Retropharyngeal Lymph Node Involvement in Oropharyngeal Carcinoma: Impact upon Risk of Distant Metastases and Survival Outcomes
Abstract
1. Introduction
2. Results
2.1. Whole Cohort
2.2. Cohort with p16 Status
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Identification of Retropharyngeal Lymph Nodes
4.3. Chemotherapy
4.4. Radiotherapy
4.5. Response Assessment and Follow-Up
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Anderson, C.M.; Kimple, R.J.; Lin, A.; Karam, S.D.; Margalit, D.N.; Chua, M.L.K. De-Escalation Strategies in HPV-Associated Oropharynx Cancer-Are we Putting the Cart Before the Horse? Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Blanchard, P. Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin. Transl. Radiat. Oncol. 2018, 8, 4–11. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Sturgis, E.M.; Burtness, B.; Ridge, J.A.; Ringash, J.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Huang, S.H.; Siu, L.L.; Waldron, J.; Zhao, H.; Perez-Ordonez, B.; Weinreb, I.; Kim, J.; Ringash, J.; Bayley, A.; et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 2013, 31, 543–550. [Google Scholar] [CrossRef]
- Faraji, F.; Eisele, D.W.; Fakhry, C. Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2017, 2, 10–18. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Adelstein, D.L.; Huang, S.H.; Koyfman, S.A.; Thorstad, W.; Hope, A.J.; Lewis, J.S., Jr.; Nussenbaum, B. First Site of Failure Analysis Incompletely Addresses Issues of Late and Unexpected Metastases in p16-Positive Oropharyngeal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1707–1708. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.K.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.N.; Ridge, J.A.; et al. Reply to B. O’Sullivan et al. J. Clin. Oncol. 2015, 33, 1708–1709. [Google Scholar] [CrossRef]
- Coskun, H.H.; Ferlito, A.; Medina, J.E.; Robbins, K.T.; Rodrigo, J.P.; Strojan, P.; Suarez, C.; Takes, R.P.; Woolgar, J.A.; Shaha, A.R.; et al. Retropharyngeal lymph node metastases in head and neck malignancies. Head Neck 2011, 33, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Komakula, S.; Chan, C.; Murphy, J.D.; Jiang, W.; Kong, C.; Lee-Enriquez, N.; Jensen, K.C.; Fischbein, N.J.; Le, Q.T. Radiologic assessment of retropharyngeal node involvement in oropharyngeal carcinomas stratified by HPV status. Radiother. Oncol. 2013, 109, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Gunn, G.B.; Debnam, J.M.; Fuller, C.D.; Morrison, W.H.; Frank, S.J.; Beadle, B.M.; Sturgis, E.M.; Glisson, B.S.; Phan, J.; Rosenthal, D.I.; et al. The impact of radiographic retropharyngeal adenopathy in oropharyngeal cancer. Cancer 2013, 119, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
