Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment
Abstract
1. Introduction
2. Results
2.1. Ototoxicity in S1pr2−/− Mice is Dependent on ROS Production
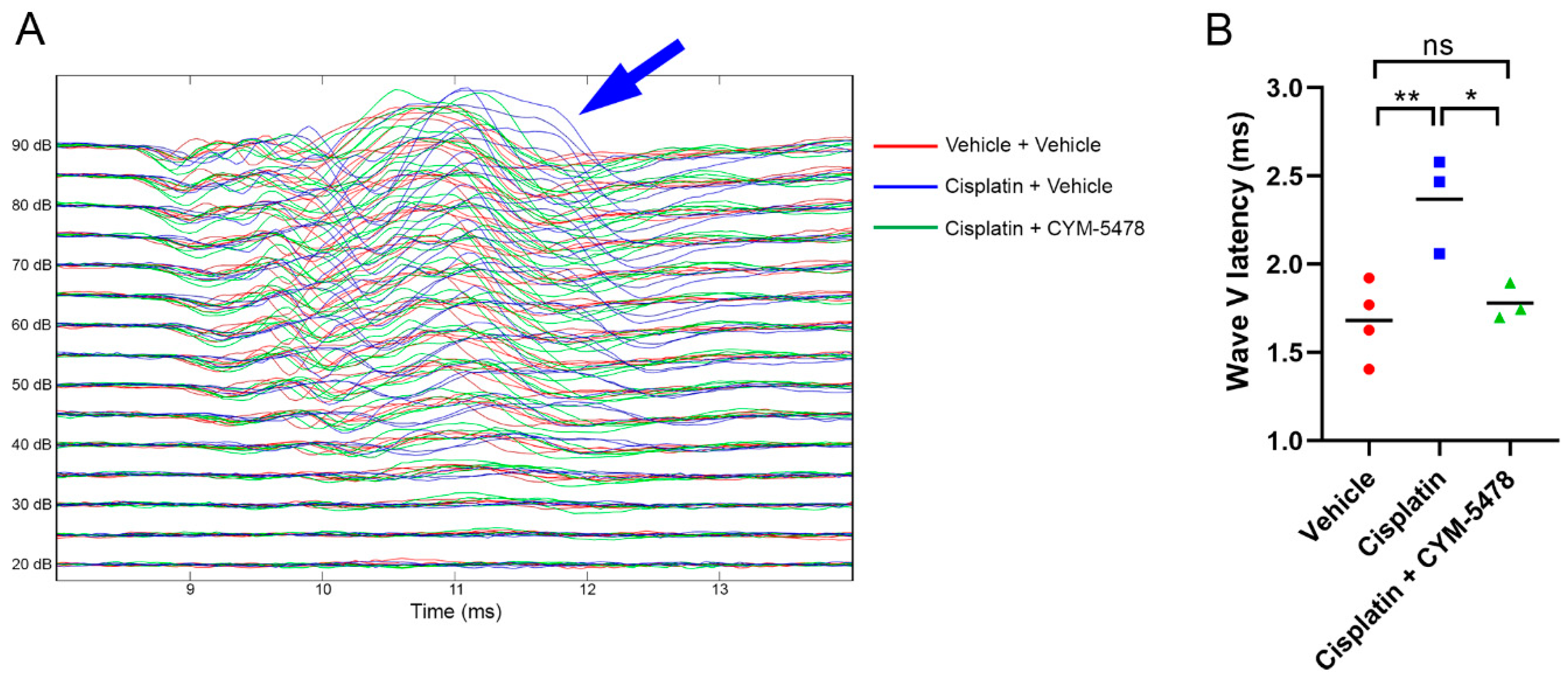
2.2. A Potent and Selective S1P2 Agonist, CYM-5478, Protects Against Cisplatin-Mediated Ototoxicity in Rats
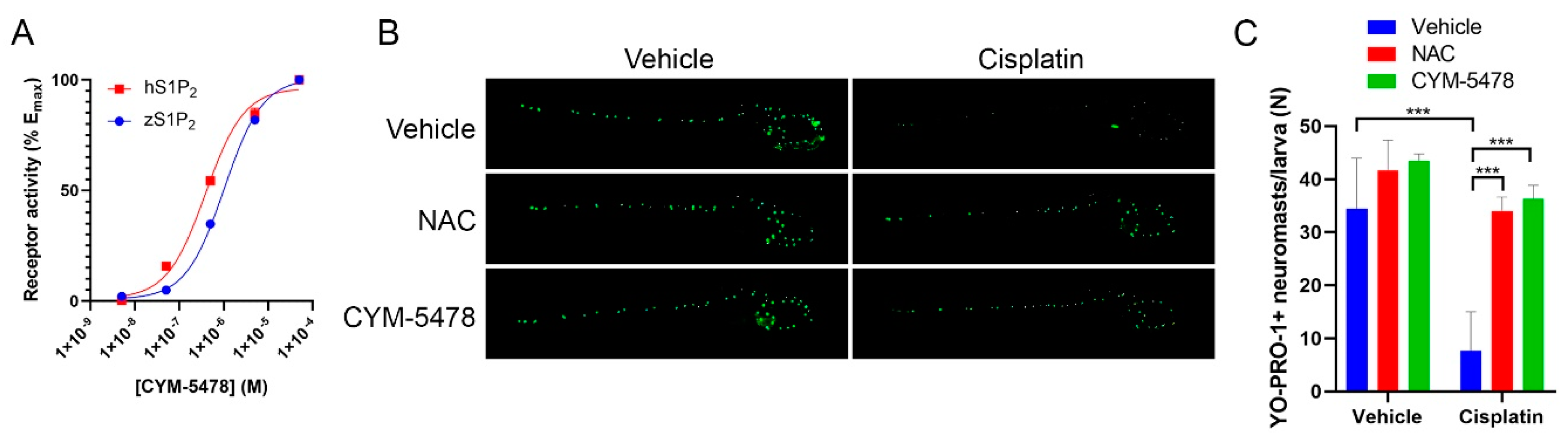
2.3. CYM-5478 Protects Against Loss of Hair Cell Viability in a Zebrafish Model for Ototoxicity
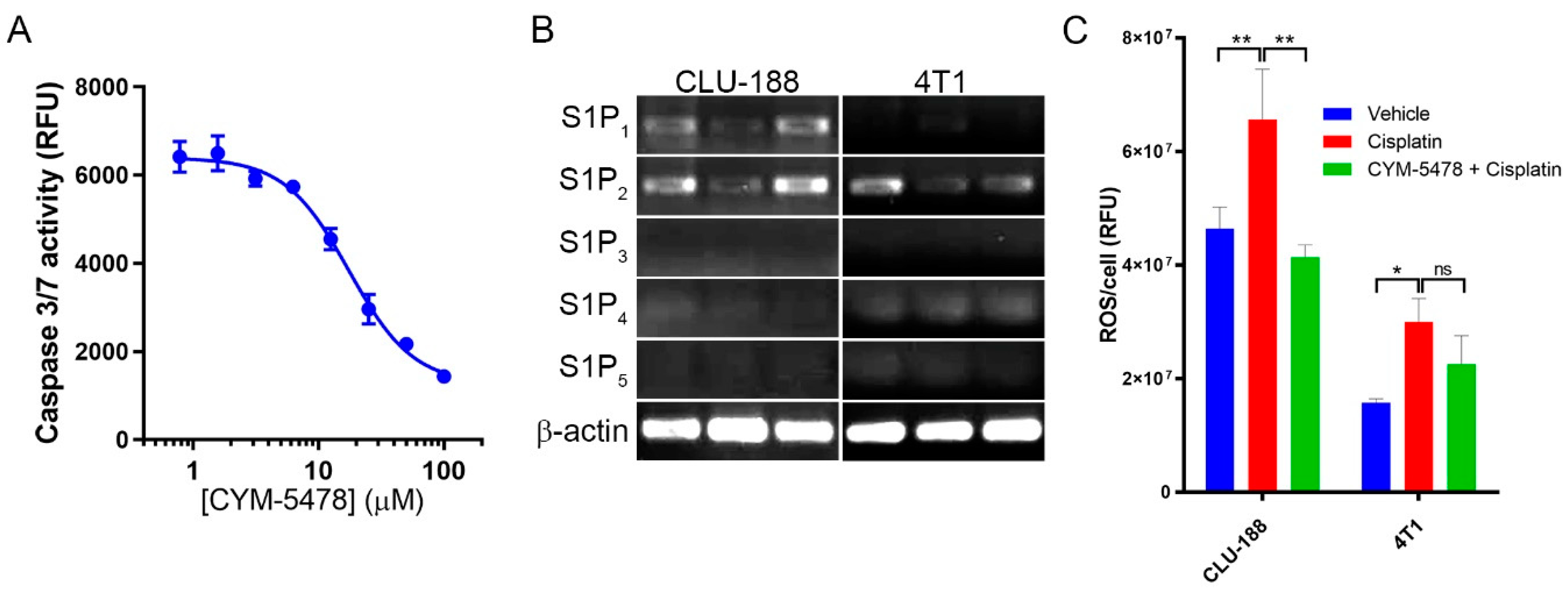
2.4. CYM-5478 Protects Neural Cells but Not Breast Cancer Cells Against Cisplatin Toxicity
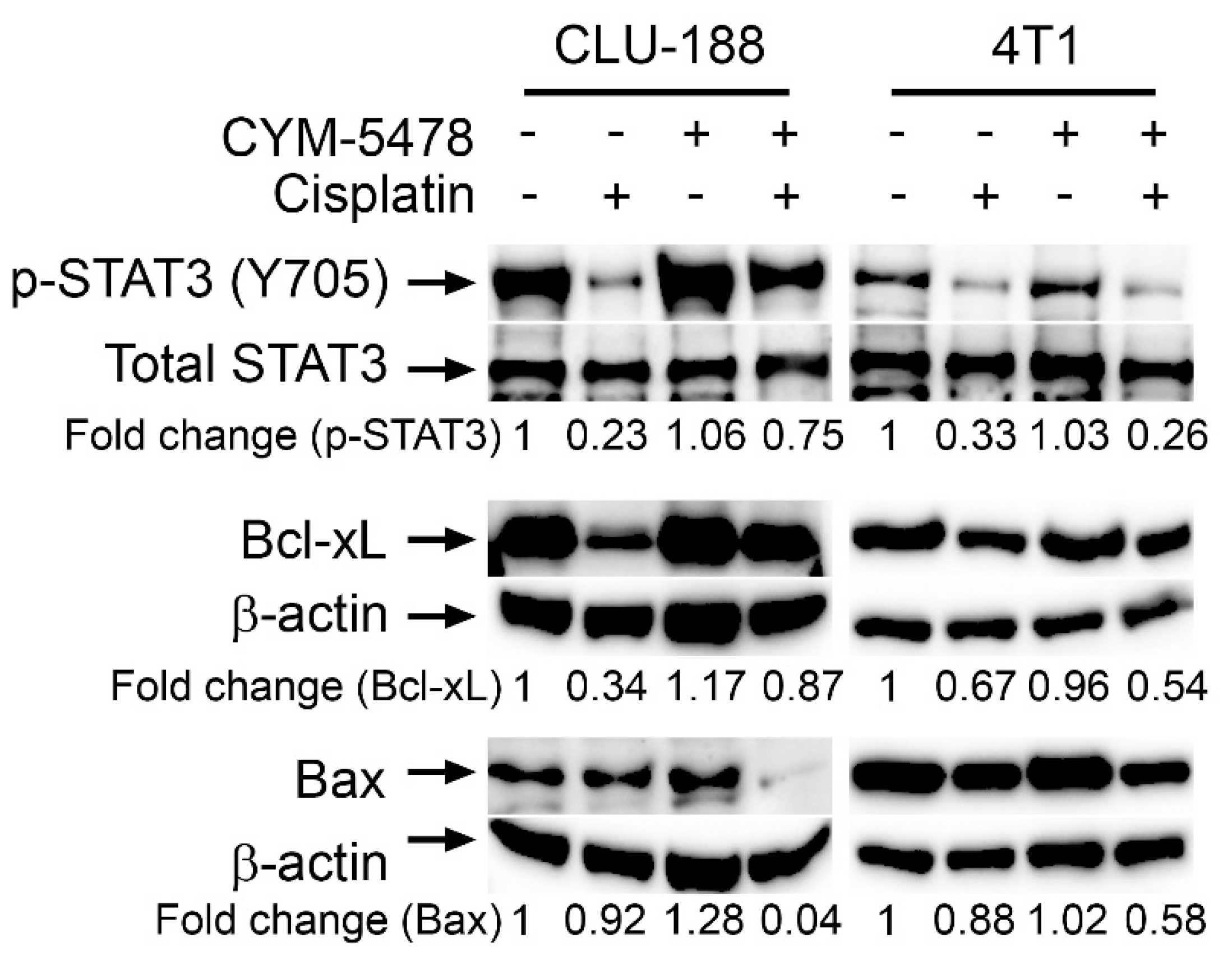
2.5. Potential Mechanisms of Action of Selective CYM-5478 Protection
3. Discussion
4. Materials and Methods
4.1. S1P2 Knockout Mice
4.2. Acoustic Startle Response (ASR)
4.3. Histology and Light Microscopy
4.4. ABR Methods
4.5. TGFα-Shedding Assay
4.6. Zebrafish Immunofluorescence Staining
4.7. Cell Treatments with Cisplatin and CYM-5478
4.8. Reactive Oxygen Species (ROS) Assay
4.9. MTT Assay
4.10. Western Blot
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Cisplatin ototoxicity and protection: Clinical and experimental studies. Tohoku J. Exp. Med. 2009, 219, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H. Curing metastatic testicular cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 4592–4595. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Lira, R.R.; Colevas, E.A.; Lavori, P.W.; Chan, C.; Shultz, D.B.; Chang, K.W. Hearing evaluation of patients with head and neck cancer: Comparison of Common Terminology Criteria for Adverse Events, Brock and Chang adverse event criteria in patients receiving cisplatin. Head Neck 2015, 37, 1102–1107. [Google Scholar] [CrossRef]
- Masters, G.A.; Temin, S.; Azzoli, C.G.; Giaccone, G.; Baker, S., Jr.; Brahmer, J.R.; Ellis, P.M.; Gajra, A.; Rackear, N.; Schiller, J.H.; et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3488–3515. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Lipp, H.P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Sastry, J.; Kellie, S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005, 22, 441–445. [Google Scholar] [CrossRef]
