Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics
Abstract
1. Introduction
2. Methods
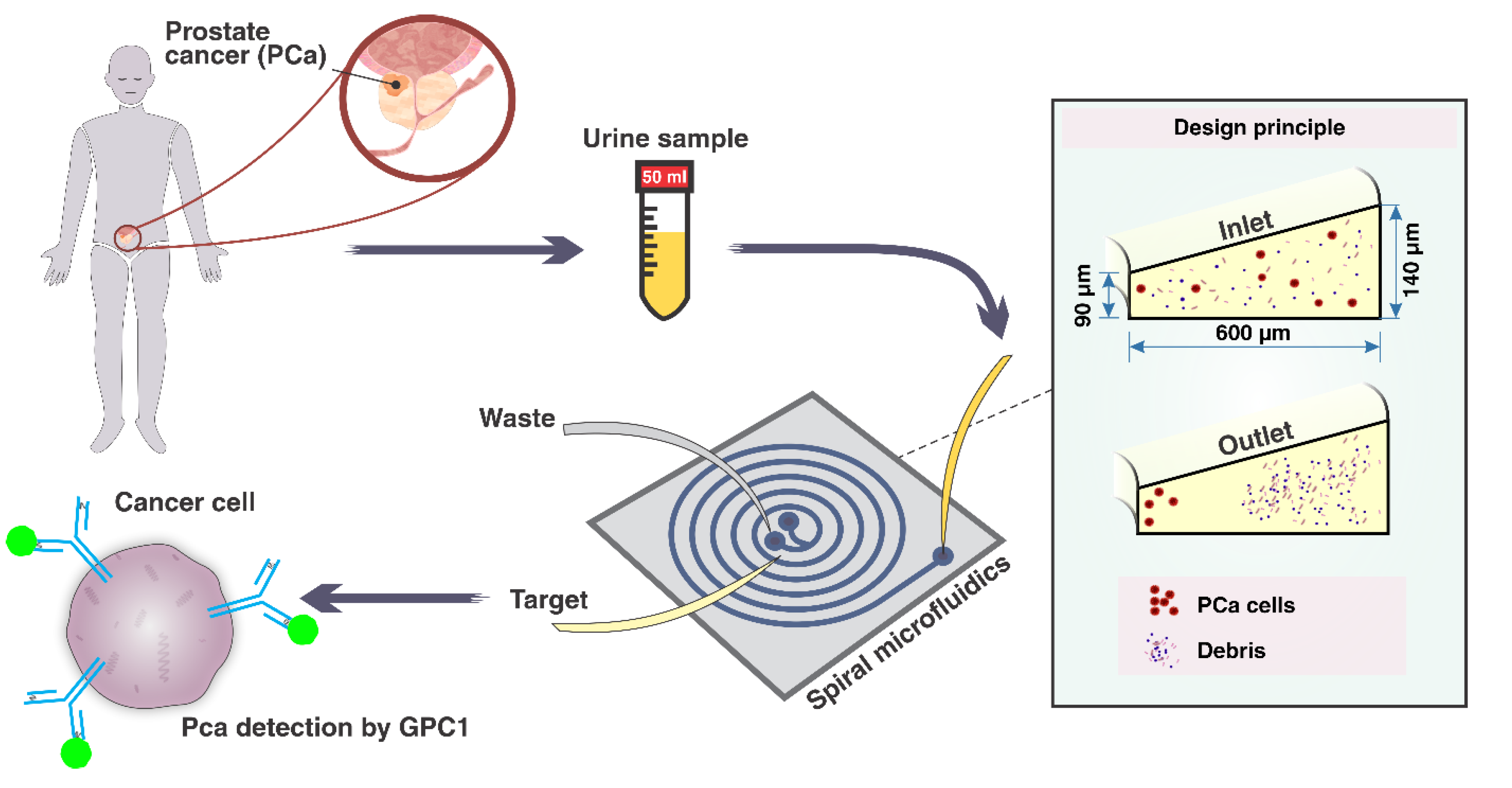
2.1. Device Design and Fabrication
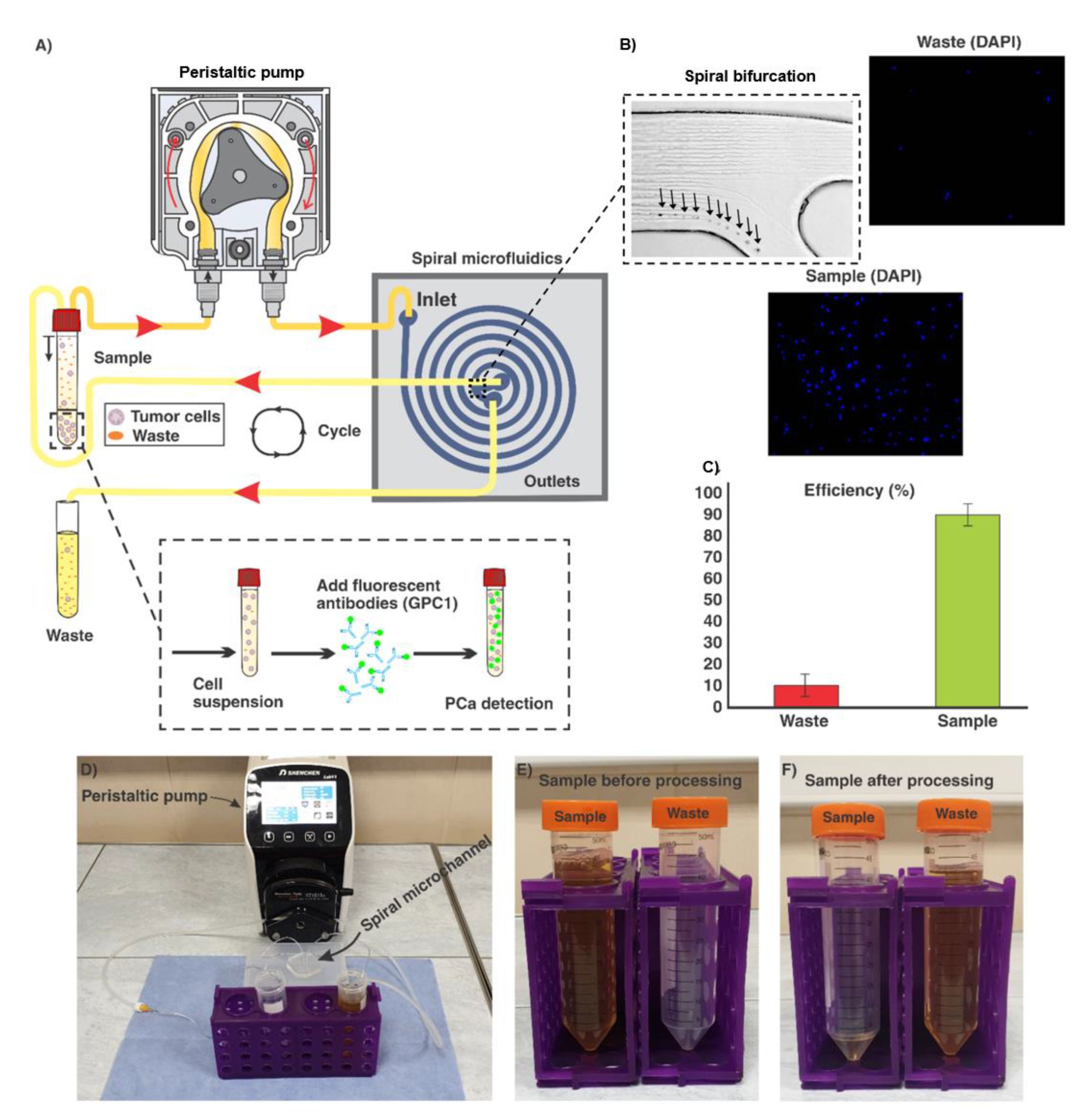
2.2. Experimental Setup and Procedure
2.3. Ethics Statement and Clinical Sample Preparation
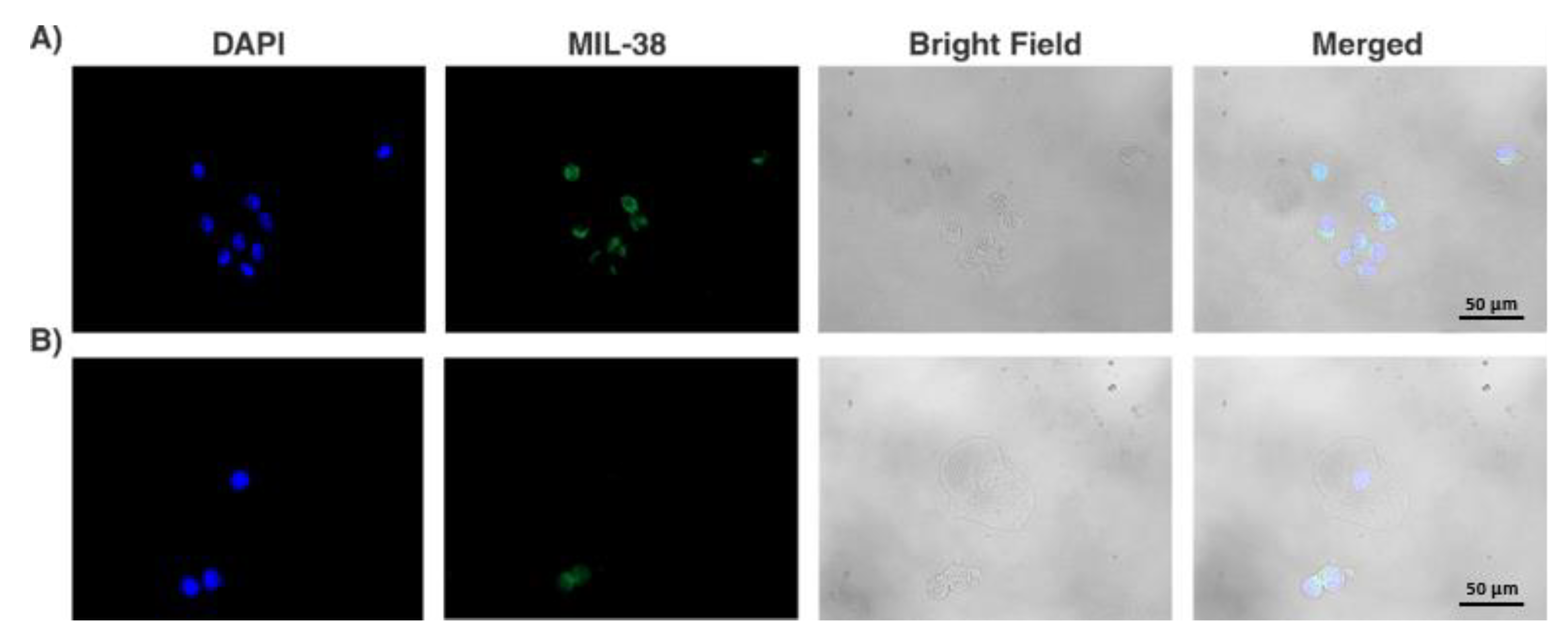
2.4. Immunofluorescence Staining
2.5. Cell Enumeration and Data Analysis
3. Results
3.1. Spiral Microfluidic Device
3.2. Collection of GPC-1+ cells from Urine
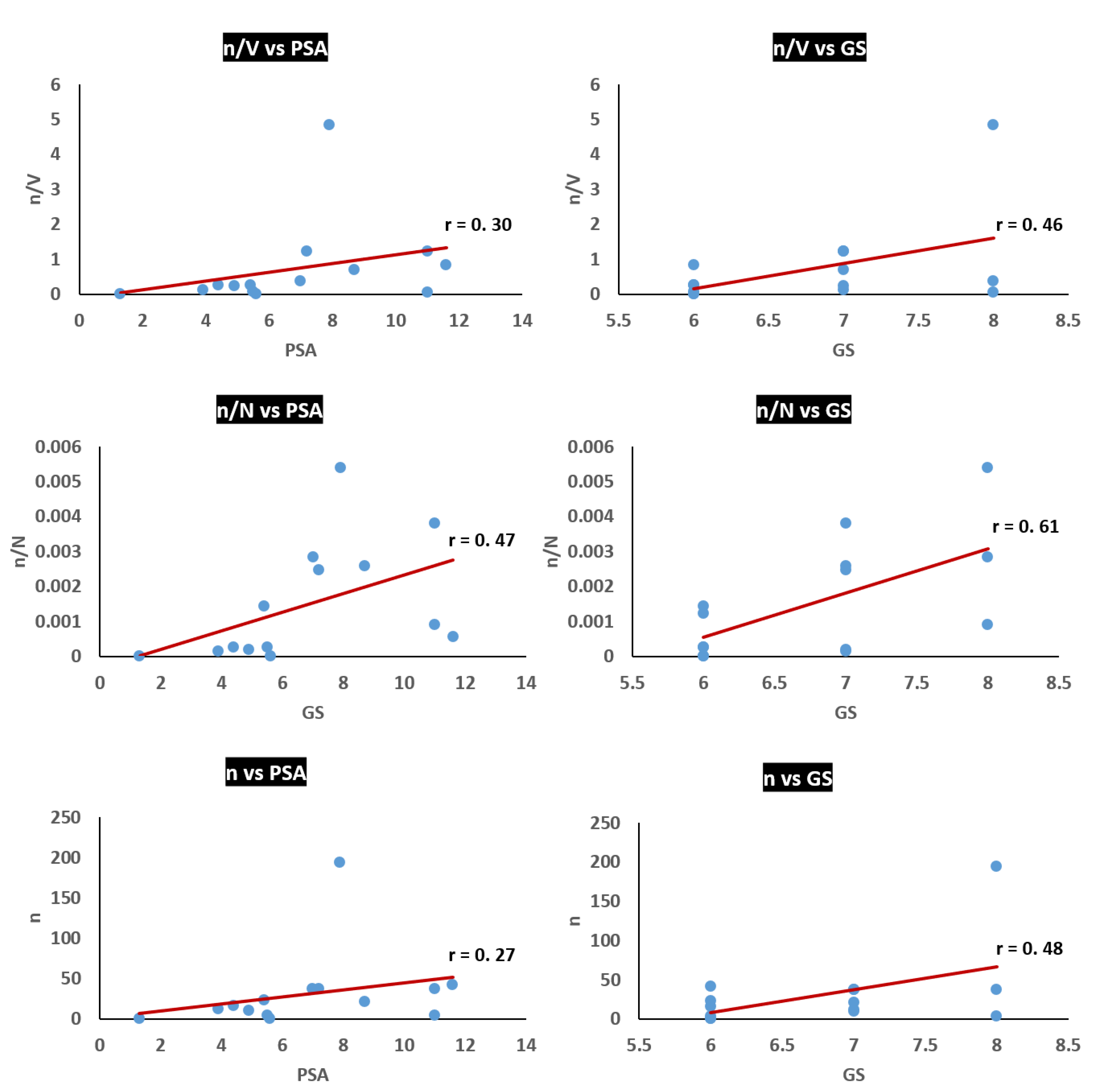
3.3. Analysis of Potential Diagnostic Applicability of the Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Thorat, M.A.; Andriole, G.; Brawley, O.W.; Brown, P.H.; Culig, Z.; Eeles, R.A.; Ford, L.G.; Hamdy, F.C.; Holmberg, L.; et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014, 15, e484–e492. [Google Scholar] [CrossRef]
- Ankerst, D.P.; Thompson, I.M. Sensitivity and specificity of prostate-specific antigen for prostate cancer detection with high rates of biopsy verification. Archivio Italiano Urologia Andrologia 2006, 78, 125–129. [Google Scholar]
- Pantel, K.; Hille, C.; Scher, H.I. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin. Chem. 2019, 65, 87–99. [Google Scholar] [CrossRef]