- Bussels, B.; Hermans, R.; Reijnders, A.; Dirix, P.; Nuyts, S.; Van den Bogaert, W. Retropharyngeal nodes in squamous cell carcinoma of oropharynx: Incidence, localization, and implications for target volume. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 733–738. [Google Scholar] [CrossRef]
- Samuels, S.E.; Vainshtein, J.; Spector, M.E.; Ibrahim, M.; McHugh, J.B.; Tao, Y.; Schipper, M.; Worden, F.; Eisbruch, A. Impact of retropharyngeal adenopathy on distant control and survival in HPV-related oropharyngeal cancer treated with chemoradiotherapy. Radiother. Oncol. 2015, 116, 75–81. [Google Scholar] [CrossRef]
- Iyizoba-Ebozue, Z.; Murray, L.J.; Arunsingh, M.; Vaidyanathan, S.; Scarsbrook, A.F.; Prestwich, R.J.D. Incidence and patterns of retropharyngeal lymph node involvement in oropharyngeal carcinoma. Radiother. Oncol. 2019. [Google Scholar] [CrossRef]
- Gregoire, V.; Ang, K.; Budach, W.; Grau, C.; Hamoir, M.; Langendijk, J.A.; Lee, A.; Le, Q.T.; Maingon, P.; Nutting, C.; et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 2014, 110, 172–181. [Google Scholar] [CrossRef]
- Chung, E.J.; Kim, G.W.; Cho, B.K.; Cho, S.J.; Yoon, D.Y.; Rho, Y.S. Retropharyngeal lymph node metastasis in 54 patients with oropharyngeal squamous cell carcinoma who underwent surgery-based treatment. Ann. Surg. Oncol. 2015, 22, 3049–3054. [Google Scholar] [CrossRef]
- Moore, E.J.; Ebrahimi, A.; Price, D.L.; Olsen, K.D. Retropharyngeal lymph node dissection in oropharyngeal cancer treated with transoral robotic surgery. Laryngoscope 2013, 123, 1676–1681. [Google Scholar] [CrossRef]
- Lin, T.A.; Garden, A.S.; Elhalawani, H.; Elgohari, B.; Jethanandani, A.; Ng, S.P.; Mohamed, A.S.; Frank, S.J.; Glisson, B.S.; Debnam, J.M.; et al. Radiographic retropharyngeal lymph node involvement in human papillomavirus-associated oropharyngeal carcinoma: Patterns of involvement and impact on patient outcomes. Cancer 2019, 125, 1536–1546. [Google Scholar] [CrossRef]
- Spector, M.E.; Chinn, S.B.; Bellile, E.; Gallagher, K.K.; Kang, S.Y.; Moyer, J.S.; Prince, M.E.; Wolf, G.T.; Bradford, C.R.; McHugh, J.B.; et al. Exploration for an Algorithm for Deintensification to Exclude the Retropharyngeal Site From Advanced Oropharyngeal Squamous Cell Carcinoma Treatment. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Chan, J.Y.; Mydlarz, W.K.; Labruzzo, S.V.; Kiess, A.; Ha, P.K.; Aygun, N.; Agrawal, N. Retropharyngeal lymph node involvement in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Laryngoscope 2015, 125, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, M.; Rabalais, A.; Hagan, J.L.; Wood, C.G.; Ferris, R.L.; Walvekar, R.R. PET-CT staging of the neck in cancers of the oropharynx: Patterns of regional and retropharyngeal nodal metastasis. World J. Surg. Oncol. 2010, 8, 70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2018.
- AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2007.
- Zhang, G.Y.; Liu, L.Z.; Wei, W.H.; Deng, Y.M.; Li, Y.Z.; Liu, X.W. Radiologic criteria of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma treated with radiation therapy. Radiology 2010, 255, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Chua, D.T.; Sham, J.S.; Kwong, D.L.; Au, G.K.; Choy, D.T. Retropharyngeal lymphadenopathy in patients with nasopharyngeal carcinoma: A computed tomography-based study. Cancer 1997, 79, 869–877. [Google Scholar] [CrossRef]
- Matsubara, R.; Kawano, S.; Chikui, T.; Kiyosue, T.; Goto, Y.; Hirano, M.; Jinno, T.; Nagata, T.; Oobu, K.; Abe, K.; et al. Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. Acad. Radiol. 2012, 19, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kanematsu, M.; Watanabe, H.; Mizuta, K.; Aoki, M. Metastatic retropharyngeal lymph nodes: Comparison of CT and MR imaging for diagnostic accuracy. Eur. J. Radiol. 2014, 83, 1157–1162. [Google Scholar] [CrossRef]
- Liao, X.B.; Mao, Y.P.; Liu, L.Z.; Tang, L.L.; Sun, Y.; Wang, Y.; Lin, A.H.; Cui, C.Y.; Li, L.; Ma, J. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1368–1377. [Google Scholar] [CrossRef]
- Lonneux, M.; Hamoir, M.; Reychler, H.; Maingon, P.; Duvillard, C.; Calais, G.; Bridji, B.; Digue, L.; Toubeau, M.; Gregoire, V. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: A multicenter prospective study. J. Clin. Oncol. 2010, 28, 1190–1195. [Google Scholar] [CrossRef]
- Prestwich, R.J.; Sykes, J.; Carey, B.; Sen, M.; Dyker, K.E.; Scarsbrook, A.F. Improving target definition for head and neck radiotherapy: A place for magnetic resonance imaging and 18-fluoride fluorodeoxyglucose positron emission tomography? Clin. Oncol. 2012, 24, 577–589. [Google Scholar] [CrossRef]
- Chu, H.R.; Kim, J.H.; Yoon, D.Y.; Hwang, H.S.; Rho, Y.S. Additional diagnostic value of (18)F-FDG PET-CT in detecting retropharyngeal nodal metastases. Otolaryngol. Head Neck Surg. 2009, 141, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Kawabata, K.; Mitani, H.; Yonekawa, H.; Beppu, T.; Fukushima, H.; Sasaki, T. Treatment results for 84 patients with base of tongue cancer. Acta Otolaryngol. 2007, 127, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Billfalk-Kelly, A.; Yu, E.; Su, J.; O’Sullivan, B.; Waldron, J.; Ringash, J.; Bartlett, E.; Perez-Ordonez, B.; Weinreb, I.; Bayley, A.; et al. Radiologic Extranodal Extension Portends Worse Outcome in cN+ TNM-8 Stage I Human Papillomavirus-Mediated Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Westra, W.H. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010, 116, 2166–2173. [Google Scholar] [CrossRef]