- Callejo, A.; Sedo-Cabezon, L.; Juan, I.D.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Buhrer, C.; Weinel, P.; Sauter, S.; Reiter, A.; Riehm, H.; Laszig, R. Acute onset deafness in a 4-year-old girl after a single infusion of cis-platinum. Pediatr. Hematol. Oncol. 1990, 7, 145–148. [Google Scholar] [CrossRef]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.H.; Beijnen, J.H.; Balm, A.J.; Schellens, J.H. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat. Rev. 2006, 32, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J. Toxicol. 2016, 2016, 1809394. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Prevention. Semin. Hear. 2019, 40, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Sphingosine 1-Phosphate Receptor 1 Signaling in Mammalian Cells. Molecules 2017, 22, 344. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Wang, W.; Yap, L.F.; Herr, D.R.; Paterson, I.C. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell Signal. 2017, 34, 66–75. [Google Scholar] [CrossRef]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Ohotski, J.; Bittman, R.; Pyne, S. The role of sphingosine 1-phosphate in inflammation and cancer. Adv. Biol. Regul. 2014, 54, 121–129. [Google Scholar] [CrossRef]
- Lidgerwood, G.E.; Pitson, S.M.; Bonder, C.; Pebay, A. Roles of lysophosphatidic acid and sphingosine-1-phosphate in stem cell biology. Prog. Lipid Res. 2018, 72, 42–54. [Google Scholar] [CrossRef]
- Patmanathan, S.N.; Johnson, S.P.; Lai, S.L.; Panja Bernam, S.; Lopes, V.; Wei, W.; Ibrahim, M.H.; Torta, F.; Narayanaswamy, P.; Wenk, M.R.; et al. Aberrant expression of the S1P regulating enzymes, SPHK1 and SGPL1, contributes to a migratory phenotype in OSCC mediated through S1PR2. Sci. Rep. 2016, 6, 25650. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hind, T.; Lam, B.W.S.; Herr, D.R. Sphingosine 1-phosphate signaling induces SNAI2 expression to promote cell invasion in breast cancer cells. FASEB J. 2019, 33, 7180–7191. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Wang, E.Y.; Yi, Y.; Thakur, A.; Tsai, S.H.; Hoodless, P.A. S1P Stimulates Proliferation by Upregulating CTGF Expression through S1PR2-Mediated YAP Activation. Mol. Cancer Res. 2018, 16, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C.; Wallington-Beddoe, C.T.; Powell, J.A.; Pitson, S.M. Targeting sphingolipid metabolism as an approach for combination therapies in haematological malignancies. Cell Death Discov. 2018, 4, 4. [Google Scholar] [CrossRef]
- Du, W.; Takuwa, N.; Yoshioka, K.; Okamoto, Y.; Gonda, K.; Sugihara, K.; Fukamizu, A.; Asano, M.; Takuwa, Y. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010, 70, 772–781. [Google Scholar] [CrossRef]
- Asghar, M.Y.; Kemppainen, K.; Lassila, T.; Tornquist, K. Sphingosine 1-phosphate attenuates MMP2 and MMP9 in human anaplastic thyroid cancer C643 cells: Importance of S1P2. PLoS ONE 2018, 13, e0196992. [Google Scholar] [CrossRef]
- Yamashita, H.; Kitayama, J.; Shida, D.; Yamaguchi, H.; Mori, K.; Osada, M.; Aoki, S.; Yatomi, Y.; Takuwa, Y.; Nagawa, H. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: Differential regulation on the migration and proliferation. J. Surg. Res. 2006, 130, 80–87. [Google Scholar] [CrossRef]
- El Buri, A.; Adams, D.R.; Smith, D.; Tate, R.J.; Mullin, M.; Pyne, S.; Pyne, N.J. The sphingosine 1-phosphate receptor 2 is shed in exosomes from breast cancer cells and is N-terminally processed to a short constitutively active form that promotes extracellular signal regulated kinase activation and DNA synthesis in fibroblasts. Oncotarget 2018, 9, 29453–29467. [Google Scholar] [CrossRef]
- Herr, D.R.; Grillet, N.; Schwander, M.; Rivera, R.; Muller, U.; Chun, J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J. Neurosci. 2007, 27, 1474–1478. [Google Scholar] [CrossRef]
- Kono, M.; Belyantseva, I.A.; Skoura, A.; Frolenkov, G.I.; Starost, M.F.; Dreier, J.L.; Lidington, D.; Bolz, S.S.; Friedman, T.B.; Hla, T.; et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 2007, 282, 10690–10696. [Google Scholar] [CrossRef]
