrence | |
| 1 site | 77 (32.49) |
| ≥2 sites | 160 (67.51) |
| Site of Metastasis | |
| Lung | 154 (64.98) |
| Lymph nodes | 133 (56.12) |
| Bone | 80 (34.04) |
| Liver | 53 (22.36) |
| Brain | 20 (8.44) |
| IMDC Risk Group | |
| Good | 57 (24.05) |
| Intermediate | 146 (61.60) |
| Poor | 34 (14.35) |
| First-Line Therapy | |
| Sunitinib | 141 (59.49) |
| Pazopanib | 81 (34.18) |
| Immunotherapy combinations | 9 (3.80) |
| Other | 6 (2.53) |
| Second-Line Therapy | 237 (100) |
| Cabozantinib | 112 (47.26) |
| Nivolumab | 89 (37.55) |
| Axitinib | 19 (8.01) |
| Everolimus | 14 (5.91) |
| Other | 3 (1.27) |
| Third-Line Therapy | 178 (100) |
| Cabozantinib | 125 (70.22) |
| Nivolumab | 29 (16.29) |
| Other | 24 (13.49) |
| IMDC Criteria | N of Patients (%) |
|---|---|
| <1 y from Diagnosis to Systemic Therapy | |
| Yes | 120 (50.63) |
| No | 117 (49.37) |
| Performance Status < 80% (Karnofsky) | |
| Yes | 19 (8.02) |
| No | 214 (91.98) |
| Hb Level < LLN | |
| Yes | 88 (37.13) |
| No | 149 (62.87) |
| Calcium Level > ULN | |
| Yes | 21 (8.86) |
| No | 216 (91.14) |
| Neutrophil > ULN | |
| Yes | 29 (12.24) |
| No | 208 (87.76) |
| Platelets > ULN | |
| Yes | 31 (13.08) |
| No | 206 (86.92) |
| Groups | Second-Line Cabozantinib | Third-Line Cabozantinib | ||
|---|---|---|---|---|
| All Patients | PFS [Median (95% CI)] | OS [Median (95% CI)] | PFS [Median (95% CI)] | OS [Median (95% CI)] |
| 7.76 (6.51–10.88) | 11.57 (10.90–NR) | 11.38 (5.79–NR) | NR (11.5–NR) | |
| Second-line Cabozantinib (29 patients, 25.9%) | Third-line Cabozantinib (28 patients, 22.4%) | |||
| Favourable Group | PFS [Median (95% CI)] | OS [Median (95% CI)] | PFS [Median (95% CI)] | OS [Median (95% CI)] |
| 11.28 (7.89–NR) | 12.53 (11.57–NR) | 11.38 (4.24–NR) | NR (7.40–NR) | |
| Second-line Cabozantinib (64 patients, 57.1%) | Third-line Cabozantinib (78 patients, 68.4%) | |||
| Intermediate Group | PFS [Median (95% CI)] | OS [Median (95% CI)] | PFS [Median (95% CI)] | OS [Median (95% CI)] |
| 7.59 (5.52–NR) | 10.95 (9.11–NR) | 7.63 (5.56–NR) | NR (11.51–NR) | |
| Second-line Cabozantinib (19 patients, 17.0%) | Third-line Cabozantinib (19 patients, 9.2%) | |||
| Poor-Risk Group | PFS [Median (95% CI)] | OS [Median (95% CI)] | PFS [Median (95% CI)] | OS [Median (95% CI)] |
| 7.13 (2.66–NR) | 11.05 (7.46–NR) | 5.75 (3.19–NR) | NR (4.01–NR) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoni, M.; Heng, D.Y.; Bracarda, S.; Procopio, G.; Milella, M.; Porta, C.; Matrana, M.R.; Cartenì, G.; Crabb, S.J.; De Giorgi, U.; et al. Real-World Data on Cabozantinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma: Focus on Sequences and Prognostic Factors. Cancers 2020, 12, 84. https://doi.org/10.3390/cancers12010084
Santoni M, Heng DY, Bracarda S, Procopio G, Milella M, Porta C, Matrana MR, Cartenì G, Crabb SJ, De Giorgi U, et al. Real-World Data on Cabozantinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma: Focus on Sequences and Prognostic Factors. Cancers. 2020; 12(1):84. https://doi.org/10.3390/cancers12010084
Chicago/Turabian StyleSantoni, Matteo, Daniel Y. Heng, Sergio Bracarda, Giuseppe Procopio, Michele Milella, Camillo Porta, Marc R. Matrana, Giacomo Cartenì, Simon J. Crabb, Ugo De Giorgi, and et al. 2020. "Real-World Data on Cabozantinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma: Focus on Sequences and Prognostic Factors" Cancers 12, no. 1: 84. https://doi.org/10.3390/cancers12010084
APA StyleSantoni, M., Heng, D. Y., Bracarda, S., Procopio, G., Milella, M., Porta, C., Matrana, M. R., Cartenì, G., Crabb, S. J., De Giorgi, U., Basso, U., Masini, C., Calabrò, F., Vitale, M. G., Santini, D., Massari, F., Galli, L., Fornarini, G., Ricotta, R., ... Battelli, N. (2020). Real-World Data on Cabozantinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma: Focus on Sequences and Prognostic Factors. Cancers, 12(1), 84. https://doi.org/10.3390/cancers12010084