- Nickens, K.P.; Ali, A.; Scoggin, T.; Tan, S.; Ravindranath, L.; McLeod, D.G.; Dobi, A.; Tacha, D.; Sesterhenn, I.A.; Srivastava, S.; et al. Prostate cancer marker panel with single cell sensitivity in urine. Prostate 2015, 75, 969–975. [Google Scholar] [CrossRef]
- Woo, H.K.; Park, J.; Ku, J.Y.; Lee, C.H.; Sunkara, V.; Ha, H.K.; Cho, Y.K. Urine-based liquid biopsy: Non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab Chip 2019, 19, 87–97. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef]
- Winter, M.; Hardy, T.; Rezaei, M.; Nguyen, V.; Zander-Fox, D.; Ebrahimi Warkiani, M.; Thierry, B. Isolation of circulating fetal trophoblasts using inertial microfluidics for noninvasive prenatal testing. Adv. Mater. Technol. 2018, 3. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.; Chaudhuri, P.K.; Tan, D.S.; Lim, W.T.; Lee, S.C.; Chen, P.C.; et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 2014, 14, 128–137. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.; Bhagat, A.A.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134. [Google Scholar] [CrossRef]
- Condina, M.R.; Dilmetz, B.A.; Razavi Bazaz, S.; Meneses, J.; Warkiani, M.E.; Hoffmann, P. Rapid separation and identification of beer spoilage bacteria by inertial microfluidics and MALDI-TOF mass spectrometry. Lab Chip 2019, 19, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Rafeie, M.; Zhang, J.; Asadnia, M.; Li, W.; Warkiani, M.E. Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 2016, 16, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Tran, T.H.; Blick, T.; O’Byrne, K.; Thompson, E.W.; Warkiani, M.E.; Nelson, C.; Kenny, L.; Punyadeera, C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci. Rep. 2017, 7, 42517. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.S.; Rafeie, M.; Vandamme, D.; Asadnia, M.; Henderson, R.; Taylor, R.A.; Warkiani, M.E. Selective separation of microalgae cells using inertial microfluidics. Bioresour. Technol. 2018, 252, 1–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiu, Y.; Bai, B. The Expression, Regulation, and Biomarker Potential of Glypican-1 in Cancer. Front. Oncol. 2019, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.H.; Lund, M.E.; Nocon, A.L.; Cozzi, P.J.; Frydenberg, M.; De Souza, P.; Schiller, B.; Beebe-Dimmer, J.L.; Ruterbusch, J.J.; Walsh, B. Detection of glypican-1 (GPC-1) expression in urine cell sediments in prostate cancer. PLoS ONE 2018, 13, e0196017. [Google Scholar] [CrossRef]