- MacLennan, A.J.; Benner, S.J.; Andringa, A.; Chaves, A.H.; Rosing, J.L.; Vesey, R.; Karpman, A.M.; Cronier, S.A.; Lee, N.; Erway, L.C.; et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear. Res. 2006, 220, 38–48. [Google Scholar] [CrossRef]
- Ingham, N.J.; Carlisle, F.; Pearson, S.; Lewis, M.A.; Buniello, A.; Chen, J.; Isaacson, R.L.; Pass, J.; White, J.K.; Dawson, S.J.; et al. S1PR2 variants associated with auditory function in humans and endocochlear potential decline in mouse. Sci. Rep. 2016, 6, 28964. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Reolo, M.J.; Peh, Y.X.; Wang, W.; Lee, C.W.; Rivera, R.; Paterson, I.C.; Chun, J. Sphingosine 1-phosphate receptor 2 (S1P2) attenuates reactive oxygen species formation and inhibits cell death: Implications for otoprotective therapy. Sci. Rep. 2016, 6, 24541. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.; Cunningham, L.L.; Raible, D.W.; Rubel, E.W.; Ou, H.C. Using the zebrafish lateral line to screen for ototoxicity. J. Assoc. Res. Otolaryngol. 2008, 9, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.C.; Cunningham, L.L.; Francis, S.P.; Brandon, C.S.; Simon, J.A.; Raible, D.W.; Rubel, E.W. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J. Assoc. Res. Otolaryngol. 2009, 10, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Rizzo, I.M.; Romero-Guevara, R.; Bernacchioni, C.; Cencetti, F.; Donati, C.; Bruni, P. Sphingosine 1-phosphate signaling axis mediates fibroblast growth factor 2-induced proliferation and survival of murine auditory neuroblasts. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 814–824. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Bruno, M.; Squecco, R.; Idrizaj, E.; Berbeglia, M.; Bruni, P.; Donati, C. Sphingosine 1-phosphate-mediated activation of ezrin-radixin-moesin proteins contributes to cytoskeletal remodeling and changes of membrane properties in epithelial otic vesicle progenitors. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 554–565. [Google Scholar] [CrossRef]