- Prestwich, R.J.; Oksuz, D.C.; Dyker, K.; Coyle, C.; Sen, M. Feasibility and Efficacy of Induction Docetaxel, Cisplatin, and 5-Fluorouracil Chemotherapy Combined With Cisplatin Concurrent Chemoradiotherapy for Nonmetastatic Stage IV Head-and-Neck Squamous Cell Carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e237–e243. [Google Scholar] [CrossRef]
- Prestwich, R.J.; Kancherla, K.; Oksuz, D.C.; Williamson, D.; Dyker, K.E.; Coyle, C.; Sen, M. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat. Oncol. 2010, 5, 121. [Google Scholar] [CrossRef]
- Oksuz, D.C.; Prestwich, R.J.; Carey, B.; Wilson, S.; Senocak, M.S.; Choudhury, A.; Dyker, K.; Coyle, C.; Sen, M. Recurrence patterns of locally advanced head and neck squamous cell carcinoma after 3D conformal (chemo)-radiotherapy. Radiat. Oncol. 2011, 6, 54. [Google Scholar] [CrossRef]
- Bayman, E.; Prestwich, R.J.; Speight, R.; Aspin, L.; Garratt, L.; Wilson, S.; Dyker, K.E.; Sen, M. Patterns of failure after intensity-modulated radiotherapy in head and neck squamous cell carcinoma using compartmental clinical target volume delineation. Clin. Oncol. 2014, 26, 636–642. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Gregoire, V.; Levendag, P.; Ang, K.K.; Bernier, J.; Braaksma, M.; Budach, V.; Chao, C.; Coche, E.; Cooper, J.S.; Cosnard, G.; et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother. Oncol. 2003, 69, 227–236. [Google Scholar] [CrossRef]
- Spencer, C.R.; Gay, H.A.; Haughey, B.H.; Nussenbaum, B.; Adkins, D.R.; Wildes, T.M.; DeWees, T.A.; Lewis, J.S., Jr.; Thorstad, W.L. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer 2014, 120, 3994–4002. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total Cohort (Column %) n = 402 | RP Lymph Node Negative (Column %) n = 362 | RP Lymph Node Positive (Column %) n = 40 | p-Value |
|---|---|---|---|---|
| Age | 0.297 | |||
| Median (range) | 57 (24–84) | 57 (24–84) | 56 (37–73) | |
| Gender | 0.393 | |||
| Male | 310 (77.1) | 277 (76.5) | 33 (82.5) | |
| Female | 92 (22.9) | 85 (23.5) | 7 (17.5) | |
| Smoking | 0.868 | |||
| Never | 114 (28.4) | 103 (28.5) | 11 (27.5) | |
| Former | 137 (34.1) | 124 (34.3) | 13 (32.5) | |
| Current | 133 (33.1) | 118 (32.6) | 15 (37.5) | |
| Not recorded | 18 (4.5) | 17 (4.7) | 1 (2.5) | |
| p16 status | 0.266 | |||
| Positive | 192 (47.8) | 171 (47.2) | 21 (52.5) | |
| Negative | 34 (8.5) | 28 (7.7) | 6 (15.0) | |
| Unknown | 176 (43.8) | 163 (45.0) | 13 (32.5) | |
| Tumour subsite | 0.002 * | |||
| Tonsil | 241 (60.0) | 219 (60.5) | 22 (55.0) | |
| Base of Tongue | 135 (33.6) | 123 (34.0) | 12 (30.0) | |
| Vallecula | 9 (2.2) | 9 (2.5) | 0 (0) | |
| Postpharyngeal | 5 (1.2) | 4 (11.0) | 1 (2.5) | |
| Soft palate | 12 (3.0) | 7 (1.9) | 5 (12.5) | |
| T stage | 0.194 | |||
| T1 | 85 (21.1) | 78 (21.5) | 7 (17.5) | |
| T2 | 168 (41.8) | 156 (43.1) | 12 (30.0) | |
| T3 | 76 (18.9) | 66 (18.2) | 10 (25.0) | |