- Quach, N.D.; Kaur, S.P.; Eggert, M.W.; Ingram, L.; Ghosh, D.; Sheth, S.; Nagy, T.; Dawson, M.R.; Arnold, R.D.; Cummings, B.S. Paradoxical Role of Glypican-1 in prostate cancer cell and tumor Growth. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Tay, A.K.; Khoo, B.L.; Xiaofeng, X.; Han, J.; Lim, C.T. Malaria detection using inertial microfluidics. Lab Chip 2015, 15, 1101–1109. [Google Scholar] [CrossRef]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.; Bhagat, A.A.; Irwin, D.; Lau, D.P.; Lim, A.S.; Lim, K.H.; Krisna, S.S.; Lim, W.T.; et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS ONE 2014, 9, e99409. [Google Scholar] [CrossRef]
- Bazaz, R.B.; Kashaninejad, N.; Azadi, S.; Patel, K.; Asadnia, M.; Jin, D.; Warkiani, M.E. Rapid Softlithography Using 3D-Printed Molds. Adv. Mater. Technol. 2019, 8. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Gorin, M.A.; Verdone, J.E.; Van der Toom, E.; Bivalacqua, T.J.; Allaf, M.E.; Pienta, K.J. Circulating tumour cells as biomarkers of prostate, bladder, and kidney cancer. Nat. Rev. Urol. 2017, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Knoblaugh, S.; True, L. Male reproductive system. In Comparative Anatomy and Histology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 335–363. [Google Scholar]