- Romero-Guevara, R.; Cencetti, F.; Donati, C.; Bruni, P. Sphingosine 1-phosphate signaling pathway in inner ear biology. New therapeutic strategies for hearing loss? Front. Aging Neurosci. 2015, 7, 60. [Google Scholar] [CrossRef]
- Campbell, K.C.; Rybak, L.P.; Meech, R.P.; Hughes, L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear. Res. 1996, 102, 90–98. [Google Scholar] [CrossRef]
- Authier, N.; Gillet, J.P.; Fialip, J.; Eschalier, A.; Coudore, F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp. Neurol. 2003, 182, 12–20. [Google Scholar] [CrossRef]
- De Lauretis, A.; De Capua, B.; Barbieri, M.T.; Bellussi, L.; Passali, D. ABR evaluation of ototoxicity in cancer patients receiving cisplatin or carboplatin. Scand. Audiol. 1999, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Nicol, T.; Zecker, S.G.; Kraus, N. Developmental plasticity in the human auditory brainstem. J. Neurosci. 2008, 28, 4000–4007. [Google Scholar] [CrossRef]
- Sharma, M.; Bist, S.S.; Kumar, S. Age-Related Maturation of Wave V Latency of Auditory Brainstem Response in Children. J. Audiol. Otol. 2016, 20, 97–101. [Google Scholar] [CrossRef] [PubMed]
- van Ruijven, M.W.; de Groot, J.C.; Smoorenburg, G.F. Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. A quantitative histological study. Hear. Res. 2004, 197, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Cattoretti, G.; Mandelbaum, J.; Lee, N.; Chaves, A.H.; Mahler, A.M.; Chadburn, A.; Dalla-Favera, R.; Pasqualucci, L.; MacLennan, A.J. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 2009, 69, 8686–8692. [Google Scholar] [CrossRef]
- Bruno, G.; Cencetti, F.; Pini, A.; Tondo, A.; Cuzzubbo, D.; Fontani, F.; Strinna, V.; Buccoliero, A.M.; Casazza, G.; Donati, C.; et al. beta3-adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation. Oncogene 2019. [Google Scholar] [CrossRef]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef]
- Subramaniam, A.; Shanmugam, M.K.; Ong, T.H.; Li, F.; Perumal, E.; Chen, L.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br. J. Pharmacol. 2013, 170, 807–821. [Google Scholar] [CrossRef]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef]