| T4 | 73 (18.2) | 62 (17.1) | 11 (27.5) | |
| N stage (AJCC 7th) | 0.005 | |||
| N0 | 41 (10.2) | 39 (10.8) | 1 (5.0) | |
| N1 | 45 (11.2) | 44 (12.2) | 2 (2.5) | |
| N2a | 30 (7.5) | 30 (8.3) | 0 (0) | |
| N2b | 199 (57.0) | 179 (49.4) | 20 (50.0) | |
| N2c | 74 (18.4) | 59 (16.3) | 15 (37.5) | |
| N3 | 13 (3.2) | 11 (3.0) | 2 (5.0) | |
| Stage (AJCC 7th) | 0.207 | |||
| 1 | 1 (0.2) | 1 (0.3) | 0 (0) | |
| 2 | 19 (4.7) | 17 (4.7) | 2 (5.0) | |
| 3 | 47 (11.7) | 47 (13.0) | 0 (0) | |
| 4 | 335 (83.3) | 297 (82.0) | 38 (95.0) | |
| Histological grade | 0.061 | |||
| Well | 3 (0.8) | 3 (0.8) | 0 (0) | |
| Moderate | 74 (18.4) | 62 (17.1) | 12 (30.0) | |
| Poor | 297 (73.9) | 272 (75.1) | 25 (62.5) | |
| Unclassified | 28 (6.9) | 25 (6.9) | 3 (7.5) | |
| Induction chemotherapy | 0.524 | |||
| No | 384 (95.6) | 345 (95.3) | 39 (97.5) | |
| PF | 1 (0.2) | 1 (0.3) | 0 (0) | |
| TPF | 17 (4.2) | 16 (4.4) | 1 (2.5) | |
| Concurrent chemotherapy | 0.707 | |||
| No | 77 (19.2) | 70 (19.3) | 7 (17.5) | |
| Yes | 325 (80.8) | 292 (80.7) | 33 (82.5) |
| Overall Survival | ||||||
| Univariate | Multivariate * | |||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.02 | 1.00–1.05 | 0.022 | |||
| Sex | 0.780 | |||||
| T stage | ||||||
| T2 vs. T1 | 2.27 | 1.05–4.88 | 0.037 | 2.30 | 1.02–5.20 | 0.046 |
| T3 vs. T1 | 2.91 | 1.28–6.64 | 0.011 | 2.40 | 1.00–5.79 | 0.051 |
| T4 vs. T1 | 4.62 | 2.11–10.43 | <0.001 | 3.64 | 1.58–8.43 | 0.003 |
| N stage | ||||||
| N1 vs. N0 | 0.37 | 0.11–1.19 | 0.094 | |||
| N2a vs. N0 | 0.230 | |||||
| N2b vs. N0 | 0.991 | |||||
| N2c vs. N0 | 0.344 | |||||
| N3 vs. N0 | 2.37 | 0.84–6.65 | 0.103 | |||
| RPLN status (+ve vs. −ve) | 2.13 | 1.22–3.71 | 0.008 | 2.00 | 1.13–3.54 | 0.018 |
| Concurrent chemotherapy (no vs. yes) | 2.17 | 1.39–3.38 | 0.001 | 2.02 | 1.29–3.19 | 0.002 |
| Smoking | ||||||
| Former vs. never | 1.92 | 0.97–3.83 | 0.063 | |||
| Current vs. never | 4.23 | 2.27–8.00 | <0.001 | 3.58 | 1.89–6.77 | <0.001 |
| Distant Metastasis–Free Survival | ||||||
| Univariate | Multivariate * | |||||
| Age | 0.513 | |||||
| Sex | 0.314 | |||||
| T stage | ||||||
| T2 vs. T1 | 2.01 | 0.75–5.38 | 0.166 | |||
| T3 vs. T1 | 2.44 | 0.83–7.14 | 0.104 | |||
| T4 vs. T1 | 3.84 | 1.38–10.7 | 0.010 | |||
| N stage | ||||||
| N1 vs. N0 | 0.582 | |||||
| N2a vs. N0 | 0.792 | |||||
| N2b vs. N0 | 2.82 | 0.67–11.88 | 0.157 | |||
| N2c vs. N0 | 3.76 | 0.85–11.67 | 0.081 | |||
| N3 vs. N0 | 4.13 | 0.58–2.32 | 0.156 | |||
| RPLN status (+ve vs. −ve) | 2.48 | 1.20–5.13 | 0.014 | 2.68 | 1.29–5.57 | 0.008 |
| Concurrent chemotherapy (no vs. yes) | 1.94 | 1.04–3.62 | 0.037 | 1.94 | 1.03–3.65 | 0.039 |
| Smoking | ||||||
| Former vs. never | 2.66 | 0.79–5.37 | 0.138 | |||
| Current vs. never | 4.36 | 1.79–10.55 | 0.001 | 4.17 | 1.71–10.16 | 0.002 |