- Saini, S. PSA and beyond: Alternative prostate cancer biomarkers. Cell Oncol. 2016, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; Yoo, J.; Carter, H.B.; Pearson, J.D.; Chan, D.W.; Epstein, J.I.; Walsh, P.C. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J. Urol. 1993, 150, 110–114. [Google Scholar] [CrossRef]
| Patient Number | n | Urine Sample Volume (mL) | N | Blood PSA level (ng/mL) | Total GS |
|---|---|---|---|---|---|
| 1 | 23 | 90 | 1.6 × 104 | 5.4 | 6 |
| 2 | 21 | 30 | 8.1 × 103 | 8.7 | 7 |
| 3 | 37 | 100 | 1.3 × 104 | 7 | 8 |
| 4 | 16 | 60 | 6.3 × 104 | 4.4 | 6 |
| 5 | 4 | 60 | 4.4 × 103 | 11 | 8 |
| 6 | 10 | 40 | 5.2 × 104 | 4.9 | 7 |
| 7 | 37 | 30 | 1.5 × 104 | 7.2 | 7 |
| 8 | 12 | 90 | 8.3 × 104 | 3.9 | 7 |
| 9 | 194 | 40 | 3.6 × 104 | 7.9 | 8 |
| 10 | 42 | 50 | 7.4 × 104 | 11.6 | 6 |
| 11 | 0 | 40 | 1.9 × 104 | 1.3 | 6 |
| 12 | 37 | 30 | 9.7 × 103 | 11 | 7 |
| 13 | 11 | 50 | 1.5 × 104 | 5.5 | 6 |