- Lee, M.; Hirpara, J.L.; Eu, J.Q.; Sethi, G.; Wang, L.; Goh, B.C.; Wong, A.L. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2018, 101073. [Google Scholar] [CrossRef]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that gamma-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-kappaB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Kumar, A.P.; Arfuso, F.; Chng, W.J.; Sethi, G. The Role of Signal Transducer and Activator of Transcription 3 (STAT3) and Its Targeted Inhibition in Hematological Malignancies. Cancers 2018, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, H.; Yin, X.; Long, L.; Chen, X.; Feng, F.; Liu, Y.; Zhao, P.; Xu, Y.; Li, M.; et al. IL-6R/STAT3/miR-204 feedback loop contributes to cisplatin resistance of epithelial ovarian cancer cells. Oncotarget 2017, 8, 39154–39166. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.; Bas, E.; Dinh, J.; Vu, L.; Gupta, C.; Van De Water, T.R. Short interfering RNA against Bax attenuates TNFalpha-induced ototoxicity in rat organ of Corti explants. Otolaryngol. Head Neck Surg. 2013, 148, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, S.H.; Kim, T.H.; Go, Y.Y.; Song, J.J. Anti-apoptotic effect of dexamethasone in an ototoxicity model. Biomater. Res. 2017, 21, 4. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Han, J. Sphingosine-1-phosphate receptor 2 modulates pain sensitivity by suppressing the ROS-RUNX3 pathway in a rat model of neuropathy. J. Cell Physiol. 2020, 235, 3864–3873. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, P.; Chew, W.S.; Torta, F.; Bandla, A.; Lopez, V.; Seow, W.L.; Lam, B.W.S.; Chang, J.K.; Wong, P.; et al. Activation of sphingosine 1-phosphate receptor 2 attenuates chemotherapy-induced neuropathy. J. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Ishii, I.; Ye, X.; Friedman, B.; Kawamura, S.; Contos, J.J.; Kingsbury, M.A.; Yang, A.H.; Zhang, G.; Brown, J.H.; Chun, J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J. Biol. Chem. 2002, 277, 25152–25159. [Google Scholar] [CrossRef]