| Overall Survival | ||||||
| Univariate | Multivariate * | |||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.03 | 0.99–1.06 | 0.111 | |||
| Sex | 0.51 | 0.20–1.30 | 0.158 | |||
| T stage | ||||||
| T2 vs. T1 | 0.324 | |||||
| T3 vs. T1 | 0.379 | |||||
| T4 vs. T1 | 4.59 | 1.51–13.97 | 0.007 | 3.67 | 1.19–11.44 | 0.024 |
| N stage | ||||||
| N1 vs. N0 | 0.942 | |||||
| N2a vs. N0 | 0.843 | |||||
| N2b vs. N0 | 0.660 | |||||
| N2c vs. N0 | 0.427 | |||||
| N3 vs. N0 | 0.244 | |||||
| p16 status (−ve vs. +ve) | 2.48 | 1.19–5.14 | 0.014 | 2.46 | 1.11–5.45 | 0.026 |
| RPLN status (+ve vs. −ve) | 2.11 | 0.97–4.59 | 0.060 | |||
| Concurrent chemotherapy (no vs. yes) | 2.01 | 0.95–4.24 | 0.066 | |||
| Smoking | ||||||
| Former vs. never | 0.553 | |||||
| Current vs. never | 3.43 | 1.53–7.67 | 0.003 | 2.41 | 1.03–5.65 | 0.043 |
| Distant Metastasis–Free Survival | ||||||
| Univariate | Multivariate * | |||||
| Age | 0.37 | |||||
| Sex | 0.64 | |||||
| T stage | ||||||
| T2 vs. T1 | 0.242 | |||||
| T3 vs. T1 | 6.24 | 0.75–51.80 | 0.090 | |||
| T4 vs. T1 | 10.55 | 1.32–84.43 | 0.026 | 11.36 | 1.42–91.04 | 0.022 |
| N stage | ||||||
| N1 vs. N0 | 0.934 | |||||
| N2a vs. N0 | 0.95 | |||||
| N2b vs. N0 | 0.935 | |||||
| N2c vs. N0 | 0.934 | |||||
| N3 vs. N0 | 0.93 | |||||
| p16 status (−ve vs. +ve) | 2.84 | 1.16–6.98 | 0.023 | 3.09 | 1.24–7.68 | 0.015 |
| RPLN status (+ve vs. −ve) | 2.41 | 0.86–6.53 | 0.084 | |||
| Concurrent chemotherapy (no vs. yes) | 2.07 | 0.76–5.61 | 0.153 | |||
| Smoking | ||||||
| Former vs. never | 0.833 | |||||
| Current vs. never | 3.32 | 1.15–9.56 | 0.026 | |||
| % RPLN+ | Imaging | Outcomes (RPLN+ versus RPLN−) | Summary | ||
|---|---|---|---|---|---|
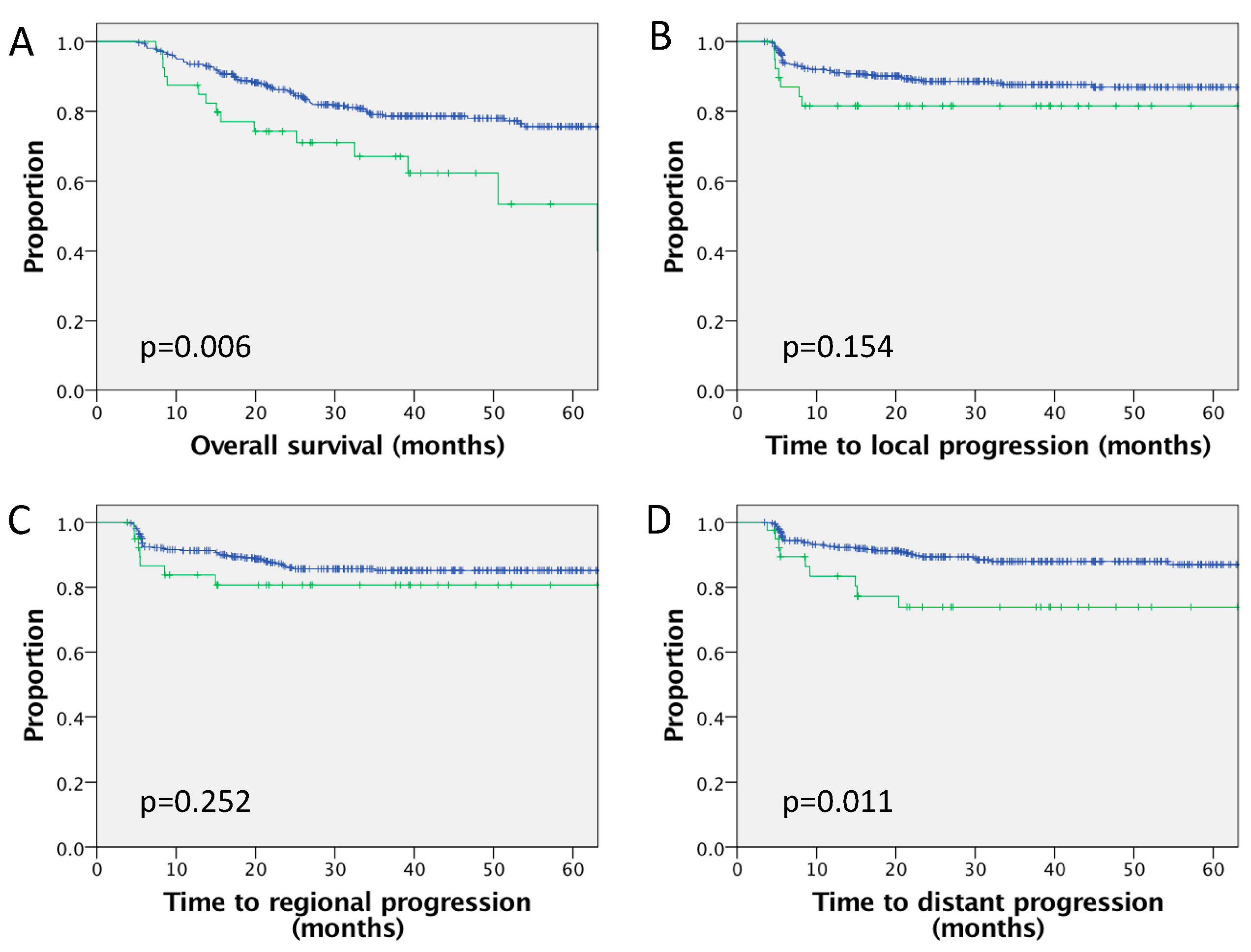
| Current study | n = 402 (p16 available in 226, of which 85% p16+) | 10% | PET-CT 100% MRI 84% CT 21% | Five-year LC 81.6% vs. 87.7%, p = 0.154 Five-year RC 80.7% vs. 85.4%, p = 0.47 Five-year DMFS 73.9% vs. 88.0%, p = 0.011 Five-year PFS 62.0% vs. 75.4%, p = 0.002 Five-year OS 67.1% vs. 79.1%, p = 0.006 Significant on MVA for DMFS, OS Nonsignificant trend for inferior DMFS, OS in p16+ve subgroup | RPLN associated with increased risk DM and inferior OS outcomes |
| Lin et al., 2019 * [17] | n = 739 N+ and HPV+ | 9% | RPLN identified by CT in 66%, PET-CT in 7%, CT and PET-CT in 26%, CT and MRI in 1% | Five-year LC 96% vs. 94%, p = 0.57 Five-year RC 95% vs. 93%, p = 0.47 Five-year DMFS 84% vs. 93%, p = 0.033 Five-year OS 74% vs. 87%, p = 0.008 Not significant for MVA RP+ associated with inferior DMFS in subgroup of smoking pack years < 10 or concurrent chemotherapy | Difficult to demonstrate association with outcome in HPV+ group. RPLN+ may not be suitable for deintensification |