| 14 | 0 | 70 | 2.7 × 103 | 5.6 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzhevskiy, A.S.; Razavi Bazaz, S.; Ding, L.; Kapitannikova, A.; Sayyadi, N.; Campbell, D.; Walsh, B.; Gillatt, D.; Ebrahimi Warkiani, M.; Zvyagin, A.V. Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics. Cancers 2020, 12, 81. https://doi.org/10.3390/cancers12010081
Rzhevskiy AS, Razavi Bazaz S, Ding L, Kapitannikova A, Sayyadi N, Campbell D, Walsh B, Gillatt D, Ebrahimi Warkiani M, Zvyagin AV. Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics. Cancers. 2020; 12(1):81. https://doi.org/10.3390/cancers12010081
Chicago/Turabian StyleRzhevskiy, Alexey S., Sajad Razavi Bazaz, Lin Ding, Alina Kapitannikova, Nima Sayyadi, Douglas Campbell, Bradley Walsh, David Gillatt, Majid Ebrahimi Warkiani, and Andrei V. Zvyagin. 2020. "Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics" Cancers 12, no. 1: 81. https://doi.org/10.3390/cancers12010081
APA StyleRzhevskiy, A. S., Razavi Bazaz, S., Ding, L., Kapitannikova, A., Sayyadi, N., Campbell, D., Walsh, B., Gillatt, D., Ebrahimi Warkiani, M., & Zvyagin, A. V. (2020). Rapid and Label-Free Isolation of Tumour Cells from the Urine of Patients with Localised Prostate Cancer Using Inertial Microfluidics. Cancers, 12(1), 81. https://doi.org/10.3390/cancers12010081