- Misawa, H.; Sherr, E.H.; Lee, D.J.; Chetkovich, D.M.; Tan, A.; Schreiner, C.E.; Bredt, D.S. Identification of a monogenic locus (jams1) causing juvenile audiogenic seizures in mice. J. Neurosci. 2002, 22, 10088–10093. [Google Scholar] [CrossRef][Green Version]
- Inoue, A.; Ishiguro, J.; Kitamura, H.; Arima, N.; Okutani, M.; Shuto, A.; Higashiyama, S.; Ohwada, T.; Arai, H.; Makide, K.; et al. TGFalpha shedding assay: An accurate and versatile method for detecting GPCR activation. Nat. Methods 2012, 9, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Mellon, P.L.; Windle, J.J.; Goldsmith, P.C.; Padula, C.A.; Roberts, J.L.; Weiner, R.I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990, 5, 1–10. [Google Scholar] [CrossRef]
- Belsham, D.D.; Fick, L.J.; Dalvi, P.S.; Centeno, M.L.; Chalmers, J.A.; Lee, P.K.; Wang, Y.; Drucker, D.J.; Koletar, M.M. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 2009, 23, 4256–4265. [Google Scholar] [CrossRef]
- Uehara, S.; Abe, H.; Hoshiai, H.; Yajima, A.; Suzuki, M. Establishment and characterization of ovarian endometrioid carcinoma cell line. Gynecol. Oncol. 1984, 17, 314–325. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Ye, S.; Liu, Y.; Chen, J.; Xue, Q.; Huang, X.; Chen, J.; Bao, W.; Yang, J.; et al. [Establishment of human hepatocellular carcinoma cell line with spontaneous pulmonary metastasis through in vivo selection]. Zhonghua Yi Xue Za Zhi 2002, 82, 601–605. [Google Scholar] [PubMed]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2004, 5, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, F.; Kalinec, G.; Boukhvalova, M.; Kachar, B. Establishment and characterization of conditionally immortalized organ of corti cell lines. Cell Biol. Int. 1999, 23, 175–184. [Google Scholar] [CrossRef]
- Srikanth, M.; Chandrasaharan, K.; Zhao, X.; Chayaburakul, K.; Ong, W.Y.; Herr, D.R. Metabolism of Docosahexaenoic Acid (DHA) Induces Pyroptosis in BV-2 Microglial Cells. Neuromol. Med. 2018, 20, 504–514. [Google Scholar] [CrossRef]