| Billfalk-Kelly et al., 2019 [32] | n = 257, T1–2, N1 HPV+ (TNM8 stage 1 only) | 8% | CT 67%MRI 33% | DFS: HR 2.62, p = 0.021. Not significant for MVA Risk DM: bivariable analysis HR 3.2, p = 0.013 | RP+ associated with higher risk DM |
| Baxter et al., 2015 [19] | n = 165 HPV+ | 10% | 100% PET-CT | OR recurrence/death 5.2 No significant association for MVA with outcome | RP+ not independently associated with outcome |
| Samuels et al., 2015 [12] | n = 185 HPV+ | 16% | 67% PET-CT | Five-year OS 57% vs. 81% (p = 0.02) Five-year FFS 63% vs. 80% (p = 0.015) Five-year DMFS 70% vs. 91% (p = 0.002) No difference local or regional failure MVA: T4, N3 and RP+ independently associated with OS and DMFS | RP+ independent prognostic factor for DM, translating into inferior FFS/OS |
| Gunn et al., 2013 * [10] | n = 981 (No HPV status) | 10% | CT 96% PET 29% MRI 9% | Five-year LC 79% vs. 92%, p < 0.01 Five-year RC 80% vs. 93%, p < 0.01 Five-year DMFS 66% vs. 89%, p < 0.01 Five-year OS 52% vs. 82%, p < 0.01 Significant for MVA for LC (p = 0.023), DMFS (p = 0.003), OS (p = 0.001) | RPLN associated with inferior local recurrence and OS, increased risk of DM |
| Tang et al., 2013 [9] | n = 160 (p16+ve: n = 134) | 12% | MRI 46% PET-CT 48% CT 6% | Whole cohort: Two-year OS 71% vs. 89% (HR 2.4, p = 0.08) Two-year EFS 71% vs. 81% (HR 2.1, p = 0.08) p16+ve only: Two-year OS HR 2.1, p = 0.23 Two-year EFS HR 2.0, p = 0.16 | Nonsignificant trend to worse OS, EFS for RP+ in whole cohort and p16+ subgroup |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyizoba-Ebozue, Z.; Murray, L.J.; Arunsingh, M.; Dyker, K.E.; Vaidyanathan, S.; Scarsbrook, A.F.; Prestwich, R.J.D. Retropharyngeal Lymph Node Involvement in Oropharyngeal Carcinoma: Impact upon Risk of Distant Metastases and Survival Outcomes. Cancers 2020, 12, 83. https://doi.org/10.3390/cancers12010083
Iyizoba-Ebozue Z, Murray LJ, Arunsingh M, Dyker KE, Vaidyanathan S, Scarsbrook AF, Prestwich RJD. Retropharyngeal Lymph Node Involvement in Oropharyngeal Carcinoma: Impact upon Risk of Distant Metastases and Survival Outcomes. Cancers. 2020; 12(1):83. https://doi.org/10.3390/cancers12010083
Chicago/Turabian StyleIyizoba-Ebozue, Zsuzsanna, Louise J. Murray, Moses Arunsingh, Karen E. Dyker, Sriram Vaidyanathan, Andrew F. Scarsbrook, and Robin J. D. Prestwich. 2020. "Retropharyngeal Lymph Node Involvement in Oropharyngeal Carcinoma: Impact upon Risk of Distant Metastases and Survival Outcomes" Cancers 12, no. 1: 83. https://doi.org/10.3390/cancers12010083
APA StyleIyizoba-Ebozue, Z., Murray, L. J., Arunsingh, M., Dyker, K. E., Vaidyanathan, S., Scarsbrook, A. F., & Prestwich, R. J. D. (2020). Retropharyngeal Lymph Node Involvement in Oropharyngeal Carcinoma: Impact upon Risk of Distant Metastases and Survival Outcomes. Cancers, 12(1), 83. https://doi.org/10.3390/cancers12010083