| Cell Origin | Cell Line | EC50 Vehicle | EC50 CYM-5478 | p Value |
|---|---|---|---|---|
| Neural-derived | C6 | 1.34 | 4.54 | <0.001 |
| GT1-7 | 5.47 | 17.0 | <0.001 | |
| SK-N-BE2 | 4.06 | 7.44 | <0.001 | |
| CLU188 | 3.23 | 5.54 | 0.00160 | |
| SH-SY5Y (undifferentiated) | 6.13 | 8.35 | 0.203 | |
| SH-SY5Y (differentiated) | 14.1 | 25.3 | 0.00830 | |
| iNHA | 18.6 | 17.5 | 0.821 | |
| Breast cancer | MDA-MB-231 | 66.1 | 53.2 | 0.178 |
| MDA-MB-436 | 8.72 | 8.16 | 0.882 | |
| MDA-MB-453 | 8.92 | 4.74 | <0.001 | |
| MCF7 | 57.9 | 36.7 | 0.0429 | |
| 4T1 | 8.16 | 4.09 | <0.001 | |
| BT474 | 24.4 | 18.4 | 0.0670 | |
| Ovarian cancer | CHO | 10.36 | 9.12 | 0.358 |
| OVK18 | 9.64 | 13.9 | 0.0729 | |
| Prostate cancer | DU145 | 1.687 | 1.27 | 0.204 |
| Liver cancer | HCCLM3 | 12.5 | 11.7 | 0.590 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Shanmugam, M.K.; Xiang, P.; Yam, T.Y.A.; Kumar, V.; Chew, W.S.; Chang, J.K.; Ali, M.Z.B.; Reolo, M.J.Y.; Peh, Y.X.; et al. Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment. Cancers 2020, 12, 211. https://doi.org/10.3390/cancers12010211
Wang W, Shanmugam MK, Xiang P, Yam TYA, Kumar V, Chew WS, Chang JK, Ali MZB, Reolo MJY, Peh YX, et al. Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment. Cancers. 2020; 12(1):211. https://doi.org/10.3390/cancers12010211
Chicago/Turabian StyleWang, Wei, Muthu K. Shanmugam, Ping Xiang, Ting Yu Amelia Yam, Vineet Kumar, Wee Siong Chew, Jing Kai Chang, Muhammad Zulfaqar Bin Ali, Marie J. Y. Reolo, Yee Xin Peh, and et al. 2020. "Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment" Cancers 12, no. 1: 211. https://doi.org/10.3390/cancers12010211
APA StyleWang, W., Shanmugam, M. K., Xiang, P., Yam, T. Y. A., Kumar, V., Chew, W. S., Chang, J. K., Ali, M. Z. B., Reolo, M. J. Y., Peh, Y. X., Abdul Karim, S. N. B., Tan, A. Y. Y., Sanda, T., Sethi, G., & Herr, D. R. (2020). Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment. Cancers, 12(1), 211. https://doi.org/10.3390/cancers12010211






